1 Introduction
Rosemary (Rosmarinus officinalis L.) is a very rich source of bioactive phenols that are primarily responsible for the plant's high bioactivity, leading to its use in gastronomy and traditional medicine for centuries [1]. Rosemary extracts are used in a broad range of applications, including food preservation [2], nutraceuticals, phytomedicines [3], and cosmetics [4]. Consequently, the scientific community has shown great interest in attaining these bioactive extracts by means of efficient and ecological processes. R. officinalis L. (Labiatae), extensively found in Western Mediterranean countries, is well known for its many uses in the kitchen and for its pharmacological properties. Essential oil is obtained from the leaves of the plant which are also used to prepare phenolic extracts that are natural remedies for a number of common diseases [5]. Besides strong antioxidant activity, hydroalcoholic extracts are recognized to have choleretic, cholagogue, hepatoprotective, antitumor, and antiviral properties [6]. The biological activity attributed to these extracts is closely related to their phenolic fractions with rosmarinic and carnosic acids being the main constituents, together with several minor flavonoids.
Designing more efficient extraction processes, which can address the dual requirements of process intensification and energy savings, is currently becoming an increasingly important research topic. Safety, sustainability, environmental, and economic factors are all forcing industries to turn to non-conventional technologies and greener protocols [7].
UAE can be considered as an ecological process as it helps to greatly accelerate the extraction process and reduce energy consumption. Extraction enhancement using UAE has been attributed to the propagation of ultrasound (US) pressure waves, and the resulting cavitation phenomena. The method is clean and, thanks to low bulk temperatures and rapid execution, it helps to prevent thermal degradation phenomena; it usually leaves no residue in the extract and uses no moving mechanical parts. It also offers advantages in productivity, yield, and selectivity; it improves processing time, and enhances quality while reducing chemical and physical hazards [8]. Despite there being few reports in the scientific literature, industrial applications have been made available since the 1990s with batch reactors, from 100 up to 500 L, mainly used in the preparation of extracts for the phyto-pharmaceutical, cosmetic, and liqueur industries.
In recent years, MAE has also been the subject of significant research across numerous fields, but especially that of medicinal plants as its unique heating mechanism, moderate capital cost, and good performance under atmospheric conditions provide a variety of benefits [9]. The main advantage of MAE resides in the performance of its heating source. In addition to the base closed (sealed-vessel above atmospheric pressure) and open MAE systems [10], many modifications have been introduced to enhance performance over the last decade. Recent technological advances have led to dramatic improvements in analyte recovery and MAE reproducibility, making it an irreplaceable plant extraction tool.
Numerous extraction methods for the efficient recovery of phenols from rosemary leaves have so far been proposed [11]; however, little data on the use of UAE and MAE are currently available. The effect of various solvents and US on the extraction of carnosic acid from rosemary has, however, been investigated [12]; ethanol was significantly less effective than ethyl acetate and butanone in a conventional stirred extraction while US improved the relative performance of ethanol. High-intensity US may therefore reduce solvent dependence [13] and facilitate the use of alternative solvents with more attractive economic, environmental, health, and safety benefits.
Although microwaves have mostly been used in rosemary steam distillation [14], methanol/water, acetone/water, ethyl acetate/water, and ethanol/water mixtures have been proposed for total polyphenol recovery [15,16]. Dielectric heating has also been proposed as a means to dry rosemary leaves; this method minimizes the decrease in quality by providing rapid and effective heat distribution throughout the material [17]. MAE has more recently been compared with Soxhlet and US extraction methods; however, the paper only discussed total phenol content and not the different phenolic classes that are typically present in rosemary [18]. A comparison of ten different rosmarinic and carnosic acid extraction processes, which only use a 9:1 v/v ethanol/water mixture, has recently been published [19]. Intensified extraction processes at various extraction temperatures gave similar yields to conventional processes (heat reflux extraction and maceration). Carnosic acid was efficiently enhanced by UAE whereas MAE was more suitable for rosmarinic acid recovery.
