1 Introduction
Crops are widely infected by fungi around the world. Diseases caused by Phoma sorghina and Fusarium moniliforme produce considerable losses on cereals in the field and during storage mainly in the tropical regions [1, 2]. However, the application of large quantities of chemical fungicides in agriculture to control these diseases affects humans and wildlife through environmental pollution [3, 4]. For these reasons, the discovery of natural antifungal products with less toxic effects is desirable. Natural products obtained from plants are an attractive alternative for disease control in agricultural practices since they could be found less toxic and biodegradable. Several secondary metabolites occurring in plants such as terpenoids, coumarins, and flavonoids showed antifungal properties [5, 6]. Among these compounds, flavonoids are an interesting group due to their wide distribution in all part of plants and their various properties. Anti-inflammatory [7], antimalarial [8], antimicrobial [9], antibacterial [10], and antioxidant [11] activities have been also reported for these natural products. For example, an antifungal activity of gnaphaliin A, a flavonoid isolated from Pseudognaphalium robustum, displayed inhibition of Botrytis cinerea conidial germination [12]. The hesperidin as a major flavonoid found in citrus species was proved to play an important role in the defense of these plants against Penicillium digitatum [13]. Flavonoids extracted from Tephrosia apollinea displayed interesting properties against four phytopathogenic fungi [14].
Due to the role played by functional groups of organic compounds in their biological activities, chemical transformation is sometimes used to improve physico-chemical properties. Thus, many studies deal with chemical conversion of natural flavonoids into corresponding amine and oxime derivatives [15–17]. As an example, the modification of 5, 7-dimethoxyflavone carbonyl to oxime derivatives was found to enhance its antifungal activity against Candida albicans [17].
When exploring plant extracts for biological applications, we found, in previous work, that the butanolic fraction of Mentha piperita was a fraction enriched in flavonoids [18].
Thus the aim of the present study was to evaluate the antifungal effect of this butanolic fraction of M. piperita against P. sorghina and F. moniliforme. In addition, the activity of its oxime derivatives previously prepared has been assessed [19].
2 Materials and methods
2.1 Plant material
The aerial part of fresh peppermint was collected on March, 26th 2010 in Ouagadougou (Burkina Faso) at the following geographical coordinates: N 12°23′35, 8”; W 001°32′31, 3”. The plant was identified by Prof Jeanne Millogo from Laboratory of Biology and Vegetal Ecology (LABEV), University of Ouagadougou.
2.2 Preparation of tested fractions
The plant material was washed with distilled water, air dried at room temperature (72 h) and then powdered (size ≤ 1 mm). The powdered material (100 g) was defatted with hexane (3 × 300 ml) and the resulting powder was macerated with 1000 ml of ethanol-water mixture (7/3; V/V) for 20 h at room temperature. The filtrate was collected, filtered on paper, concentrated with a rotary evaporator and lyophilized to obtain 17 g of hydro-alcoholic crude fraction (EB). 15 g of crude extract were dissolved in 100 ml of distilled water and extracted with butanol (3 × 50 ml). The butanolic fraction (FR) and water soluble fraction (Aq) were evaporated to dryness to give (3 g) and (10 g), respectively.
The flavonoid contents in the butanolic fraction (FR) were in a previous study characterized by MALDI-MS and LC-MS [18].
For the preparation of an oxime derivative from the butanolic fraction, the following protocol was used. To a solution of 1 g of butanolic fraction (FR) in 15 ml distilled water, NH2OH.HCl (0.25 g) and 10 ml of KOH (1M) were added. The mixture was heated at 70 °C for 20 h and then neutralized with a solution of HCl (1M) to neutral pH. The reaction mixture was extracted with butanol (3 × 50 ml); the obtained butanolic fraction was washed several times with distilled water, dried over anhydrous Na2SO4 and evaporated to dryness to give 0,7 g of dry oxime of the butanolic fraction called (OX).
The formation of the oxime derivative (OX) was monitoring by MALDI-MS and LC-MS analysis under the same experimental conditions used for butanolic fraction (FR) analysis [19].
2.3 Fungal strains
F. moniliforme and P. sorghina were isolated from sorghum samples (1341So07) at Bobo-Dioulasso, Burkina Faso, in 2007. These isolates were subcultured and routinely maintained on potato dextrose agar (PDA) at 25 °C. The 5-day isolates of both fungi were used to conduct the various tests.
