1 Introduction
Oxidation of sulfides is the most straightforward method for the synthesis of sulfoxides and sulfones [1]. Sulfoxides and sulfones are important intermediates for pharmaceuticals, polymer materials and ligands in asymmetric catalysis. Also, these compounds could be used as oxotransfer reagents and biologically active molecules [1,2].
Many kinds of oxidants such as nitric acid, organic peroxides, and heavy metal oxidants have been employed as effective oxidants to form sulfoxides and/or sulfones in high yields [1]. Among various kinds of oxidants, hydrogen peroxide (H2O2) is one of the most straightforward, clean, and versatile oxidants from both an environmental and economic perspective because H2O2 has a high content of active oxygen and its byproduct is water [3]. It is well known that sulfides are oxidized to generate sulfoxides and sulfones by H2O2 at an elevated temperature and/or with a long reaction time without a metal catalyst [4]. However, highly selective oxidation of sulfides under mild reaction conditions is achieved by the application of optimized catalysts and/or reaction systems [5].
Schiff bases have played an important role in the development of coordination chemistry; for example, they readily form stable complexes with most of the transition metals. Also, these compounds are often used as catalysts for oxidation reactions under different conditions [6–10]. Although Schiff base complexes are often used as efficient homogeneous catalysts for the oxidation reaction, some problems often exist in these types of oxidation studies such as (1) deactivation, (2) instability and (3) expensive recycling of these homogeneous systems [11]. So, for improving the performance of catalytic activity, scientists tried to convert them into heterogeneous ones in some different ways. The greatest advantage of heterogeneous catalysis is the facile separation of catalysts from the reaction media and products [12]. In order to fix catalysts into heterogeneous supports, different ways can be used, classified as: (1) immobilization in zeolites, (2) grafting onto inorganic supports such as silica, and (3) copolymerization and attachment of the catalyst onto an organic polymer and use of insoluble complexes as heterogeneous catalysts without any supports [12,13]. The latter is a very good way for synthesis of heterogeneous catalysts because of removal of some synthesis steps. However, a major challenge is design of insoluble catalysts with high yields, selectivity, and cost effectiveness. According to all the above discussion, insoluble Schiff base complexes with good stability and catalytic activity are still rare and lack generality. Most of the Schiff base complexes that are used as heterogeneous catalysts form covalent bonds or electrostatic interactions with organic polymers.
This paper is a continuation of our previous study on Schiff base ligands and complexes with ortho-aminophenyl diamines [14]. In our previous study, we reported the crystal and molecular structure of the precursory 1,2-bis(2′-nitrophenoxy)-4-methylbenzene prepared from an achiral molecule (4-methylcatechol), in which the two phenoxy groups are placed to one another in such a way that the molecule is C1 symmetrical, i.e., chiral. The chiral conformation of 1,2-bis(2′-nitrophenoxy)-4-methylbenzene after reduction is maintained in complexation with zinc(II) and cobalt(II). This implies that in Schiff base ligands derived from ortho-aminophenyl diamines, the two α-diimine systems form an octahedral cavity having the same chirality as that of the precursory diamine [14]. In a continuation of this study, herein we report the synthesis and characterization of four new Schiff base complexes, CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) derived from 1,2-bis(2′-aminophenoxy)benzene and 2-pyridinecarbaldehyde. These complexes have very poor solubility in all polar and non-polar solvents. This parameter (insolubility) causes these complexes to be suitable for acting as heterogeneous catalysts without attachment to organic/inorganic supports. Therefore, these complexes have been used for selective heterogeneous oxidation of sulfides in acetonitrile. The results showed high stability and reusability in oxidation reactions.
2 Experimental
2.1 Physical methods
Infrared spectra (KBr pellets) were recorded on a JASCO, FT/IR-6300 instrument. The elemental analysis (CHN analysis) was carried out on Leco, CHNS-932 and PerkinElmer 7300 DV elemental analyzers. Powder X-ray diffraction (XRD) data were obtained on a D8 Advanced Bruker using Cu Kα radiation (2θ = 5–70°). The oxidation products were quantitatively analyzed by gas chromatography (GC) on a Shimadzu GC-16A instrument using a 2 m column packed with a silicon DC-200 and an FID detector. 1H and 13C NMR spectra of the ligand were recorded on a Bruker Avance 400 spectrometer using CDCl3 as the solvent.
2.2 Reagents
All chemicals used were of analytical grade and were used as received without any further purification and were obtained from Sigma–Aldrich. 1,2-bis(2′-aminophenoxy)benzene was prepared according to literature methods [15].
2.2.1 Synthesis of the Schiff base ligand (L)
2-Pyridinecarboxaldehyde (4 mmol) in absolute EtOH (50 mL) was added dropwise to a boiling solution of 1,2-bis(2′-aminophenoxy)benzene (2 mmol) in absolute EtOH (25 mL). The solution was gently refluxed for 12 h. Then, the solvent volume was reduced by using a rotary evaporator (∼5 mL) and cooled in an ice bath for 5 h. The precipitate was filtered off and washed with cold EtOH and dried in vacuo. Yield: 58%. Anal. Calcd for C30H22N4O2 C, 76.4; H, 4.7; N, 11.8. Found: C, 76.5; H, 4.8; N, 11.8%. 1H NMR δH (300 MHz, CDCl3): 9.29 (2H, HCN), 8.87 (2H, pyridine), 8.16 (2H, pyridine), and 8.01–6.63 (aromatic (12H) and pyridine (4H)) ppm. 13C NMR δC (300 MHz, CDCl3): 163.51 (CN)imi, and 150.34–115.34 (aromatic and pyridine rings) ppm. IR: 1637 (CHN)imi, 1601 (CHN)py, and 1488 (CC)py.