The aim of this work is to investigate US potency in the rapid and selective recovery of the phenolic compounds in dried rosemary leaves, a material not suitable for recovering volatile terpenes, but that is still rich in powerful antioxidant compounds. Simultaneously, the efficacy of MW extraction with ethanol and water was preliminarily evaluated and compared with the US results. A very short process time (10 min) was applied to the recovery of the phenolic fractions from rosemary leaves. Furthermore, the aim of improving extraction yields and process selectivity was tackled using sequential UAE procedures and n-hexane, ethanol, acetone, and water as the only extraction solvents. Extraction yields and final dried extract (DE) quality were determined by HPLC/DAD, which measured dried extract weight over dried leaf weight (w/w%) and the phenolic content of the dried sample.
2 Results and discussion
2.1 Extraction yield and time
This work investigates US potency for the rapid and selective recovery of phenolic compounds from rosemary leaves. MW efficacy in this extraction has also been preliminarily evaluated. An extraction time of only 10 min was used to shorten the process and only solvents that are suitable for food applications were used. Extraction efficiency was evaluated across a series of single-extraction steps (UAE and MAE) and in some sequential procedures (only UAE) that were carried out on the same dried leaf batch. Table 1 compares yields, in terms of DE weight over dried leaves (DL) weight for each sample. As can be seen, the UAE and MAE single extraction steps showed similar results (values close to 18–21% w/w) in water and ethanol.
Relative yields expressed as a percentage over DL. Each UAE extractive sequence is indicated by a number and the sequential order of the extractive steps is from top to bottom.
| Samples | Yields (% w/w) | |
| MAE single step | MW-H2O | 18.0 |
| MW-EtOH | 20.0 | |
| MW-EtOH 70% | 19.0 | |
| UAE single step | US-Hexa | 6.4 |
| US-Aceb | 14.1 | |
| US-EtOH | 18.7 | |
| US-H2O | 21.1 | |
| US-H2O+βCD | 15.0 | |
| UAE Extraction sequences | 1-US-Ace | 15.1 |
| 1-US-EtOH | 8.3 | |
| 1-US-H2O | 22.0 | |
| 2-US-Ace | 13.0 | |
| 2-US-H2O | 21.4 | |
| 3-US-Hex | 6.1 | |
| 3-US-Ace | 11.4 | |
| 3-US-EtOH | 6.4 | |
| 3-US-H2O | 25.7 | |
| 4-US-H2O | 21.4 | |
| 4-US-EtOH | 17.3 | |
| 5-US-Hex | 6.6 | |
| 5-US-H2O | 21.4 | |
| 5-US-EtOH | 13.1 |
a Mean of 3-US-Hex and 5-US-Hex.
b Mean of 1-US-Ace and 2-US-Ace.
These values are in agreement with those recently obtained by Jacotet-Navarro et al. [19] who applied UAE and MAE to rosemary leaves after hydrodistillation; their work was carried out with an ethanol/water 9:1 v/v mixture, 30 min of extraction and a solid/liquid ratio of 20. The authors obtained final yields ranging from 13% to 18% which increased (up to 25%) at the higher extraction temperature of 150 °C. Our findings suggest that it is possible to obtain comparable DE yields after only 10 min extraction time and a lower solid/liquid ratio of 10. More lipophilic solvents, such as hexane and acetone, gave lower yields as is also the case with more traditional liquid/solid extraction processes.
The identification of the various compounds in the extract has been carried out according to previous studies [11,20], and confirmed by HPLC-MS-TOF. The chromatographic profiles, at 330 nm of aqueous, ethanol, and acetone extracts (Fig. 1) obtained using UAE and MAE, show the distribution of the rosmarinic acid and minor flavonoids in the different samples.
