1 Introduction
d-Limonene belongs to the family of terpenes and is the major constituent of the essential oils resulting from citrus fruit skins. With a production of more than 50 million tons, the orange juice industry represents an important source for d-limonene and a challenging research pilot platform for the valorisation of by-products. It is considered as a GRAS (Generally Recognized as Safe) material by the U.S. Food and Drug Administration and plays an important role in flavors and fragrances as well as acts as a cleaning/degreasing agent in industrial and in household applications [1,2]. The growing interest in d-limonene has emerged since its cleaner and degreaser qualities were recognized and taken into consideration [3]. In this respect, this molecule has been designated as an efficient alternative for halogenated carbon hydrates or conventional degreasing agents used in industry and households. In their attempt to develop an industrial application for d-limonene, different authors [4–6] have indicated, recently, the high suitability of this molecule as a synthon and solvent for the synthesis and extraction of natural compounds to replace petroleum-based chemicals and solvents such as n-hexane. Likewise, d-limonene is a versatile compound that can be oxidized to produce diverse compounds used in the production of fragrances, perfumes and food additives.
Ever since the first reaction carried out by Bordenca in 1951 [7] using nitrosyl-chloride, background information on the catalytic oxidation of d-limonene can be found in many papers. In recent years, many attempts have been reported to transform d-limonene into high-added-value compounds. In this context, Penicillium digitatum was used to carry out the biotransformation of d-limonene to α-terpineol [8]. Likewise, Maróstica Jr. et al. [9] investigated the production of R-(+)-α-terpineol by the biotransformation of d-limonene from orange essential oils, using cassava waste water as a suitable substrate for mycelial growth medium. Moreover, the bioconversion of (+)-limonene to get (+)-α-terpineol using Pseudomonas gladioli as the biocatalyst was carried out [10].
α-Terpineol, or its acetate analog, has also been synthesized using various chemical methods [11] through different acids (chloroacetic acid, acetic acid, formic acid, etc.) using different catalysts (cation exchange resin, zeolite, ferric sulphate) and oxymercuration followed by reduction with sodium borohydride. Further, catalytic oxidation of d-limonene leads to oxidized compounds with more or less efficiency and selectivity, up to the catalyst, such as metal complexes [12]. Liquid-phase oxidation (LPO) of hydrocarbons is another important methodology for the manufacturing of valuable organic compounds that has been developed in several industrial processes. LPO can be performed with different oxidants and, in the presence of a suitable catalyst, it can take place under relatively mild conditions of temperature and pressure. Pena et al. [13] carried out the LPO of limonene with molecular oxygen as the sole oxidizing agent under non-solvent conditions in the presence of three different nickel-aluminium hydrotalcites. Several oxygenated limonene derivatives such as endo- and exo-epoxides, carveol and carvone represented 45–60% of the whole amount of products obtained.
Due to the need for environmentally friendly processes and products, sustainable chemistry has prompted a great amount of research into the processing of renewable feedstock to obtain platform molecules and downstream end products [14]. In this context, the methodology applied in this study was focused on the biorefinery concept because d-limonene was obtained by solvent-free microwave extraction from orange peels, an agricultural by-product from the orange juice industry, followed by a deterpenation process (Fig. 1). Finally, d-limonene was used as feedstock in two different procedures. First, this compound was employed as the starting material (agro-synthon) to synthesize other high value products with industrial interest. This process was performed through an oxidation reaction using three iron supported catalysts, two mesoporous supports (Fe-SBA 15 and Fe-F16SM) and a double lamellar hydroxide support (Fe-LDH). This process works on the principle of generating free radicals (such as HO•).

General experimental design. SFME (Solvent-free microwave extraction), EO (essential oil), Fe-LDH, Fe-SBA 15 and Fe-F16SM (commercial mesoporous material iron and double lamellar hydroxide iron).
Otherwise, alternatives to conventional extraction procedures or to conventional petroleum solvents may increase production efficiency and contribute to environmental preservation by replacing the use of petroleum solvents by bio-solvents and reducing fossil energy and generation of hazardous substances. The drive to reduce CO2 and volatile organic compound (VOC) emissions, chemical and food industries are in search of new technologies in order to reduce energy and solvent consumption, to meet legal requirements on emissions, product/process safety and increased quality as well as functionality. Solvent extraction of natural products is one of the promising innovation themes that could contribute to the sustainable growth of chemical and food industries. So, d-limonene was used as an alternative solvent to substitute n-hexane in the extraction of bioactive natural products (carotenoids, oils and aromas). In addition, the study of the extraction of bioactive compounds combines an experimental procedure with a theoretical approach carried out by means of two computational simulation methods, Hansen solubility parameters (HSP) and predictive computational COSMO-RS (Conductor-like Screening Model for Real Solvents) software.