2.2.2 Preparation of complexes
2.2.2.1 General synthesis
2-Pyridinecarboxaldehyde (4 mmol) in dry ethanol (50 mL) was added dropwise to a boiling solution of 1,2-bis(2′-aminophenoxy)benzene (2 mmol) in the same solvent (25 mL). The solution was stirred and refluxed for 12 h and then the appropriate salt (2 mmol) in EtOH (20 mL) was added dropwise. Upon addition, immediate precipitation of complexes occurs. The solution was refluxed for 6 h, and concentrated in a rotary evaporator until approximately 10–15 mL. The obtained precipitate was filtered, subsequently washed with EtOH and then dried in air.
2.2.2.2 Ni complex NiLCl2
Yield: (87%). Anal. Calcd for C30H22Cl2N4NiO2: C, 60.04; H, 3.70; N, 9.34. Found: C, 60.09; H, 3.74; N, 9.39%. IR: 1635 (CHN)imi, 1595 (CHN)py, and 1483 (CC)py, 404 (M − N).
2.2.2.3 Co complex CoL(NO3)2
Yield: (81%). Anal. Calcd for C30H22CoN6O8: C, 55.14; H, 3.39; N, 12.86. Found: C, 55.06; H, 3.43; N, 12.79%. IR: 1628 (CHN)imi, 1595 (CHN)py, 1485 (CC)py, 1456 and 1318 (NO3), 403 (M − N).
2.2.2.4 Zn complex ZnL(NO3)2
Yield: (94%). Anal. Calcd for C30H22N6O8Zn: C, 54.60; H, 3.36; N, 12.73. Found: C, 54.65; H, 3.41; N, 12.69%. IR: 1633 (CHN)imi, 1595 (CHN)py, 1485 (CC)py,1456 and 1323 (NO3), 407 (M − N).
2.2.2.5 Pd complex Pd2LCl4
Yield: (92%). Anal. Calcd for C30H22Cl4N4O2Pd2: C, 43.67; H, 2.69; N, 6.79. Found: C, 43.69; H, 2.68; N, 6.83%. IR: 1619 (CHN)imi, 1587 (CHN)py, and 1488 (CC)py, 412 (M − N).
2.3 Catalytic activity and optimization of reaction conditions
After the synthesis of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes, we decided to investigate the catalytic activity of the complexes in oxidation of sulfides with H2O2 as the oxidant under different reaction conditions.
2.3.1 Oxidation of thioanisole with H2O2 catalyzed by Co, Ni, Zn and Pd Schiff base complexes
To find the optimized conditions, we investigated the oxidation of thioanisole as a model substrate using hydrogen peroxide (Scheme 1). Generally, oxidation of thioanisole gives a sulfoxide and sulfone. In our catalytic reaction sulfone is formed as the major product (Scheme 1). The effect of different reaction parameters (H2O2 amount, temperature and amount of catalyst) was studied on the thioanisole oxidation.
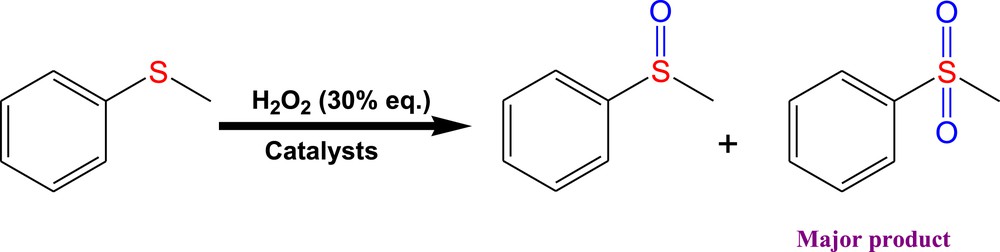
Oxidation of thioanisole with hydrogen peroxide in the presence of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes as catalysts.
2.3.2 General procedure for catalytic oxidation of sulfides in acetonitrile
A mixture of 1 mmol sulfide and H2O2 (3 mmol) was added to a stirring solution of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes (0.01 mmol) in acetonitrile (3 ml) at 50 °C for the required period of time (4 h). After completion of the reaction (TLC), the catalyst was separated by filtration, washed three times with acetonitrile and then dried under vacuum and used for the next oxidation cycle. The products were analyzed by GC using diphenyl sulfide as the internal standard. NMR spectra of the products (sulfone) are available in Supplementary data.
3 Results and discussion
3.1 Characterization of Schiff base ligand and complexes
The procedure for the synthesis of the Schiff base ligand, L, is reported in Scheme 2. The ligand was obtained by the self-condensation reaction between 1,2-bis(2′-aminophenoxy)benzene and 2-pyridinecarboxaldehyde with ethanol as the solvent under reflux conditions. The chemical structure of the ligand was confirmed by elemental analysis and IR, 1H NMR and 13C NMR spectroscopy. Formation of the Schiff base ligand is evidenced by the presence of a strong IR band at 1637 cm−1 due to ν(CN), while no bands attributable to ν(CO) or ν(NH2) have been detected. The bands at 1601 and 1488 cm−1 of the pyridine ring vibrations are also present in the IR spectrum of the ligand [16]. The 1H NMR spectrum is consistent with the IR observations. The 1H NMR spectrum in CDCl3 shows a peak at 9.29 ppm corresponding to the imine protons of the ligand. Cobalt(II), Nickel(II), Zinc(II) and Palladium(II) Schiff base complexes, CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4), were synthesized by the reaction of Co(NO3)2.6H2O, NiCl2·6H2O, Zn(NO3)2·6H2O and PdCl2 with the tetradentate Schiff base ligand, L, in ethanol under reflux conditions, respectively (Scheme 2). All of the Schiff base complexes are stable in air. Their photographic images are shown in Fig. 1. The bands in the range of 1619–1635 cm−1 were assigned to the –CHN– group. The imine peak of the complexes showed a red-shift of ca. 2–18 cm−1 compared to that of the ligand, indicating the coordination of the imine nitrogen to the metal ions (Scheme 2) [17,18]. This feature can be explained by the withdrawal of electrons from the nitrogen atom to the metal ion due to the coordination process. The IR spectra exhibit medium to strong bands at ∼1595 and ∼1480 cm−1 as expected for the two highest energy pyridine-ring vibrations. The shift of the imine and pyridine bands by complexation suggests coordination via the imine and pyridine nitrogen atoms. Another conclusive evidence for the formation of the M–N bond is also shown by the appearance of a new band at around 405 cm−1 which could be assigned to the M–N stretching vibrations [19].