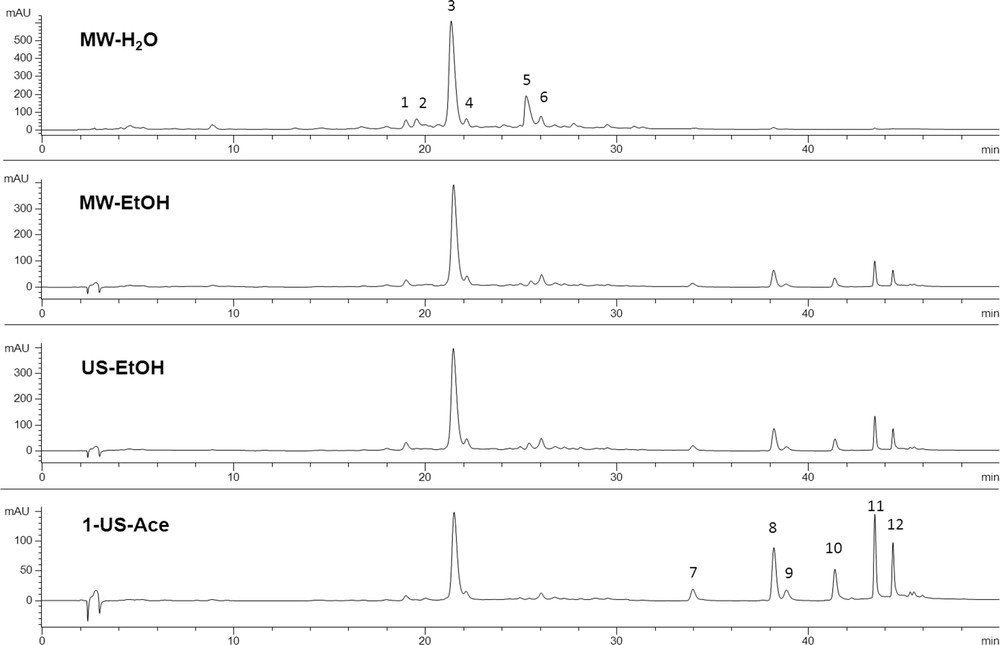
HPLC profiles at 330 nm of aqueous, ethanol and acetone extracts obtained using UAE and MAE. Peaks on the chromatogram correspond to: 1, flavonoid; 2, flavonoid monoglucoside; 3, rosmarinic acid; 4, flavonoid; 5, flavonoid diglycoside; 6, isoscutellarein 7-O-glu; 7, flavonoid; 8, cirsimaritin; 9, flavonoid; 10, genkwanine; 11 and 12, flavonoids.
The quantitative data are expressed both as phenolic content/DL, and phenolic amount/DE. The term “total phenols” in the text indicates the sum of non-volatile terpenoids, flavonoids, and rosmarinic acid in each extract. Only carnosic and rosmarinic acids were selected as external standards as we aim to use a relatively simple method to correctly quantify the main constituents. It is not easy to choose the most suitable and representative standard for rosemary flavonoids because they are characterized by numerous minor components with various structures. In light of these considerations and according to our previous studies [11,20], rosmarinic acid was also used to determine the flavonoid content.
2.2 Phenolics recovery from leaves
An overview of the results, in terms of rosmarinic acid and flavonoids, is shown in Fig. 2, while terpenoid and the total phenol content can be found in Fig. 3. It is worth nothing that the total phenol contents from UAE and MAE using ethanol were up to three times higher than more traditional solid–liquid extractions (reference extract) and gave final contents of 35.0 mg/g and 36.6 mg/g DL, respectively (Fig. 3). It is worth remembering that these values were obtained from processes that are consistently rapid, with the extraction completing in ten minutes rather than several hours, and that consume less energy. Acetone also provided high amounts of total phenol contents, close to 34 mg/g DL.
