1 Introduction
Harpagophytum procumbens D.C. (Pedaliaceae), commonly known as Devil's claw, is an herbaceous plant growing in the south of Africa, mainly in the Kalahari Desert and in the Namibian Steppes. H. procumbens is traditionally used as an anti-inflammatory and analgesic particularly to address painful osteoarthritis and inflammation [1,2]. The herbal drug consists of secondary roots which contain iridoids as the active compound. The herbal drug in powder form or the aqueous and hydroalcoholic extracts are generally used in phytotherapy [3]. The iridoid glycoside fraction contains mainly harpagoside, procumbide, harpagide and 8-p-coumaroylharpagide. Harpagoside (HS) (Fig. 1), the main iridoid glycoside of H. procumbens, has been extensively investigated with respect to its pharmacological properties including anti-inflammatory and analgesic activities [4,5].

Chemical structure of harpagoside.
The H. procumbens herbal drug and H. procumbens extract have been documented in a monograph in the European Pharmacopoeia [6,7] for the quality control. In the herbal drug monograph, the extraction of HS, before HPLC analysis, is performed by shaking a mixture of the powdered drug with methanol. The H. procumbens extract, described in the monograph, is produced from the herbal drug by an appropriate procedure using either water or a hydroalcoholic solvent equivalent in strength to a maximum of 95% ethanol, in order to obtain a content of minimum 1.5% of HS. In both monographs, the HS content is determined with a Spherisorb ODS 2 column at an elevated flow rate of 1.5 ml/min.
Extraction is the first crucial step in the qualitative and quantitative analysis of medicinal plant constituents in order to ensure and provide high-quality herbal medicinal products [http://www.techniques-ingenieur.fr/base-documentaire/archives-th12/archives-operations-unitaires-genie-de-la-reaction-chimique-tiajb/archive-3/extraction-solide-liquide-aspects-theoriques-j2780]. The design of an efficient, sustainable and green extraction method for the vegetal material is currently a hot research topic [8].
Conventional extraction processes are quite laborious, time- or energy-consuming, involve large amounts of solvents, and ultimately, may cause some target molecule degradation.
Great improvements can be achieved with the use of non-conventional techniques [9] such as microwave-assisted extraction (MAE) [10] and ultrasound-assisted extraction (UAE) [11,12]. The main advantages of UAE and MAE are the large reduction in extraction time, the improved selectivity and the higher yield of the extracts, high reproducibility in a shorter time, simplified manipulation and reduced solvent consumption [13,14].
Each matrix needs to have carefully optimized operating conditions in order to achieve high-quality standards and yields.
The decreased solvent consumption in HPLC analysis can also be proposed. The use of HPLC analytical columns with core-shell particles can significantly reduce solvent consumption and greatly shorten analysis times. These particles consist of a non-porous silica core and a porous silica layer. The thin porous layer significantly improves the mass transfer of the analytes. These columns offer a high speed and a good separation resolution compared to the traditional large porous particles used earlier in liquid chromatography [15].
The first aim of this study is to apply two innovative, green and non-conventional techniques, MAE and UAE, for the extraction of HS in H. procumbens, and compare these techniques with conventional methods (percolation and reflux). The second aim is to optimize the assay of HS by HPLC using a core-shell technology chromatographic column. This new analytical method will be validated to test its reliability. The final objective is to propose a “green” methodology.
2 Materials and methods
2.1 Material, standard and reagents
Dried secondary roots of H. procumbens have been purchased from Cailleau Herboristerie, batch 19995 (Chemillé, France). Until required, roots were protected from light and humidity and were then ground to a powder (18 mesh sizes) before use.
HPLC-grade methanol, RPE-ACS grade methanol and RPE-ACS grade ethanol were purchased from Carlo Erba (Val de Reuil, France). HPLC-grade water (18.2 MΩ) was obtained from the Milli-Q Reference A+ system (Millipore Co., Bedford, MA, USA). Standard HS was purchased from Extrasynthese (Genay, France), purity was >99%.