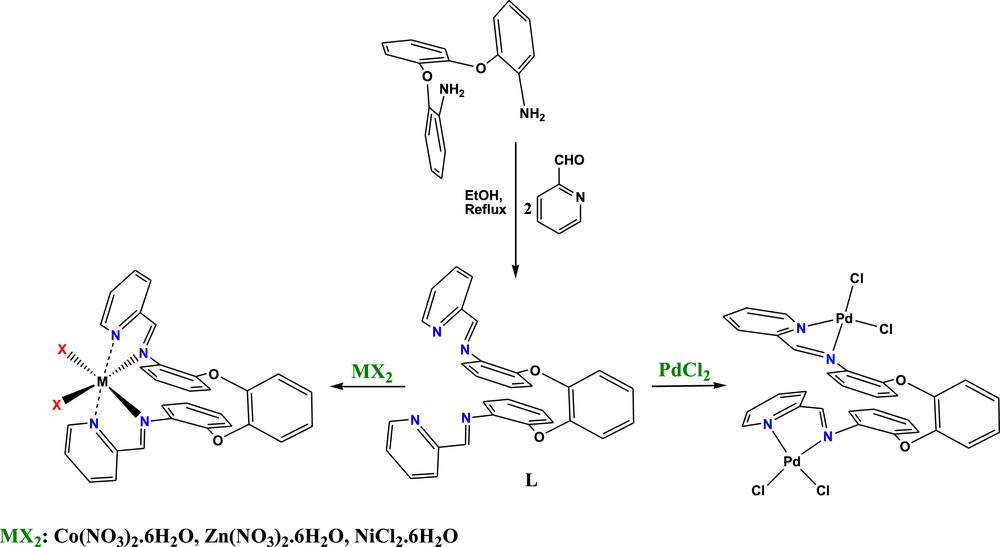
Synthetic routes for the preparation of Schiff base complexes.

The photographic images of CoL(NO3)2 (1), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes from left to right. The color of NiLCl2 (2) and ZnL(NO3)2 (3) Schiff base complexes are identical.
When the fundamental IR bands of the NO3− group would be identified, it is possible to tell if the NO3− group is ionic or coordinated. The occurrence of two strong absorption bands in the complexes under study, 1 and 2, at ∼1450 and ∼1320 cm−1 is attributed to ν4 and ν1 modes of vibrations of the covalently bonded nitrate groups, respectively. This suggests that nitrate groups are present inside the coordination sphere [20,21]. Also, if the (ν4 − ν1) difference is taken as an approximate measure of the covalency of the nitrate group [22,23], a value of ∼200 cm−1 for the complexes studied suggests strong covalency of the metal-nitrate bonding. The strong and sharp band at ∼1384 cm−1 is characteristic of ionic nitrate. As depicted in Fig. 2, we do not observe this peak in the IR spectrum of zinc(II) and cobalt(II) complexes.
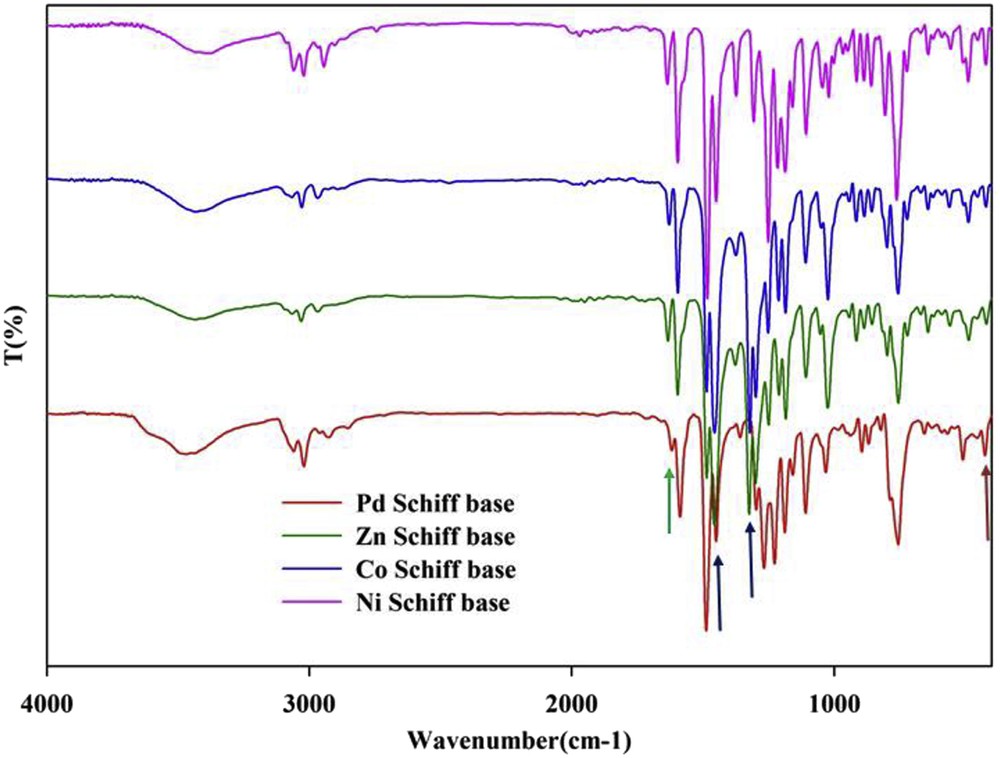
FT-IR spectra of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes. Important peaks are indicated by arrows (see the text).