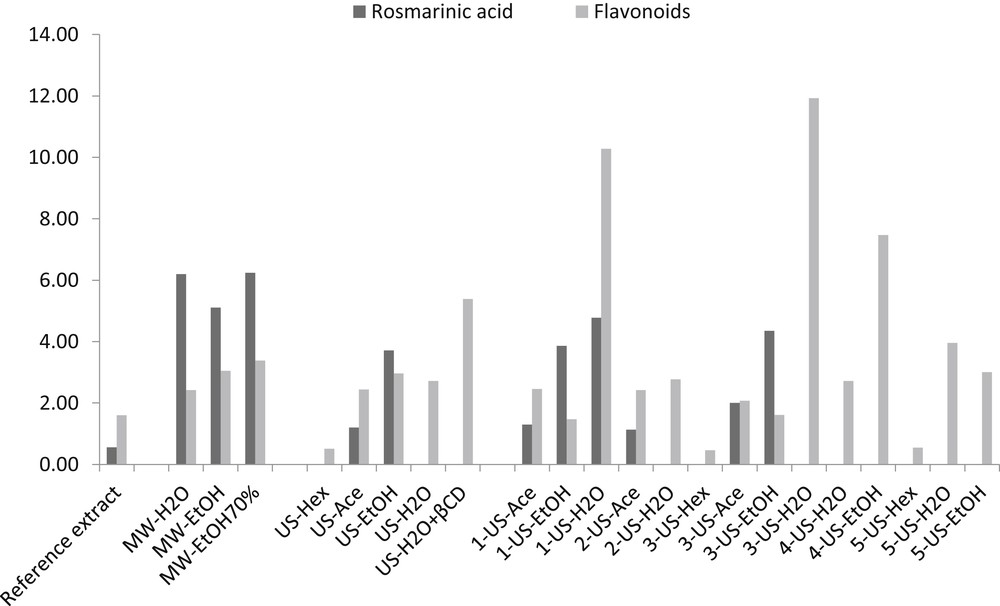
Rosmarinic acid and flavonoid contents (mg/g DL) of all extracts present in Table 1.

Total terpenoid and phenol contents (mg/g DL) of all extracts present in Table 1.
On the other hand, very low total phenol amounts were obtained when water was used as the first solvent (MW-H2O and US-H2O), which is mainly due to the absence of the terpenoid fraction (Fig. 3). Water's overall poor extractive capacity was observed in the UAE sequential procedures, while a preliminary test with water and β-cyclodextrin (β-CD) [21,22] showed a slightly higher recovered flavonoid amount. As expected, extraction with ethanol as the second (1-US and 4-US) or third step (3-US), was less effective and gave consistently lower phenol recovery than ethanol used alone or after the first extraction with water (4-US-EtOH). The highest recoveries in the sequential extractions were obtained with sequences 1-US and 3-US (Fig. 4), which were found to be the most suitable processes for producing exhausted wastes.
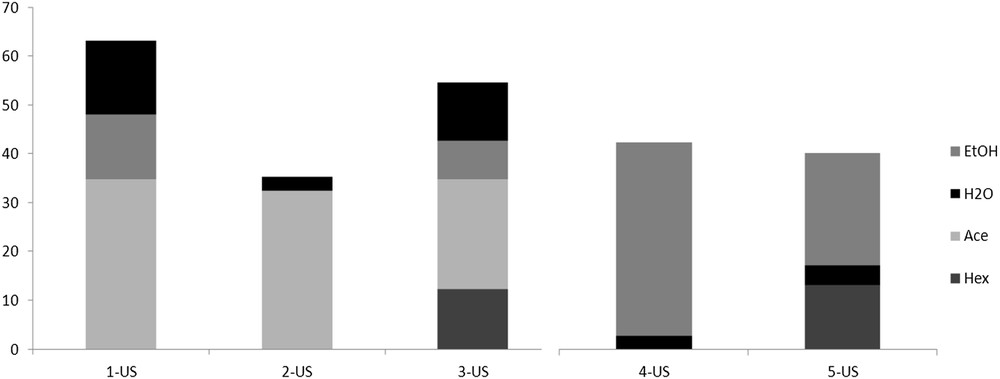
Percentage distribution of total phenol in the extractive sequences (Table 1).