2 Materials and methods
2.1 Plant material and chemicals
In this study, about 1 kg of orange (Citrus sinensis L. Osbeck, Valencia late cultivar from Blida, Algeria) peels was collected locally after juice extraction (which separates the external part of the orange (peel), giving a yield of approximately 20% (w/w) of orange peels with respect to the whole fruit). Moisture content determination was carried out by a conventional Dean–Stark distillation apparatus. The initial moisture content of the orange peels was 90%.
Carrots (Daucus carota) cut into small pieces and dried were provided by Naturex Company (Avignon, France); rapeseed, belonging to the Astrid variety (Euralis Semences), was provided by the Centre Technique Interprofessionnel des Oléagineux et du Chanvre industriel (CETIOM, Pessac, France) and caraway seeds (Carum nigrum) were purchased from “Herbier du Diois”, SARL Touret, 26410 Châtillon-en-Diois, France. n-Hexane (HPLC grade) was supplied by VWR International (Darmstadt, Germany). β-Carotene (95–99%), used as the standard, and d-limonene were provided by Sigma-Aldrich, Germany, and carvone from MerckTM (Darmstadt, Germany).
2.2 Solvent-free microwave extraction (SFME)
SFME was performed using an open vessel multimode MW extractor (NEOS-GR, Milestone Srl, Italy), operating at 2.45 GHz and a maximum power of 900 W, with a 1.5 L Pyrex extraction vessel. Temperature was monitored using an external infrared (IR) sensor. The experiment was conducted at atmospheric pressure. 250 g of fresh orange peels were heated using a fixed power density of 1 W/g for 30 min without the addition of solvents or water. A cooling system outside the microwave cavity condensed the distillate continuously. Condensed water was refluxed to the extraction vessel in order to provide uniform conditions of temperature and humidity for extraction. The distillation was continued at 100 °C until no more essential oils were obtained. The essential oils were collected, dried over anhydrous sodium sulphate and stored at 4 °C until use. Extractions were performed at least three times and the mean values are reported.
2.3 Deterpenation experimental design
The concentration was carried out by distillation in an UIC KDL4 apparatus of 0.043 m2 of a heat-exchange surface [15]. It is an evaporator with a wiped film, provided with an internal condenser and being able to work under very thorough vacuum (1–10−3 mmHg). The choice of the experimental field was done on the basis of preliminary tests. The pressure was fixed at 100 mmHg. The experimental factors selected are the temperature of the heated fluid (water), stirring velocity and rate of feed. The weights of essential oils used (initial) and of concentrated essential oils (final) are measured to accuracy to 10−3 g. A sequential step was required by the simplex methods, which finally determined the optimal conditions for the concentration of the sample of orange essential oil by wiped film evaporator.
2.4 Oxidation method
To a solution of d-limonene (2.3 mL, 14.18 mmol) in ethanol (12.7 mL), 10 mL of H2O2 and 50 mg of each catalyst were added (Fe-SBA 15, Fe-FSM, and Fe-LDH, separately). The reaction mixture was stirred at 60 °C under N2 pressure during 4 h. The level N2 flow rate was high at the beginning (20 min) to purge atmospheric O2, and then it was lowered.
2.5 Computational methods: theoretical prediction
The solubility parameters of d-limonene and n-hexane used to dissolve the main carotenoids from carrots, triacylglycerides and diacylglycerides from rapeseed oil and aromas from caraway seeds have been studied by means of Hansen solubility parameter (HSP) theoretical prediction and COSMO-RS.
2.5.1 Hansen solubility parameters (HSPs)
Hansen solubility parameters provide a convenient and efficient way to characterize solute-solvent interactions according to the classical “like dissolves like” rule [16]. HSP is based on the concept that the total cohesive energy density is approximated by the sum of the energy densities required to overcome the atomic dispersion forces (), molecular polar forces arising from dipole moments () and hydrogen bonds (exchange of electrons and proton donors/acceptors) between molecules (), as given by:
| (1) |
| (2) |
The chemical structures of the solvents and solutes (Fig. 2) discussed in this article could be mutually transformed by JChemPaint version 3.3 (GitHub Pages, San Francisco, CA, USA) to their simplified molecular input line entry syntax (SMILES) notations, which were subsequently used to calculate the solubility parameters of the solvents and compounds. These solubility parameters were further modelled to a three-dimensional HSP sphere for better visualization of the solute/solvent interactions (HSPiP Version 4.0, Hansen Solubility, Hørsholm, Denmark).