Devi et al. have shown that the number and relative energies of nitrate combination frequencies (ν1 + ν4) in the 1700–1800 cm−1 region of the infrared spectrum may be used as an aid to distinguish the various coordination modes of the nitrate groups [24]. According to Agarwal et al. bidentate coordination involves a greater distortion from D3h symmetry than monodentate coordination; therefore, bidentate complexes should show a larger separation of (ν1 + ν4) [25]. By studying the spectra of a number of compounds with known crystal structures, Devi et al. [24] showed that the separation of monodentate nitrate groups appeared to be 5–26 cm−1 and that of bidentate groups to be 25–66 cm−1. However, these absorptions are very weak and can only be used for diagnosis when there are no other absorptions in this region. The authors have tried to apply this method for cobalt (II) and zinc(II) complexes. In both cases, CoL(NO3)2 (1) and ZnL(NO3)2 (3), a separation of 20–25 cm−1 in the combination bands (ν1 + ν4) in the 1700–1800 cm−1 region concludes the monodentate nitrate coordination. On the basis of the above discussion, a six-coordinated structure is proposed for cobalt (II), zinc(II) and nickel(II) complexes in which the metal ions coordinate via four azomethine nitrogens and the two remaining coordination sites are occupied by two oxygen atoms of two monodentate NO3− groups in zinc and cobalt complexes and by two Cl atoms in the nickel complex (Scheme 2). As we know, six-coordinate structures are very common for Co(II), Ni(II) and Zn(II) complexes. It should be noted that these structures were confirmed in our previous work by X-ray crystallography [14].
Because of the great size of the palladium atom and impossibility of the octahedral structure for palladium complex, we suggest different coordination modes for the Pd(II) complex. This proposed structure was confirmed by elemental analysis. It should be noted that in our previous work, we proved that the four azomethine nitrogen atoms of the ligand are not able to provide a square planar conformation when the metal atom lies in the ligand cavity [14]. For this reason, the metal atom must be out of the ligand cavity if a square planar configuration is assumed for the metal ion (Scheme 2). As shown in Scheme 2, we suggest a bimetallic structure for the Pd(II) complex. Each Pd(II) atom is coordinated to one pyridine nitrogen and one azomethine nitrogen. Two remaining coordination sites are occupied by two Cl atoms.
Fig. 3 shows the X-ray powder diffraction pattern of four Schiff base complexes. As depicted in Fig. 3, the XRD patterns of CoL(NO3)2 (1) NiLCl2 (2) and ZnL(NO3)2 (3) are almost identical.
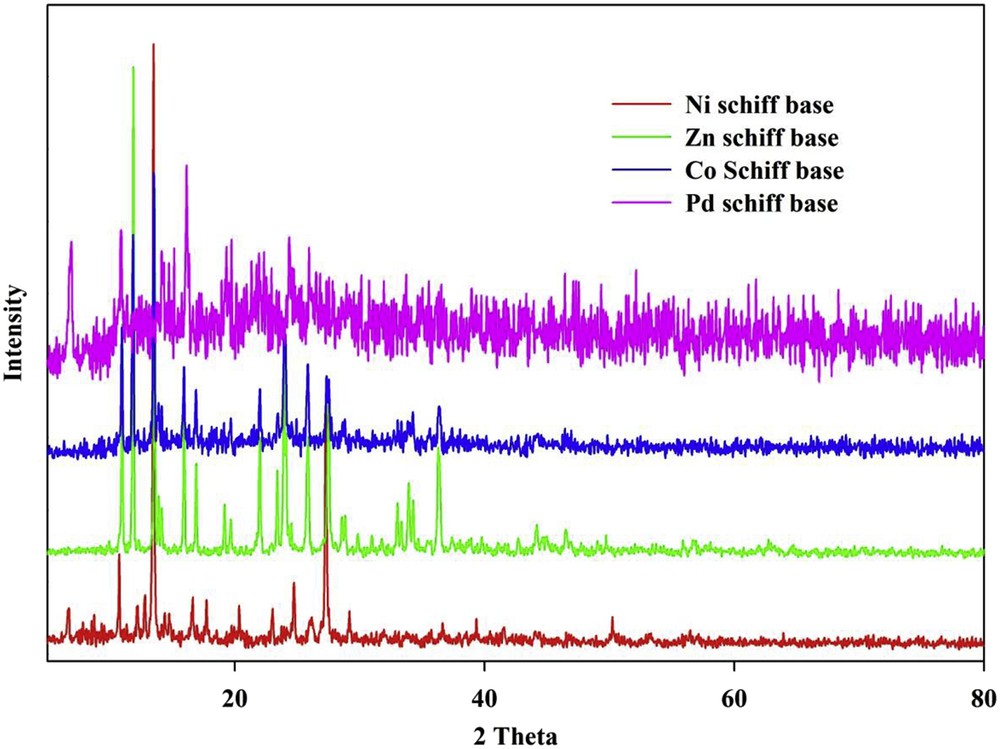
XRD pattern of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) Schiff base complexes.
3.2 Application of Schiff base complexes for the oxidation of sulfides
In order to optimize the reaction conditions, we surveyed the oxidation reaction of thioanisole as a model compound using 30% H2O2 under various reaction conditions in terms of time and product yield.
3.2.1 Effect of H2O2 amounts on the oxidation of thioanisole
In order to determine the role of H2O2 amounts on the oxidation of thioanisole to the corresponding sulfone and sulfoxide, the mole ratio of H2O2/thioanisole was changed, keeping all other parameters fixed: catalysts (5 mg), temperature (50 °C) and reaction time (4 h). The results are shown in Table 1.