US-Ace and US-EtOH samples were the best extracts, in terms of total terpenoids; comparable amounts were found in MW-EtOH, although they were slightly lower and close to 2.8 mg/g% DL (Fig. 3). Fig. 5 focuses on the terpenoid fraction, bringing together carnosic acid and carnosol, in all extracts except those from water. The histogram highlights a consistent decrease in carnosic acid amounts in the ethanol extracts obtained after pretreatment with water (4-US-EtOH and 5-US-EtOH). This contact accelerates the loss of carnosic acid as the radicals generated from water sonolysis promote the oxidation of carnosic acid to carnosol, as already described during ethanol extraction on fresh leaves [11] and in other studies on rosemary [23,24]. Ethanol is not useful if hexane or acetone is previously applied during sequential steps (sequences 1 and 3), because these solvents are able to efficiently recover most of the terpenoids in rosemary leaves. Unexpected and interesting findings were obtained from UAE in n-hexane. Although the recovery of total phenols in n-hexane was around three times lower than that in acetone or ethanol (Fig. 3), this solvent showed high selectivity for the terpenoid fraction. The use of n-hexane in UAE consistently differs from its use in traditional liquid/solid extraction where terpenoids are not recovered at all. Moreover, these new extracts are extremely rich in carnosic acid, as discussed in the next paragraph.

Amounts of carnosic acid and carnosol (expressed as mg/g DL) recovered from dried leaves.
Solvent choice clearly plays a major role in UAE and MAE, where ethanol and acetone were found to be the most suitable solvents for guaranteeing high total phenolic compound recovery from dried rosemary leaves. At the same time, UAE in n-hexane was found to be the most selective in terpenoid recovery, mainly of carnosic acid.
2.3 Quality of DE from rosemary
Knowledge of the bioactive constituent composition is fundamental in determining the commercial value of botanicals. The concentration of compounds selected as phytochemical markers and the total phenolic amount, both expressed as mg/g DE, need to be taken into account when evaluating the overall quality of rosemary extracts. Data on the richest extracts are discussed below and mainly focus on total phenols and the terpenoidic fraction.
The composition of DE that contain at least 120 mg/g DE is shown and compared in Table 2. The total phenol content was spread across a wide range, from 123.6 mg/g DE (3-US-EtOH) to 249.4 mg/g DE (2-Us-Ace) and the highest total terpenoid amounts were obtained in acetone (1-US-Ace and 2-US-Ace) with concentrations similar to those measured for the oleoresin that was selected as the reference. Nevertheless, an important difference must be pointed out; the UAE extracts contain carnosic acid in the concentration range from 107.5 to 172.4 mg/g DE, while the maximum content in the oleoresin is 31.6 mg/g DE. This well-known antioxidant is commonly used as a chemical marker when defining the quality of rosemary oleoresins, which are widely used as food preservatives.
Comparison of the richest phenolic extracts (over 120 mg/g DE) and a commercial powdered oleoresin from rosemary. Data are expressed as mg/g DE.