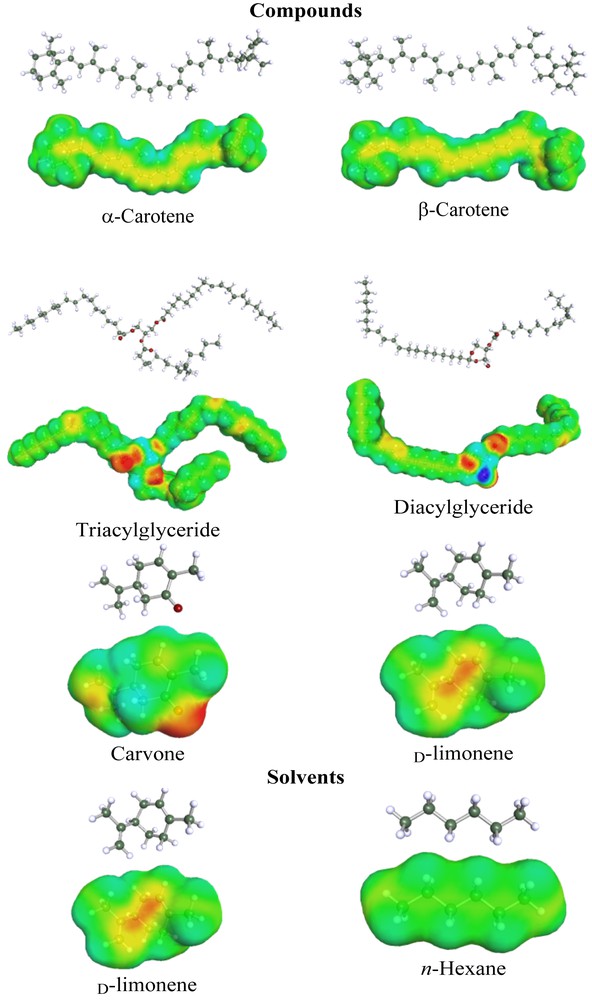
Structures and α-surfaces of the solvents and main compounds used in the theoretical study.
2.5.2 COSMO-RS
The Conductor-like Screening Model for Real Solvents (COSMO-RS) uses a statistical thermodynamic approach based on the result of quantum chemical calculations for an understanding of the dissolving mechanism. COSMO-RS combines quantum chemical considerations (COSMOs) and statistical thermodynamics (RS) to determine and predict the thermodynamic properties without experimental data. The COSMO-RS developed by Klamt [17] is known as a powerful method for molecular description and solvent screening based on a quantum-chemical approach.
COSMO-RS is a two-step procedure including microscopic and macroscopic steps. In the first step, the COSMO model is applied to simulate a virtual conductor environment for the molecule of interest. The molecule is embedded into a virtual conductor (Fig. 3a). In such an environment, the molecule induced a polarization charge density on its surface (Fig. 3b). Thus, during the quantum calculation self-consistency algorithm, the solute molecule is converged to its energetically optimal state in the conductor with respect to its electron density and geometry. The standard quantum chemical methods, triple zeta valence polarized basis set (TZVP) was used in this study.