Oxidation of thioanisole with various amounts of H2O2 in the presence of Co, Ni, Zn and Pd Schiff base complexes.a
| Entry | Catalyst | mmol H2O2 | Conversion (%)b | Selectivity (%)c |
| 1 | Co | 1 | 9 | 60 |
| 2 | Co | 2 | 14 | 67 |
| 3 | Co | 3 | 19 | 71 |
| 4 | Co | 4 | 23 | 80 |
| 5 | Ni | 1 | 24 | 54 |
| 6 | Ni | 2 | 31 | 66 |
| 7 | Ni | 3 | 36 | 70 |
| 8 | Ni | 4 | 41 | 75 |
| 9 | Zn | 1 | 27 | 57 |
| 10 | Zn | 2 | 34 | 64 |
| 11 | Zn | 3 | 39 | 69 |
| 12 | Zn | 4 | 41 | 73 |
| 13 | Pd | 1 | 51 | 80 |
| 14 | Pd | 2 | 57 | 92 |
| 15 | Pd | 3 | 70 | 99 |
| 16 | Pd | 4 | 72 | 99 |
a Reaction conditions: thioanisole (1 mmol), catalyst (5 mg), acetonitrile (3 mL) and H2O2 30% at 50 °C for 4 h.
b Conversion based on sulfide substrates.
c Selectivity for sulfone.
For all four complexes, H2O2/thioanisole molar ratios from 1:1 to 4:1 resulted in 9%–72% conversion. When this molar ratio was changed to 1:4, conversion increased but sulfoxide selectivity decreased for four complexes. However, the conversion was found to decrease for thioanisole/H2O2 molar ratios of 1:1 and 1:2. Therefore, a 1:3 molar ratio of thioanisole/H2O2 was found to be the optimum.
3.2.2 Effect of catalyst amounts
The amount of catalyst had a considerable effect on the conversion of thioanisole to the corresponding sulfone. The results are given in Table 2. Four various amounts, 1, 5, 10 and 20 mg, were used for all the catalysts and thioanisole (1 mmol), H2O2 (3 mmol), temperature (50 °C) and the reaction time (4 h) were fixed. For all complexes the lower conversion of thioanisole into the corresponding sulfone with 1 mg of catalysts may be as a result of fewer catalytic sites. The maximum conversion was observed with 5 mg of catalysts for all four complexes, but there was no considerable difference in the conversion when 10 and 20 mg of catalysts were employed. Therefore, 5 mg of catalysts were taken as the optimum amount.
Oxidation of thioanisole with different amounts of catalyst in the presence of H2O2.a
| Entry | Catalyst | mg of catalyst | Conversion (%)b | Selectivity (%)c |
| 1 | Co | 1 | 7 | 45 |
| 2 | Co | 5 | 19 | 71 |
| 3 | Co | 10 | 21 | 65 |
| 4 | Co | 20 | 22 | 63 |
| 5 | Ni | 1 | 16 | 51 |
| 6 | Ni | 5 | 36 | 70 |
| 7 | Ni | 10 | 38 | 64 |
| 8 | Ni | 20 | 40 | 61 |
| 9 | Zn | 1 | 19 | 53 |
| 10 | Zn | 5 | 39 | 69 |
| 11 | Zn | 10 | 42 | 66 |
| 12 | Zn | 20 | 45 | 63 |
| 13 | Pd | 1 | 36 | 79 |
| 14 | Pd | 5 | 70 | 99 |
| 15 | Pd | 10 | 70 | 80 |
| 16 | Pd | 20 | 72 | 81 |
a Reaction conditions: thioanisole (1 mmol), acetonitrile (3 mL) and H2O2 (3 mmol) at 50 °C for 4 h.
b Conversion based on sulfide substrates.
c Selectivity for sulfone.
3.2.3 Effect of temperature on the reaction
In the present work for optimization of the reaction conditions, we also studied the effect of temperature on the catalytic performance of CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4) complexes (Table 3). The effect of temperature on the catalytic activity of the four catalysts was studied at four different temperatures, 25 °C, 50 °C, 60 °C and reflux (85 °C) with a constant amount of thioanisole (1 mmol), H2O2 (3 mmol) and catalyst (5 mg) in 3 mL of acetonitrile (Table 3). The maximum conversion, 77%, with 99% selectivity for sulfones was obtained when the reaction was carried out at 85 °C for the Pd complex. At lower temperatures, the conversion was low but the selectivity for sulfoxide was higher for all complexes. At the reflux temperature, the initial conversion of thioanisole was higher than the conversion at 50 °C, but when the reaction would continue the conversion of thioanisole was almost the same as the conversion at 50 °C. Therefore, 50 °C was selected as the optimum temperature and all the catalytic oxidation reactions were carried out at this temperature. These results showed that the Pd Schiff base complex, Pd2LCl4 (4), under optimal reaction conditions exhibits much better catalytic activity compared to the other Schiff base complexes. Therefore, the applicability of the Pd Schiff base complex was studied by oxidation of several types of sulfides with different electronic and steric effects to corresponding sulfones under the optimized conditions (3 mmol H2O2, 5 mg catalyst at 50 °C) (Table 4).
Oxidation of thioanisole with different catalysts in various temperatures.a
| Entry | Catalyst | Temperature °C | Conversion (%)b | Selectivity (%)c |
| 1 | Co | 25 | 7 | 25 |
| 2 | Co | 50 | 19 | 71 |
| 3 | Co | 60 | 24 | 76 |
| 4 | Co | 85 | 32 | 91 |
| 5 | Ni | 25 | 12 | 31 |
| 6 | Ni | 50 | 36 | 70 |
| 7 | Ni | 60 | 38 | 76 |
| 8 | Ni | 85 | 44 | 88 |
| 9 | Zn | 25 | 15 | 30 |
| 10 | Zn | 50 | 39 | 69 |
| 11 | Zn | 60 | 44 | 73 |
| 12 | Zn | 85 | 48 | 89 |
| 13 | Pd | 25 | 34 | 53 |
| 14 | Pd | 50 | 70 | 99 |
| 15 | Pd | 60 | 73 | 99 |
| 16 | Pd | 85 | 77 | 99 |
a Reaction conditions: thioanisole (1 mmol), acetonitrile (3 mL) and H2O2 (3 mmol), 5 mg catalyst for 4 h.
b Conversion based on sulfide substrates.
c Selectivity for sulfone.