2.2 Methods
2.2.1 Conventional extraction
Extraction by percolation was performed with 10.0 g of powdered herbal drug by adding 100 mL of different extraction solvents (methanol, ethanol and methanol/water mixtures) in the percolation column, maintained at room temperature for 16 h, followed by the slow flow of the solvent extraction through the column. Extracts were filtered and concentrated under reduced pressure to dryness. The dried residue was suspended in water and freeze-dried.
Extraction by reflux was carried out with 10.0 g of the powdered material for 30 min with 100 mL of solvent. After the extraction, the solution was filtered and evaporated to dryness under reduced pressure. The dried residue was suspended in water and then freeze-dried.
2.2.2 MAE
MAE was executed in a multimode Microwave apparatus using an open-vessel system (Mars X-press instrument, CEM Corporation, Matthiews, NC, USA). The microwave extractor was equipped with a magnetron of 2450 MHz, delivering a maximal power of 1200 W in 10 W increments, a reflux unit, a time controller, a temperature control system with a temperature probe, an exhaust system, a beam reflector and a stirring device. The temperature was controlled with feedback to the microwave power regulator.
For MAE, an accurately weighed sample (10 g) of the plant powder was mixed with 100 mL of different solvents and binary mixtures. The suspension was irradiated using microwave under different experimental conditions for the optimization of the extraction parameters. After extraction, the samples were filtered through paper and the solvent was concentrated under reduced pressure to dryness. The residue was suspended in water and then freeze-dried.
2.2.3 UAE
Ultrasound Assisted Extraction was performed with a PEX 05 Sonifier (REUS, Contes, France) composed of an inox jug with 150 × 137 mm internal dimensions and a maximal capacity of 500 mL, as well as a transducer, in the base of jug, operating at a frequency of 25 kHz and with a maximum input power of 150 W. The double layered mantle facilitated the temperature control of the medium by means of a cooling/heating system. The drug powder (10 g) was sonicated in 100 mL of different solvents, under different experimental conditions for the optimization of the extraction parameters.
After ultrasonic treatment, the extracts were filtered and concentrated to dryness by vacuum. The residue was suspended in water and then freeze-dried.
2.2.4 HPLC analysis
Analyses were performed using an Agilent 1100 series apparatus (degasser G1379A, autosampler G1313A, quaternary pump G1311A, and Diode Array Detector (DAD) (detector G1315B) with a Kinetex C18, 2.6 μm, 3 × 100 mm (Phenomenex, USA) with a SecurityGuard C18, 3 × 4 mm cartridge (Phenomenex). The acquisition and calculation of data were performed with the Chemstation Agilent data acquisition system. The mobile phase consisted of a mixture of water-methanol (42/58, v/v). The flow-rate was 0.4 mL/min. The detector was operated at 278 nm. The injection volume was 10 μl. A typical HPLC chromatogram of H. procumbens extract is presented in Fig. 2.
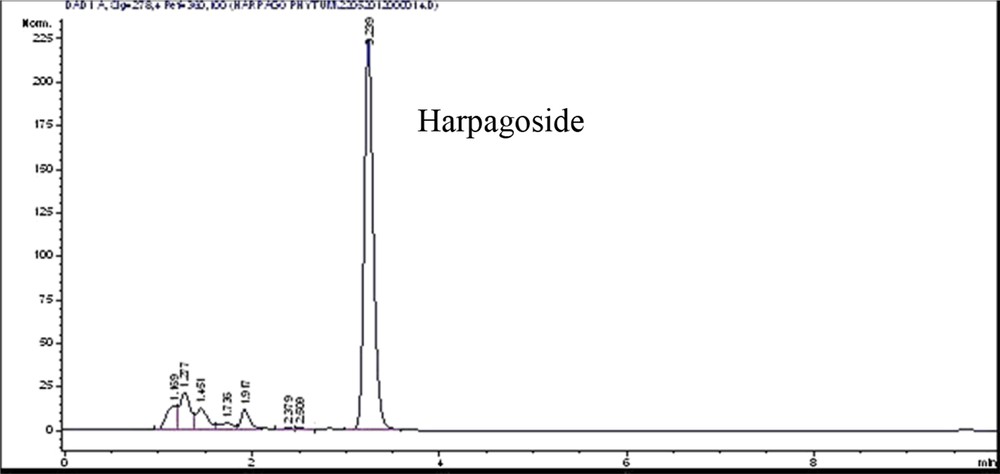
Isocratic HPLC profile of the UAE extract of H. procumbens.