| Compounds | Single step | Extraction sequences | ||||||||||
| MW-EtOH | MW-70% EtOH | US-EtOH | 1-US-Ace | 1-US-EtOH | 2-US-Ace | 3-US-Ace | 3-US-EtOH | 4-US-EtOH | 5-US-EtOH | Oleoresin | ||
| 1 | Flavonoid | – | – | – | 0.4 | 2.6 | 0.4 | – | 1.8 | 1.1 | – | 0.1 |
| 2 | Flavonoid | 1.2 | 1.6 | 1.1 | 0.2 | 0.4 | 0.2 | 0.6 | 4.4 | 1.4 | – | 0.2 |
| 3 | Rosmarinic acid | 25.6 | 32.9 | 19.8 | 8.6 | 46.5 | 8.7 | 17.5 | 67.7 | – | – | 0.2 |
| 4 | Flavonoid | 1.7 | 1.2 | 0.9 | 0.3 | 2.1 | 0.6 | 0.7 | 3.5 | 6.9 | – | 0.1 |
| 5 | Flavonoid | 0.7 | 3.7 | 0.8 | 0.6 | 2.9 | 0.5 | 0.1 | 1.5 | 1.6 | 0.9 | 0.1 |
| 6 | Flavonoid | 2.1 | 1.9 | 1.6 | 1.0 | 3.3 | 1.1 | 1.4 | 4.1 | 4.7 | 3.0 | 0.2 |
| 7 | Flavonoid | 0.8 | 0.9 | 0.4 | – | – | – | 0.2 | 4.9 | 2.1 | 1.0 | 0.4 |
| 8 | Flavonoid | – | – | 0.8 | – | – | – | 1.2 | 1.3 | 11.4 | 2.8 | 0.6 |
| 9 | Cirsimaritin | 3.0 | 2.7 | 3.3 | 4.2 | 2.1 | 4.9 | 5.1 | 1.3 | 3.7 | 5.9 | 1.8 |
| 10 | Flavonoid | 0.5 | 0.6 | 0.6 | 0.8 | 0.4 | 1.3 | 1.1 | 0.5 | 0.8 | – | 0.5 |
| 11 | Genkwanine | 1.4 | 1.5 | 1.7 | 2.5 | 1.1 | 2.7 | 3.1 | 0.7 | 2.8 | 3.1 | 1.2 |
| 12 | Flavonoid | 2.5 | 2.5 | 2.8 | 3.8 | 1.7 | 4.2 | 2.9 | 0.7 | 3.7 | 4.1 | 0.7 |
| 13 | Flavonoid | 1.5 | 1.4 | 1.7 | 2.4 | 1.1 | 2.7 | 1.7 | 0.4 | 2.7 | 2.1 | 0.7 |
| Tot. flavonoids | 15.4 | 18.0 | 15.7 | 16.2 | 17.7 | 18.6 | 18.1 | 25.1 | 42.6 | 23.0 | 6.6 | |
| 14 | Rosmanol | 6.6 | – | 7.5 | 10.1 | 4.8 | 11.2 | 11.3 | – | – | 37.9 | 70.5 |
| 15 | Carnosol | 29.4 | 48.4 | 31.8 | 35.6 | 22.9 | 38.5 | 41.6 | 24.2 | 147.0 | 114.0 | 122.3 |
| 16 | Carnosic acid | 106.3 | 84.9 | 112 | 159.4 | 67.7 | 172.4 | 107.5 | 6.5 | 38.3 | 0.0 | 31.6 |
| Tot. terpenoids | 142.3 | 133.3 | 151.3 | 205.1 | 95.5 | 222.1 | 160.4 | 30.8 | 185.3 | 151.8 | 224.3 | |
| Tot. Phenols | 183.3 | 184.2 | 186.8 | 229.9 | 159.7 | 249.4 | 196 | 123.6 | 227.9 | 174.8 | 231.1 |
The UAE acetone extracts help to avoid oxidation processes to carnosol and rosmanol and can provide more valuable commercial extracts than the oleoresins on the market. The highest rosmarinic acid concentrations across all extracts (Table 2) were obtained via UAE in ethanol, as observed for 3-US-EtOH and 1-US-EtOH, which provided 67.7 mg/g and 46.5 mg/g DE, respectively. Lower amounts were obtained from microwaves in the same solvent; only 25.6 mg/g DE for MW-EtOH and only a little better for MW-EtOH 70% (32.9 mg/g DE). Our results demonstrate that the best process for enriching an extract in rosmarinic acid is UAE in ethanol which is particularly efficient when pre-extraction with acetone is carried out (1-US-EtOH and 3-US-EtOH). MAE and UAE have recently been applied to rosemary leaves that had previously been hydro-distilled giving lower amounts of rosmarinic and carnosic acids in the final extracts, with values close to 3 mg/g DE and 15 mg/g DE, respectively [19]. These authors used extraction times (30 min) and leaves/solvent ratios (1:20) that are considerably higher than those used in our work, but the final main phenol contents were very low. This result can certainly be partially attributed to the low quality of the hydro-distilled leaves and not only to the extraction process.