2.4 Mycelial growth inhibition test
In vitro antifungal activities of the crude extract (EB), n-butanolic fraction (FR) and residual aqueous fraction (Aq) of M. piperita were assessed on the basis of mycelial growth inhibition of P. sorghina and F. moniliforme. Potato dextrose agar (PDA) was used as the basal medium for all test fungi. The observation was performed in the presence and the absence of the tested compound. The various dry fractions were dissolved in dimethylsulfoxide DMSO (1%). Each fraction was added into assay flasks containing PDA and final concentrations were adjusted to 0.5, 1 and 5 mg/ml. The mixture was sterilized in an autoclave at 120 °C for 30 min. After cooling, aliquots (25 ml) of treated medium were poured into Petri dishes (9 cm in diameter). Control Petri dishes were treated with DMSO (1%) alone. Plugs (5 mm in diameter) of the fungal mycelium cut from the edge of an active growing colony were inoculated in the centers of Petri dish containing medium PDA. For each treatment three repetitions were done. The Petri dishes were incubated at 25 °C for 10 days. The mycelium growth was measured every two days during ten days. This experiment was conducted in three replicates and the percentage of growth inhibition (±standard deviation) was calculated from mean values as: % inhibition = 100[(A−B)/A], where A = mycelium growth in control and B = mycelium growth in treatment.
2.5 Conidial germination test
One hundred ninety eight micro liters (198 μl) of the different fractions of plant extracts were prepared by dissolving in DMSO solution (1%) the extract powder in order to obtain a solution concentrated at 5 mg/ml according to the description in the paragraph “description of tested fractions”. A conidial suspension was prepared by misting pycnidia of P. sorghina or mycelium explants of Fusarium monoliforme in different essay tubes containing 3 ml of sterile distilled water. The mixture was homogenized with a vortex, filter and the conidial suspension is adjusted at 107 conidia/ml with the cell of Neubauer. Two microliters of conidial suspension are added to 198 μl of plant extract fraction solutions to obtain 200 μl of conidial suspension concentrated at 105 conidies/ml from that, aliquots of 50 μl of conidial suspension from each treatment were placed on separate hole-slide glasses. Slides containing spores were incubated in a moisture chamber at 25 °C for 5 h and approximately 40 spores were examined directly on the slides at 1 h intervals. Control culture was incubated with DMSO (1%) alone. Conidial germination assays were carried out on microscope slides. The percentage of conidial germination was estimated by counting the number of germinated or non-germinated conidia in microscope fields. Conidia were judged to be germinated when the germ tube length was equal to or greater than the conidial diameter. Each experiment was performed in triplicate.
2.6 Statistical analysis
Analysis of data was carried out by ANOVA one way. Comparison of mean values was performed with the Student-Newman-Keuls range test at the (P = 0.05) level using the SPSS 16.0 program.
3 Results
In the present study, four fractions of M. piperita such as a hydro-alcoholic crude extract (EB), residual aqueous fraction (Aq), butanolic fraction (FR) and its oxime derivative (OX) were tested against P. sorghina and F. moniliforme at three concentration levels: 0.5, 1 and 5 mg/ml. To evaluate the antifungal activity, two inhibition tests were done namely, mycelium growth inhibition and spore germination inhibition.
3.1 Mycelial growth inhibition test
The mycelium growth in PDA medium in the presence of four fractions of M. piperita was monitored for 10 days. A comparative analysis of plant extract fraction effects on P. sorghina and F. moniliforme showed a highly significant difference between the fractions (Table 1). On P. sorghina, oxime derivatives from the butanolic fraction, and butanolic fraction at 5 mg/ml significantly reduced the mycelium growth of the fungi compared to the other tested concentrations. The same two fractions and hydro-alcoholic crude fraction showed significant antifungal activity on mycelium growth of F. moniliforme when compared with water control, DMSO control, water soluble fraction and butanolic fraction at 0.5 and 1 mg/ml. The experimental data indicated that the oxime derivative from the butanolic fraction is more efficient than the other fractions tested (Table 1)
In vitro effect of plant extracts on mycelium growth of P. sorghina and F. moniliforme.
| Treatment | Mycelium growth of P. sorghina and F. moniliforme | |
| P. sorghina at 10 days | F. moniliforme at 10 days | |
| TE | 9e | 9f |
| TD | 9e | 9f |
| Aq 0.5 | 9e | 9f |
| Aq 1 | 9e | 9f |
| Aq 5 | 9e | 9f |
| Eb 0.5 | 9e | 7.03cd |
| Eb 1 | 9e | 6.5bc |
| Eb 5 | 9e | 7.63de |
| Fr 0.5 | 9e | 8.33f |
| Fr 1 | 8.93e | 8.43f |
| Fr 5 | 4.53b | 6.03b |
| OX 0.5 | 8.70d | 7.80e |
| OX 1 | 8.10c | 7.03cd |
| OX 5 | 2.4a | 2.97a |
| F value | 2.59 | 95.73 |
| Probability | 0.000 | 0.000 |
| Signification | HS | HS |
As no change was observed after four days, the results observed on the fourth day are presented in Fig. 1. Except the residual aqueous fraction, all the fractions showed antifungal activity on the fourth day of incubation against both fungi. The activity increases with the concentration of the extract. The inhibition rates of P. sorghina mycelium growth were 8% for the crude extract, 74% for the butanolic fraction and 84% for the oxime fraction at 5 mg/ml (Fig. 1(a)). The inhibition rates were respectively 26%, 55% and 65% in the case of F. moniliforme at the same concentration 5 mg/ml (Fig. 1(b)). When lower concentrations are used, 1 mg/ml and 0.5 mg/ml as shown in Fig. 1, inhibition effect on mycelium growth for both fungi decreases. In contrast, the residual aqueous fraction showed a stimulatory effect on mycelium growth for both fungi (Fig. 1).