The HS standard solution was prepared at a concentration of 0.2 mg/ml in the mobile phase. This solution was filtered through a 0.45 μm membrane filter (Millipore, refHVPL04700) before analysis.
Stock solutions of the dried extracts were prepared by dissolving the appropriate amount in methanol to obtain a final concentration of 10 mg/ml. The solutions were diluted in the mobile phase to obtain working solutions at 5 mg/ml. The solution was filtered through a 0.45 μm membrane filter (Millipore, refHVPL04700) before analysis.
2.2.5 HPLC validation
The HPLC method was validated based on the extract obtained by the UAE: extraction with ethanol, for 10 min with a ratio of 1/25, on the basis of its specificity, linearity, accuracy and precision according to ICH requirements [16].
The specificity was investigated using standard solution and sample solutions. The linearity (calibration curve) was performed for the HS standard. Five different standard solutions had been prepared and analysed in triplicate for each concentration. The concentration range was 0.015–0.045 mg/mL for HS. For extract solutions (sample solutions), the concentration range was 0.170–0.510 mg/mL. Calibration curves have been constructed by plotting peak areas against concentrations. The linearity was assessed by calculating the slope, y-intercept and coefficient of correlation (r2) using least squares regression. The intra-day precision (repeatability) and the intermediate precision (inter-day precision) were determined by six replicated analyses over a three-day period. The accuracy of the method was evaluated using the recovery test. This involved the spiking of known quantities of HS standard solutions into the real samples. The standard samples were analysed in triplicate according to the previously described chromatographic conditions.
3 Results and discussion
3.1 Optimization of extraction parameters with conventional extraction techniques: percolation and reflux
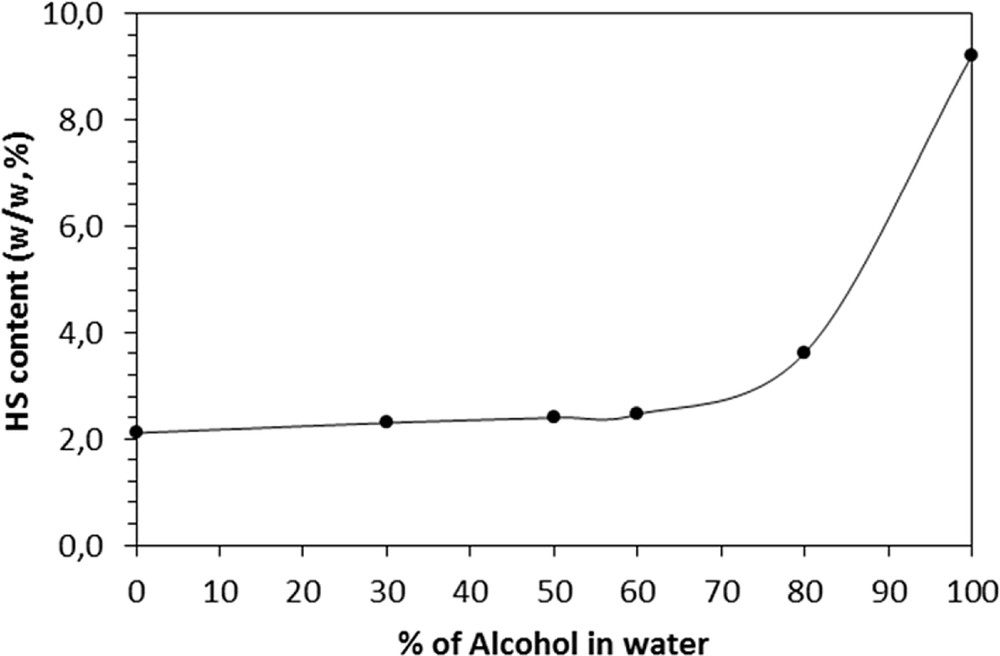
First, the selection of the most suitable solvent was investigated under standard conditions, that is, 10 g of plant powder, 100 mL extractant, percolation for 16 h and reflux for 30 min. Ethanol, methanol and hydroalcoholic solutions containing different concentrations of methanol and ethanol were tested under the same conditions. The results are illustrated in Fig. 3.