The oxidation of various sulfides with Pd Schiff base complex as the catalyst by using 30% aqueous H2O2.a
| Entry | Sulfide (Substrate) | Sulfone (Product) | Time (h) | Conversion (%)b | Selectivity(%)c |
| 1 | 5.5 | 72 | 99 | ||
| 2 | 4 | 70 | 99 | ||
| 3 | 6 | 73 | 99 | ||
| 4 | 6.5 | 69 | 98 | ||
| 5 | 4.15 | 74 | 99 | ||
| 6 | 6.5 | 76 | 99 | ||
| 7 | 8 | 75 | 98 | ||
| 8 | 4.5 | 73 | 99 | ||
| 9 | 6 | 21 | 99 |
a Reaction conditions: catalyst (5 mg), substrate (1.0 mmol), H2O2 (3 mmol), CH3CN (solvent, 3 mL), at 50 °C.
b Conversion based on sulfide substrates.
c Selectivity for sulfone.
As shown in Table 4, the sulfides with less and high steric hindrance were converted to the corresponding sulfones in good to excellent yields except DBT (Table 4, entry 9). It is obvious that these types of sulfides are completely not affected under the reaction conditions. Also, the influence of electronic effects was found in the case of 4-nitrophenyl sulfide, which has a negative effect on the reaction time. The chemoselectivity of this catalytic system was also investigated in the selective oxidation of sulfides containing hydroxyl and carbon–carbon double bond groups. These substrates selectively underwent oxidation at the sulfur atom without undergoing further structural changes in their functional group. For example, in the case of allylic sulfides, epoxidation of the double bond was not observed and only the corresponding sulfones were obtained in excellent yields (Table 4, entries 6 and 7). Also, the presence of the hydroxyl group did not interfere with the oxidation process of the sulfide, and the desired sulfone was obtained in excellent yield (Table 4, entry 8). It is clear that these kinds of sulfides are completely unaffected under the reaction conditions, indicating the good ability of this protocol in oxidation of different types of sulfides.
The recycling properties of the Pd Schiff base complex were investigated in methyl phenyl sulfide oxidation at 50 °C using aqueous 30% H2O2 for 1 mmol of substrate. After the completion of the first oxidation reaction of thioanisole to the methyl phenyl sulfone under optimized conditions, the catalyst was isolated by filtration, washed with CH3CN, dried in an oven at 80 °C for 2 h and reused for the next run. Under the described conditions, the catalyst exhibited high catalytic activity up to five times reuse without noticeable decrease in catalytic activity (Fig. 4).
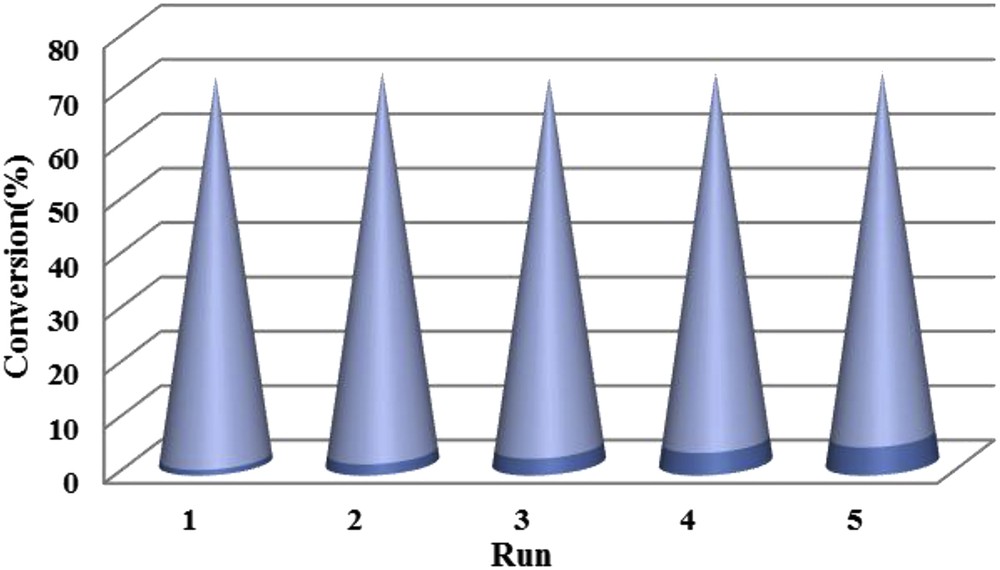
The recycling experiment of the Pd Schiff base complex in the oxidation reaction of methyl phenyl sulfide to methyl phenyl sulfone under optimized conditions.
The XRD pattern and FT-IR spectrum of the recovered Pd Schiff base complex indicated that no significant change in the catalyst took place even after reusing five times (Figs. 5 and 6).

XRD pattern of the Pd Schiff base complex recovered in run 5.