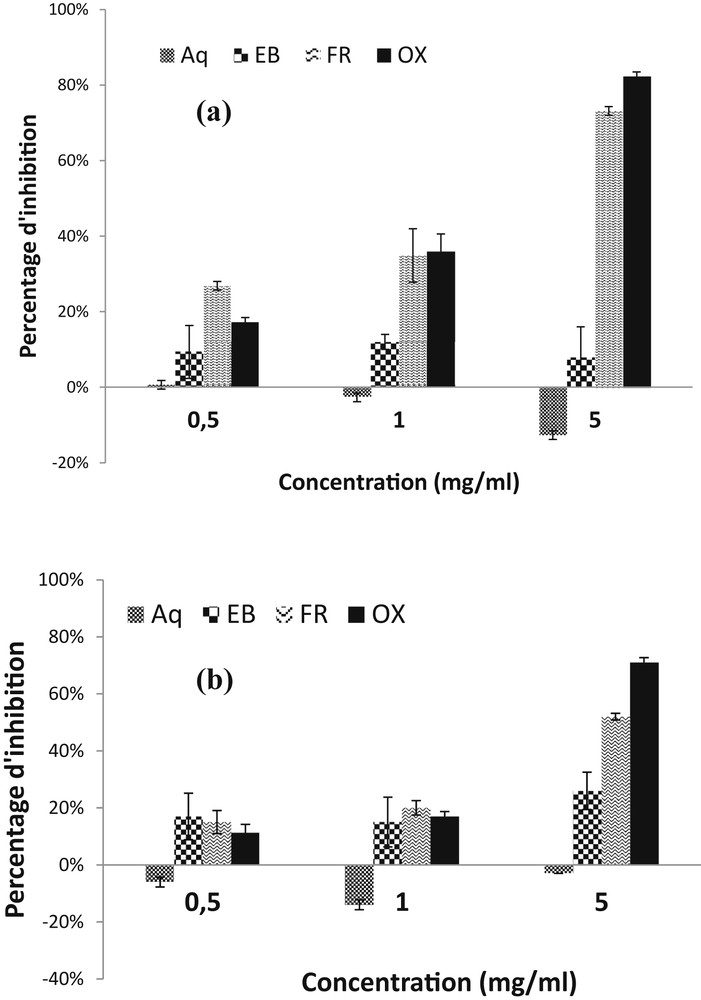
Effect of different concentrations of Mentha piperita: hydro-alcoholic extract (EB), butanolic fraction (FR), oxime fraction (OX) and residual aqueous fraction (Aq) on the mycelial growth of a) P. sorghina: b) F. moniliforme. Bars with different letters indicate statistically significant differences according to Duncan’s multiple range test at the (P = 0.05) level of confidence. Results are obtained after 4 days of incubation.
After a ten-day incubation, the oxime derivative exhibited the greatest mycelium growth inhibition (2.4 cm) for P. sorghina and (3 cm) for F. moniliforme compared to the control without treatment which reaches 9 cm (Fig. 2).

Mycelium growth inhibition by oxime fraction (OX) treatment at 5 mg/ml mycelium growth inhibition of a) P. sorghina and b) F. moniliforme. DMSO at 1% was added to the culture media as a control (TE).
3.2 Conidial germination test
Fig. 3 shows results concerning the effect on conidial germination of the four fractions used at 5 mg/ml. The butanolic fraction and its oxime derivative affect significantly the germination of both fungal conidia. In presence of these two fractions, the germination of P. sorghina was delayed by 3 h compared to the control without treatment (Fig. 3(a)). In the presence of the hydro-alcoholic crude fraction and residual aqueous fraction, this germination started just 1 h after incubation. This similar effect was observed with F. moniliforme (Fig. 3(b)) but at a different time of germination. In this case, the germination of spores began after 1 h and reached a maximum after 2 h of incubation, after which, the number of germinated spores decreases.