Effect of the concentration of alcohol (MeOH or EtOH) on the harpagoside content using conventional extraction by percolation and reflux.
The HS content of extractive solutions was determined by HPLC.
The highest HS content was obtained with both pure ethanol by reflux (9.87%) and percolation (10.23%).
On the other hand, the conventional extraction methods were carried out with ethanol at different times and with a herbal drug/ethanol ratio of 1/10. The extraction solutions were collected at different times. Optimum extraction of HS was obtained in 6 h by percolation and in 20 min by reflux.
The conventional extraction method of percolation allowed the highest content of HS in the extract with ethanol to be obtained.
Further extraction studies by MAE and UAE were conducted with ethanol and hydroalcoholic mixtures.
3.2 Optimization of microwave-assisted extraction parameters
In this study, the effects of several extraction parameters such as ethanol concentration, microwave power and the effect of solid–liquid ratio have been investigated. The content of HS was established by HPLC.
In the first study, extractions were assessed using ethanol and binary mixtures with water. Ethanol was selected because this solvent allowed the highest content in HS to be obtained with conventional extraction methods and this is a non-toxic solvent. All extractions were performed with a solid-liquid ratio of 1/10 (w/v), for 10 min at 400 W. The results are shown in Fig. 4.
Effect of the concentration of ethanol solution on the extraction of harpagoside using microwave-assisted extraction.
The ethanol solvent yielded the highest HS content in the extract with a value of 9.22%.
Ethanol was used in the following experiments.
The effect of microwave power was also studied. Extractions at different levels of irradiation, that is, 100, 200, and 400 W for 10 min with a solid-liquid ratio of 1/10 (w/v) were tested.
The HS contents were similar: 9.42%, 9.25% and 9.22% (w/w) with 100 W, 200 W and 400 W respectively.
The lowest power of 100 W, conducted with a content of 9.42% of HS in the extract, was used in the following studies. This power resulted in energy savings.
In a third study, different solid-liquid ratios were tested (1/20, 1/15, 1/10, 1/5, and 1/2.5 w/v). A power of 100 W was applied for 10 min. The best content of HS (10.36%) was obtained with the solid-liquid ratio 1/2.5 (w/v) as shown in Fig. 5.

Effect of the solid/liquid ratio on the extraction of harpagoside using microwave-assisted extraction.
In the final experiment, irradiation times of 5 and 10 min at 100 W and a solid-liquid ratio of 1/2.5 (w/v) were investigated. Optimum extraction of HS was obtained in 5 min with a content of 10.50%.
It has been found that the best extraction efficiencies were obtained with ethanol, a microwave power of 100 W, an irradiation time of 5 min and a solid-liquid ratio of 1/2.5 (w/v).
3.3 Optimization of ultrasound-assisted extraction parameters
The solvent selected was ethanol. It yielded the highest content in HS with conventional extraction methods and with microwave-assisted extraction. The content of HS was established by HPLC.