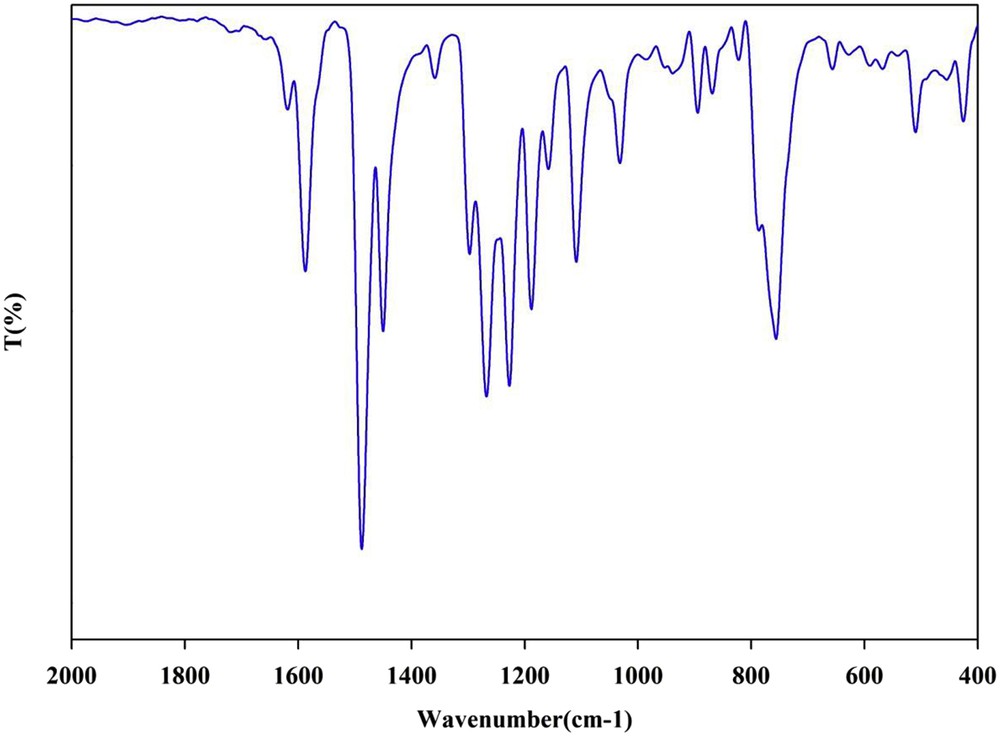
FT-IR spectrum of the Pd Schiff base complex recovered in run 5.
3.3 Comparison with other catalysts
Comparative data on the performance of various Schiff base complexes in the oxidation of sulfides are shown in Table 5. As you can see in most of them, the Schiff base complexes can do the selective oxidation of sulfide to sulfoxide. The selective oxidation of sulfides to sulfones with Schiff base complexes as catalysts is very rare.
- 1 Conte et al. reported an experimental and theoretical study concerning the effects of steric and electronic modification of the ligands on the catalytic activity of salophen and salen oxo vanadium(V) complexes in the oxidation of PhSMe with H2O2. The results indicated that steric factors play a major role in determining the outcome of the reaction (Table 5, entries 1–14) [26].
- 2 Islam et al. synthesized a new Co(III) Schiff base complex, (CoL)Cl.4H2O (L = Schiff base), and used this complex as homogeneous (without support) and heterogeneous (with polymer support) catalysts for the oxidation of alkenes and sulfides using H2O2 as the oxygen source (Table 5, entries 15–18) [27]. Comparison between catalytic activities of the homogeneous cobalt complex and the heterogeneous complex was done and it was shown that the polymer anchored cobalt complex is more active than the homogeneous complex. The active sites do not leach out from the polymer support and thus the polymer anchored cobalt catalyst can be reused without appreciable loss of activity, indicating that the anchoring procedure was effective. The reusability of this catalyst was high and can be reused seven times without significant decrease from its initial activity.
- 3 Rezaeifard et al. investigated oxidation of sulfides using urea hydrogen peroxide (UHP) under the influence of a tridentate Schiff base dioxo-molybdenum(VI) complex catalyst, [MoO2(L)(CH3OH)], in ethanol under mild conditions. Relatively high stability and desired turnover numbers have been observed for this Mo-catalyst in oxidation of sulfides to both sulfoxides and sulfones (Table 5, entries 19–22) [28].
- 4 Barman et al. reported selective oxidation of sulfide to sulfoxide with 30% H2O2 catalyzed by the copper(II)–Schiff base complex. The reactions proceed under mild conditions in acetonitrile at room temperature to provide a variety of aryl and alkyl sulfoxides in excellent yield (Table 5, entries 23–24) [10].
- 5 Liu et al. presented two chiral robust porous MOFs containing μ-oxo-bis[Fe(salen)] dimers and demonstrated their efficient enantioselective abilities to catalyze oxidation of sulfides to sulfoxides with comparable catalytic performance relative to the homogeneous catalysts. These two compounds were efficient and recyclable heterogeneous catalysts for asymmetric oxidation of sulfides to sulfoxides with an enantioselectivity up to 96% (Table 5, entries 25–26) [29].
- 6 In our previous work, we have synthesized a new bidentate NO donor ligand, 2-tert-butyliminomethyl-phenol, and its Co(II), Cu(II), Zn(II) and Pd(II) complexes [18]. Then, we have demonstrated the effectiveness of these complexes as catalysts for the green oxidation of sulfides to the corresponding sulfones with hydrogen peroxide. In this system, the reactions can be carried out under solvent-free conditions as a green sustainable method using all the catalysts in the presence of H2O2. Conversions of 20–96%, and selectivities of 98–100% for sulfones are observed with the four compounds as catalysts (Table 5, entries 27–32). The Zn(II) Schiff base complex shows a better catalytic activity for the oxidation of sulfide and the Co(II) Schiff base complex shows a lower catalytic performance under these reaction conditions. Our study indicated that Pd(II) Schiff base complex is able to convert thioanisol to the corresponding sulfone with mild conversion (74%) and high selectivity (99%) (Table 5, entry 29) [18].