Effect of the crude extract (EB), butanolic fraction (FR), oxime fraction (OX) and residual aqueous fraction (Aq) at 5 mg/ml on conidial germination of a) P. sorghina, b) F. moniliforme. The control is represented by (TE).
4 Discussion
M. piperita offers great potential as a plant, which can provide natural antifungal products, since its extracts inhibited the growth of several fungi in vitro [20, 21]. However, most published studies examined antifungal or antimicrobial activity of volatile essential oils of M. piperita rather than its other components. Anyone knows the high volatility of essential oils, which surely limits their use in long term postharvest handling. In this study, we showed a strong antifungal activity in vitro against P. sorghina and F. monoliforme of the M. piperita butanolic fraction (rich in flavonoids) at 5 mg/ml. The mycelial growth inhibition rate reached for P. sorghina 74% for the butanolic fraction at 5 mg/ml (Fig. 1(a)) and the effect was slightly lower (64%) for F. moniliforme. The hydro-alcoholic crude fraction showed in vitro a weak activity against P. sorghina and F. moniliforme at the same concentration compared to butanolic and oxime fractions. This observation further confirms that the antifungal activity of the butanolic fraction of M. piperita is due to its high content of flavonoids compared to that of the hydro-alcoholic crude fraction. In our study, the concentration of 5%m/v with high inhibition observed against P. sorghina mycelium growth is very interesting compared to some aqueous extracts from local plants which showed effective inhibition at the concentration 30% (w/v) [22].
The antifungal activity of plant extracts has been correlated with the high content in phenolic compounds and particularly in flavonoids by several authors [23–26]. As reported by Oh et al., the antifungal activity of the butanolic fraction from the Eucalyptus darlympleana methanolic crude extract was observed. A strong antifungal effect of the ethanolic extract of Mentha longifolia was observed [27] and all these fractions were rich in flavonoids.
The chemical transformation of the flavonoids contained in the butanolic fraction into oxime derivatives improved significantly the antifungal activity. This fraction (OX) displayed a higher inhibition rate (84%) at 5 mg/ml against P. sorghina compared to the butanolic fraction at the same concentration. In a similar context, oximation of 5, 7-dihydroxyflavanone extracted from Keampferia parviflora enhances antifungal activity against C. albicans [17]. The flavanone glycosides as eriocitrin and hesperidin major compounds in the FR extract could be oximated [18].
On the other hand, spore germination assays were also performed for the evaluation of antifungal properties of various compounds. In addition, this property could be used to understand the action mechanism of a product. As an example, strobilurin fungicides, which block the electron transport chain, are extremely potent inhibitors of spore germination but are less active as inhibitors of mycelial growth [28]. Interestingly, besides the inhibition of the radial growth of P. sorghina and F. moniliforme, the two fractions i.e butanolic and its oxime derivative at 5 mg/ml affected conidial germination of both fungi (Fig. 3). These results might suggest that the flavonoids contained in the butanolic fraction and their oxime derivatives might act on the electron transport chain, inducing mycelium lysis. In fact, an inhibition of spore production in the P. digitatum was observed in the presence of nobiletin and of both naringin and hesperidin from citrus species [13]. Also, amentoflavone, a flavonoid isolated from Selaginella tamariscina, induces a damageable effect on the C. albicans mitochondria [29]. 5,7-Dihydroxy-3,8-dimethoxyflavone an flavonoid isolated from P. robustum that affected significantly the germination of B. cinerea spores was found to reduce the consumption of oxygen by these spores [12]. Our results are in accordance with the general view that polyphenols are a secondary metabolite part of plant defense mechanisms against anthropods and microorganisms.
The slight stimulatory effect on mycelium growth for both fungi displayed by the residual aqueous fraction is certainly due to a mixture of other metabolites, such as polysaccharides or proteins, used as food by the fungus. This observation warrants the strategy to enhance antifungal properties through the separation of the bioactive compounds from the dietary components that can be used as nutritive substances by the fungus. These stimulatory effects on the fungus have been already observed with some plant extracts [22,30].
5 Conclusion
A significant antifungal effect of the butanolic fraction from areal parts of M. piperita was demonstrated in laboratory against P. sorghina and F. moniliforme. This fraction inhibited significantly the mycelial growth of P. sorghina and F. moniliforme and delayed the germination of the spores for both fungi. The chemical transformation of the flavonoids into their oxime derivatives improved this antifungal activity. This antifungal activity of a fraction is linked to its richness in flavonoids. The potential use of plant extracts rich in flavonoids could be a sustainable way to protect cereals against P. sorghina and F. moniliforme fungal infections. M. piperita could contribute by a local cultivation to a fungicide formulation developed for the treatment of crops.
Acknowledgments
The authors acknowledge the International Science Programme (ISP) for their financial support on antifungal natural products through the project BUF 01.