Two solid-liquid ratios (1/10, 1/2.5, w/v) and various times (5, 10 and 15 min) have been crossed. The results are illustrated in Table 1.
Effect of the solid-liquid ratio and time on harpagoside extraction in the UAE technique.
| Solid-liquid ratio (w/v) | Time (min) | Harpagoside content (w/w) |
| 1/2.5 | 5 | 10.54 |
| 1/10 | 5 | 9.79 |
| 1/2.5 | 10 | 10.88 |
| 1/10 | 10 | 10.10 |
| 1/2.5 | 15 | 10.00 |
| 1/10 | 15 | 8.80 |
The best result was obtained with the ratio 1/2.5 (w/v) for 10 min. The HS content is 10.88% (w/w) in the extract.
3.4 Comparison with conventional and innovative extraction methods
In general, percolation, heat reflux and Soxhlet extraction are the most frequently used extraction procedures. In this study, the conventional extraction methods used were reflux, a hot extraction method, because HS is usually extracted by this technique and percolation, a cold extraction, a usual and an efficient extraction method to obtain pharmaceutical preparations.
The main drawbacks of these conventional methods of extraction are the large amounts of solvent and long extraction times needed. The conditions of the different techniques and their results are summarized in Table 2.
Comparison of conventional percolation and reflux techniques with innovative MAE and UAE techniques.
| Extraction method | Extraction time | Solvent volume | Harpagoside content (w/w) |
| Percolation | 360 min | 100 mL | 10.26 |
| Reflux | 20 min | 100 mL | 9.78 |
| MAE | 5 min | 25 mL | 10.50 |
| UAE | 10 min | 25 mL | 10.88 |
It should be noted that all extraction techniques were applied under their optimized conditions. With respect to the extraction time, MAE and UAE were the fastest extraction methods with 5 and 10 min, respectively, whereas percolation required 360 min and reflux 20 min. Table 2 shows that, in terms of the content of target analyte, the best results were obtained by UAE, which gave the highest value (10.88% (w/w)).
Ultrasound-assisted extraction method saved considerable energy and time, and has been found to be suitable for the quality control of the H. procumbens plant material used on a commercial scale by the industry. In the European Pharmacopoeia monograph of the H. procumbens herbal drug, the test solution for the quality control has been prepared by extraction for a period of 4 h by shaking the powdered drug with methanol. This extraction procedure should be changed by the UAE with ethanol for 10 min in an ultrasonic bath for the assay of H. procumbens herbal drug.
3.5 HPLC development and validation
In both of the European Pharmacopoeia monographs of the H. procumbens herbal drug and extract, the HS content has been determined by HPLC using a C18 stationary phase, 5 μm, 100 × 4 mm, type Spherisorb ODS 2 column, a mobile phase with water-methanol, (50/50, v/v) and an elevated flow rate of 1.5 mL/min. Under these conditions, the retention time of HS is about 7 min and the peak is large.
The objective was to reduce the amount of solvent used and the analysis time, while having a good resolution. The mobile phase water–methanol was retained. Various reversed phase columns such as Symmetry C18 (250 × 4.6 mm, 5 μm), Luna C18 (150 × 4.6 mm, 3 μm), Uptisphere C18 (150 × 4.6 mm, 3 μm), Kinetex C18 (100 × 4.6 mm or 3 mm, 2.6 μm) were tested. The optimum separation was obtained with a Kinetex C18, a core-shell column, 100 × 3 mm, 2.6 μm. The Core-Shell particles consisted of a non-porous silica core and a porous silica layer that improved the mass transfer of the analytes.
The ratio of water–methanol and the flow-rate were modified. The optimum analytical conditions were determined, the mobile phase consisted of a mixture of water-methanol (42/58, v/v) and a flow-rate of 0.4 mL/min. Under these conditions, the retention time of HS was about 3 min and the peak width was 0.100. The retention time and the flow-rate have been considerably reduced.