Catalytic activity of various catalysts for the oxidation of sulfides.
| Entry | Catalyst | Substrate | Solvent | Sulfoxide Conv(%)/Selec(%) | Sulfone Conv(%)/Selec(%) | Ref. |
| 1 | [3,3′,5,5′-Cl4 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 96/100 | [26] | |
| 2 | [5,5′-Cl2 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 94/100 | [26] | |
| 3 | [salophenVVO].CF3SO3 | PhSCH3 | MeCN | 100/100 | [26] | |
| 4 | [5,5′-(t-Bu)2 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 53/100 | [26] | |
| 5 | [3,3′-(OMe)2 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 96/100 | [26] | |
| 6 | [5,5′-(OMe)2 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 62/100 | [26] | |
| 7 | [3,3′,5,5′-(t-Bu)4 salophenVVO].CF3SO3 | PhSCH3 | MeCN | 6/100 | [26] | |
| 8 | [3,3′,5,5′-Cl4 salenVVO].CF3SO3 | PhSCH3 | MeCN | 35/100 | [26] | |
| 9 | [5,5′-Cl2 salenVVO].CF3SO3 | PhSCH3 | MeCN | 99/100 | [26] | |
| 10 | [salenVVO].CF3SO3 | PhSCH3 | MeCN | 15/100 | [26] | |
| 11 | [5,5′-(t-Bu)2 salenVVO].CF3SO3 | PhSCH3 | MeCN | 98/100 | [26] | |
| 12 | [3,3′-(OMe)2 salenVVO].CF3SO3 | PhSCH3 | MeCN | >99/100 | [26] | |
| 13 | [5,5′-(OMe)2 salenVVO].CF3SO3 | PhSCH3 | MeCN | 93/100 | [26] | |
| 14 | [3,3′,5,5′-(t-Bu)4 salenVVO].CF3SO3 | PhSCH3 | MeCN | 98/100 | [26] | |
| 15 | PS-TETA-Co (with polymer support) | PhSPh | MeCN | 90/100 | [27] | |
| 16 | (CoL)Cl.4H2O (without support) | PhSPh | MeCN | 76/100 | [27] | |
| 17 | PS-TETA-Co (with polymer support) | PhSC2H5 | MeCN | 89/100 | [27] | |
| 18 | (CoL)Cl.4H2O (without support) | PhSC2H5 | MeCN | 73/100 | [27] | |
| 19 | [MoO2(L)(CH3OH)] | PhSPh | C2H5OH | 75/93 | 75/7 | [28] |
| 20 | [MoO2(L)(CH3OH)] | PhSPh | C2H5OH | 95/3 | 95/97 | [28] |
| 21 | [MoO2(L)(CH3OH)] | PhSCH3 | C2H5OH | 92/100 | 92/0 | [28] |
| 22 | [MoO2(L)(CH3OH)] | PhSCH3 | C2H5OH | 100/2 | 100/98 | [28] |
| 23 | Copper–Schiff base | PhSCH3 | MeCN | 100/82 | [10] | |
| 24 | Copper–Schiff base | PhSPh | MeCN | 100/92 | [10] | |
| 25 | FeL2(OAc) | PhSCH(CH3) | CH2Cl2 | 100/94.6 | [29] | |
| 26 | Fe(salen)-MOF | PhSCH(CH3) | CH2Cl2 | 94/100 | [29] | |
| 27 | CuL2 Schiff base complex | PhSCH3 | Solvent-free | 80/99 | [18] | |
| 28 | ZnL2 Schiff base complex | PhSCH3 | Solvent-free | 93/99 | [18] | |
| 29 | PdL2 Schiff base complex | PhSCH3 | Solvent-free | 74/99 | [18] | |
| 30 | CuL2 Schiff base complex | PhSPh | Solvent-free | 83/99 | [18] | |
| 31 | ZnL2 Schiff base complex | PhSPh | Solvent-free | 96/99 | [18] | |
| 32 | PdL2 Schiff base complex | PhSPh | Solvent-free | 79/99 | [18] | |
| 33 | Pd Schiff base | PhSPh | MeCN | 72/99 | Present work | |
| 34 | Pd Schiff base | PhSCH3 | MeCN | 70/99 | Present work |
So, in comparison with the above catalysts, the present Pd Schiff base catalyst, Pd2LCl4 (4), provides a desirable catalytic activity for the oxidation of sulfides to corresponding sulfone compounds than other Schiff base complexes. Although the ability of this Pd(II) Schiff base complex, Pd2LCl4 (4), in oxidation of sulfides to sulfones is similar to that of our previously reported Pd Schiff base complex derived from 2-tert-butyliminomethyl-phenol, PdL2 [18], the catalyst recyclability and stability of our new Schiff base complex, Pd2LCl4 (4), are much better than those of our previously reported Pd Schiff base complex, PdL2, and it can be used several times without any obvious loss in activity.
4 Conclusion
In this study, four new Schiff base complexes, CoL(NO3)2 (1), NiLCl2 (2), ZnL(NO3)2 (3) and Pd2LCl4 (4), have been prepared by the condensation reaction of 1,2-bis(2′-aminophenoxy)benzene with 2-pyridinecarbaldehyde in the presence of Co, Ni, Zn and Pd metal ions. All complexes have been characterized by IR and XRD spectroscopy techniques and elemental analysis. The synthesized complexes have very poor solubility in all polar and non-polar solvents such as: H2O, MeOH, EtOH, CH3CN, DMSO, DMF, CHCl3, CH2Cl2, THF, etc. This parameter (insolubility) causes these complexes to be suitable for acting as heterogeneous catalysts without any organic/inorganic support. Catalytic performance of the complexes was studied in oxidation of thioanisole using hydrogen peroxide (H2O2) as the oxidant. Various factors including the reaction temperature, amount of oxidant and catalyst amount were optimized. Among the four Schiff base complexes, the Pd(II) Schiff base complex, Pd2LCl4 (4), showed better catalytic activity in oxidation of thioanisole compared to methyl phenyl sulfone. Therefore, the Pd(II) Schiff base complex has been used as the catalyst for oxidation of different sulfides to the corresponding sulfones in acetonitrile using hydrogen peroxide as the oxidant. The Pd(II) complex, Pd2LCl4 (4), showed high activity with good selectivity for the oxidation of various sulfides. This separable catalyst can be prepared by a very simple procedure, by filtration, and the catalyst can be reused for five cycles without any significant changes in its catalytic activity and structure.
Acknowledgments
Support for this research by the University of Isfahan and Islamic Azad University (Shahrekord Branch) is acknowledged.