UAE in n-hexane was found to be extremely selective for terpenoids while also providing low amounts of lipophilic flavonoids and a large prevalence of carnosic acid, as confirmed by HPLC-DAD-MS TOF analysis (Fig. 6).

TIC and HPLC profile of 5-US-Hex at 284 nm with the chemical structures of carnosic acid and carnosol. Peaks in the chromatograms correspond to: 1, flavonoid (299.1986 m/z); 2, carnosol; 3, unknown compound; 4, flavonoid (315.2328 m/z); 5, carnosic acid; 6, methyl carnosate; 7, unknown compound.
The total terpenoid content recovered by n-hexane is close to 200 mg/g DE (Fig. 7). The process displayed good reproducibility, as observed after repeated extractions on the same batch of DL over several months. Herrero et al. [25] have proposed SFE with CO2 and ethanol as a co-solvent to obtain fractions with a composition similar to our n-hexane US extracts. Nevertheless, it is clear that the instrumentation required for SFE is certainly more expensive and complex than the system required for UAE, which is also a more convenient process for possible scale-up. Both MAE and UAE gave very low total phenol contents, <50 mg/g DE, when water was used as the extraction solvent (Table 3), while neither process yield nor selectivity were improved when a preliminary UAE test using water and β-CD was carried out.

Phenolic distribution in n-hexane extracts obtained by UAE; data (mg/g DE) are means of three independent extractions carried out on the same batch of dried leaves within one year.
Phenolic composition of the aqueous extracts. Data are expressed as mg/g DE.
| Samples | Rosmarinic acid | Total flavonoids | Total phenols |
| MW-H2O | 34.4 | 13.4 | 47.8 |
| 1-US-H2O | 21.7 | 46.7 | 68.4 |
| 2-US-H2O | – | 13.0 | 13.0 |
| 3-US-H2O | – | 46.4 | 46.4 |
| 4-US-H2O | – | 12.7 | 12.7 |
| 5-US-H2O | – | 18.5 | 18.5 |
| US-H2O+βCD | – | 17.9 | 17.9 |
3 Conclusion
In conclusion, this work highlights the possibility of efficiently extracting specific phenolic fractions from DL under UAE and MAE. The higher extraction selectivity may reduce time and the amount of solvents required for purification in accordance with the general trend towards the concept and principles of green extraction [26]. Ethanol and ethanol/water mixtures in UAE and MAE, and acetone in UAE, are solvents which either provide good final extract yields or yields that are comparable with those recently obtained with the same techniques but at longer extraction times. UAE extraction with acetone can be very favorably compared with the more traditional liquid/solid extractions in acetone that are used to prepare commercial rosemary oleoresins. Our UAE process provides similar results in much shorter times. Moreover, selectivity toward terpenoids is increased when using n-hexane which provides a higher final amount of carnosic acid, which is the typical quality marker of rosemary oleoresins. The rapidity of UAE with n-hexane and its extremely reproducible extract composition are remarkable. This volatile solvent could easily be recycled in the possible future scale-up of this extraction, as it traditionally occurs during oil extraction from edible seeds.
The results of our work may be the basis for future developments of batch and flow UAE methods, which are known to be extremely versatile and suitable for easy scale-up design and the convenient recovery of rosemary phenols.
4 Experimental
4.1 Plant materials, solvents and chemicals
R. officinalis L. leaves were collected in the Florentine countryside. The powdered, commercial oleoresin from rosemary was kindly provided by Giotti S.p.A.
Acetone and ethanol (ACS grade, ≥99%) were used for extractions (Sigma–Aldrich). For HPLC analyses, acetonitrile CHROMASOLV® (gradient grade, for HPLC, ≥99.9%) and formic acid (ACS grade, ≥99.5%) were purchased from Sigma–Aldrich, while Milli-Q H2O was obtained in the laboratory from a Milli-Q Reference A+ System (Merck Millipore). Standards of rosmarinic and carnosic acid were purchased from Sigma–Aldrich.