For HPLC validation, the specificity of the method has been investigated. Standard solution and samples were prepared and analysed in terms of the specificity. No interference and great resolution were observed for HS which was assessed by comparing the retention time and the UV spectra with the standard.
Furthermore, the purity of the investigated peak was confirmed by DAD purity studies.
With regard to the linearity, regression equations and correlation coefficients obtained for regression analysis are presented in Table 3. The calibration curves are linear in the tested concentration range.
Regression equations, correlation coefficients and precision for the evaluation of the harpagoside content in the proposed HPLC method.
| Harpagoside Standard | Extract | ||
| Linearity | Range (mg/mL) | [0.015–0.045] | [0.170–0.510] |
| Calibration curves and r2 | Y = 33155 X + 3.705 r2 = 0.9998 | Y = 34065 X − 16.076 r2 = 0.9989 | |
| Precision | Repeatability (intra-day, 3 days) % RSD | First day Second day Third day | 1.70 0.68 0.82 |
| Intermediate precision (inter-day) % RSD (n = 18) | 1.14 |
The intra-day precision (3 days, n = 6) and the intermediate precision (n = 18) have been assessed for the extract. Table 3 demonstrates that the intra-day % RSD was less than 2.00% and the inter-day % RSD was less than 1.20%, showing a good precision of the method.
The results concerning the accuracy are presented in Table 4. Recoveries range from 103.6 to 104.6%.
Accuracy for the quantification of harpagoside (HS) in the proposed HPLC method.
| Relative amount added (%) of HS | Spiked concentration (mg/mL) of HS | Observed concentration (mg/mL) of HS | Recovery (%) (n = 3) | RSD (%) |
| 50 | 0.0146 | 0.0152 ± 0.00006 | 103.58 | 0.38 |
| 100 | 0.0293 | 0.0301 ± 0.00078 | 104.21 | 2.55 |
| 150 | 0.0439 | 0.0453 ± 0.00157 | 104.57 | 3.41 |
The results, presented in Tables 3 and 4, indicate that the proposed HPLC method is adequate for the quantification of HS in the samples. This method also reduces the analysis time (5 min versus 15 min) as well as the amount of solvent used, with a low flow rate (0.4 mL/min versus 1.5 mL/min).
4 Conclusion
In this study new and time-saving extraction methods, that are also economical and ecofriendly, based on the use of microwave energy and ultrasound, have been optimized for the analysis of HS from HP by using HPLC with a core-shell column.
The optimal MAE conditions were as follows: ethanol, solid/liquid ratio 1/2.5, microwave power 100 W, an extraction time of 5 min. Under the optimal conditions, the HS content in the extract was 10.50% (w/w).
The optimal UAE conditions were: ethanol, solid/liquid ratio 1/2.5, and an extraction time of 10 min. Under these optimal conditions, the HS content in the extract was 10.88% (w/w).
The MAE and UAE developed in this study are promising methods in accordance with sustainable development. They are proposed for the first time as alternatives to classical methods, such as reflux or percolation, for the extraction of HS from H. procumbens. UAE which saves more energy than MAE could be retained for HS extraction. New commercial extracts of H. procumbens with high HS content should be produced by these methods.
An analytical method for the determination of HS in secondary roots of H. procumbens has been developed and validated. This method is specific, linear, precise and accurate. It can be used for the determination of HS in the herbal drug and different extracts. This method allows both the time of analysis and the amount of solvent to be reduced.
The objective of a “green” methodology is achieved both in the extraction and in the HPLC analysis in terms of reduction of the volume of organic solvent, reduced time and energy savings. This methodology can be proposed to the European Pharmacopeia for the quality control of the H. procumbens herbal drug and extract using HS as an active and analytical marker.
The commercial production of the extract from the HP herbal drug by UAE may also be used as an appropriate procedure. The UAE extract could be used for further isolation and purification of HS with a better yield.