β-cyclodextrin was kindly provided by Wacker Chemie Srl.
4.2 Extractions
DL from the same batch of R. officinalis L., which had been dried at room temperature in the dark for several days, were used in the various extractions. All extraction procedures used in this work are summarized in Table 1. Most of the tests were performed using UAE because this technique is currently easier and less expensive to scale-up than MAE. The plant/solvent ratio (1 g of DL/10 mL of solvent), and extraction time (10 min) were kept constant across all extractions.
UAE was carried out using a probe system (Danacamerini, Turin) equipped with a titanium horn (∅ = 15 mm) with a conical tip (∅ = 25 mm) working at 19.5 kHz (140 W). Wider tip diameters have less amplitude, but can accommodate larger extraction volumes and ensure more homogeneous sonication. MAE was performed in a closed multimode reactor (Synthwave, Milestone, Bergamo) under N2 (20 bar) at 100 °C.
Different sequential extractions were carried out. In sequence 1, acetone was used in the first extraction step, followed by ethanol (second step) and water in the last extraction cycle. The same was done for the other extraction sequences, as is described in Table 1, where the extraction order is from top to bottom; water extractions were carried out within the sequences and also a single extraction step was performed in a 1.5% β-CD water solution (US-H2O + β-CD). To prepare the “reference extract”, dried leaves (1 g) were dipped in liquid nitrogen and immediately finely ground in a laboratory mill. The leaf powder was extracted twice with ethanol, as already described in a previous study [11]. The powdered commercial oleoresin from rosemary (Giotti S.p.A.) was also analyzed for comparison with the DE prepared by UAE and MAE. All extracts were dissolved in a defined solvent volume and solutions were directly analyzed by HPLC-DAD–MS-TOF.
4.3 HPLC-DAD–MS-TOF analyses
Analyses were carried out using an HP 1100L liquid chromatograph equipped with a DAD detector coupled to a TOF MS mass spectrometer equipped with an electrospray (ESI) interface (all from Agilent Technologies, Palo Alto, CA, USA). Analysis parameters were set using a negative ion mode with spectra acquired over a mass range from m/z 80 to 800.
The ESI source conditions were as follows: drying gas, high purity nitrogen (N2); drying gas temperature, 350 °C; drying gas flow-rate, 6 L/min; nebulizer, 20 psi; capillary voltage, 4000 V; fragmentation, 80–150 V; and skimmer, 60 V. Acquisition and data analysis were controlled using Agilent LC–MS TOF Software (Agilent, USA).
A 150 mm × 2 mm i.d., 4 μm Fusion, RP18 column (Phenomenex, USA) equipped with a pre-column of the same phase was used. The mobile phases were (A) 0.1% formic acid/water and (B) CH3CN. The multi-step linear solvent gradient used was: 0–15 min 15–25% B; 15–25 min 25–35% B; 25–35 min 35–50% B; 35–40 min 50–100% B with a final plateau of 8 min at 100% B. Equilibration time 10 min; flow rate 0.2 mL min−1 and oven temperature 26 °C; injection volume 5 μL. Analysis conditions were as described in a previous study [11].
4.4 Quantitative determination
The quantitative evaluation of the main constituents was performed using two external standards; rosmarinic acid at 330 nm and carnosic acid at 284 nm. The first compound was used at 330 nm, to also quantify all flavonoids, while the second one was used at 284 nm to determine all other diterpenoids, according to previous works [11,20]. The calibration curve of rosmarinic acid was in the linearity range of between 0.1 μg and 9.4 μg with an r2 0.9999; the calibration curve of carnosic acid was in the linearity range of 0.05–3.4 μg with an r2 0.9998.
Acknowledgments
This work has been supported by the University of Turin (fondi Ricerca locale 2014), we are also grateful to Ente Cassa di Risparmio di Firenze for supplying a part of the instrumentation used in this research.