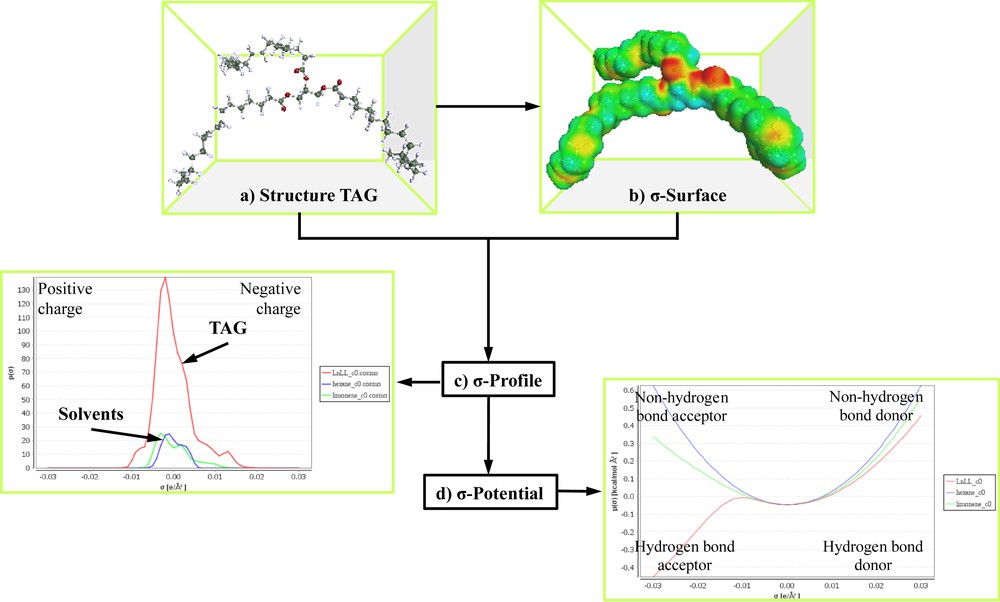
Step calculation with COSMO-RS: a) molecule emerged; b) σ-surface; c) energies of local surface interactions between σ-profiles of TAG (triacylglyceride) and solvents; d) σ-potentials of TAG and solvents.
The second step used the statistical thermodynamic calculation. This polarization charge density was used for the quantification of the interaction energy of the pairwise interacting surface segments with regard to the most important molecular interaction modes, i.e. electrostatics and hydrogen bonding. The 3D distribution of the polarization charges on the surface of each molecule was converted into a surface composition function (σ-profile). Such σ-profile provided detailed information about the molecular polarity distribution (Fig. 3c). The thermodynamics of the molecular interactions that were based on the obtained σ-profile were then used to calculate the chemical potential of the surface segment (σ-potential) as shown in Fig. 3d using the COSMOthermX program (version C30 release 13.01).
The σ-potential described the likeliness of the compound being able to interact with the solvents with polarity and hydrogen bonds (Fig. 3d). The part of the negative charge of the molecule was located on the right side (acceptor hydrogen bonds) with positive σ values while the part of the positive charged was located on the left side (donor hydrogen bonds) with negative σ values. Generally, the region σ ± 0.01 e A−2 was considered to be non-polar or weakly polar. The σ-profile and the σ-potential were used for interpreting the affinity of the solvent for surface polarity, to understand the interactions between the compound and solvent in mixed state and to estimate the thermodynamic properties of the system.
In addition, the COSMOthermX also allows the determination of the relative solubility between the solid compound and the liquid solvent in terms of the logarithm of the solubility in mole fractions (log10(x solub)). The logarithm of the best solubility was set to 0 and all other solvents were given relative to the best solvent. Also, the logarithm was transformed into the probability of solubility and was expressed in percentage.
2.6 Solid–liquid extraction
The extraction of carotenoids from carrots, the oils from rapeseed and terpenes (aromas) from caraway seeds was carried out using an ULTRA-TURRAX® Tube Drive System (IKA). The dried vegetable materials were ground immediately before extraction. One gram of each powder of vegetable materials, 20 g of ceramic balls and 10 mL of a solvent (d-limonene or n-hexane) were added to a tube with a stirring device. The mixture was mechanically stirred and macerated at 4000 rpm for 1 h at room temperature. Afterwards, the mixture was filtered and centrifuged at 9000 rpm for 10 min to separate the supernatant. The final volumes of the extracts were adjusted to 10 mL by adding of d-limonene or n-hexane, respectively. The samples were stored at −20 °C until analysis. All experiments were carried out in triplicate.
2.7 Analytical methods
2.7.1 Gas chromatography-mass spectrometry identification
Essential oil composition and oxidation products were determined by gas chromatography coupled to mass spectrometry (GC-MS) analysis on a Hewlett-Packard 6890 gas chromatograph coupled to a 5973A mass spectrometer, using two fused-silica-capillary columns with different stationary phases. The non-polar column was a HP5MS™ (30 m × 0.25 mm × 0.25 μm film thickness) and the polar one was a Stabilwax™ consisting of a Carbowax™-PEG (60 m × 0.25 mm × 0.25 μm film thickness). GC-MS spectra were obtained using the following conditions: carrier gas He; flow rate 0.7 mL min−1; split 1:20; injection volume 0.1 μL; injection temperature 250 °C; and oven temperature progress from 60 to 280 °C at 2 °C min−1. The ionization mode used was an electronic impact at 70 eV. Most constituents were tentatively identified by comparison of their GC Kovats retention indices (RI), determined with a reference to a homologous series of C5–C28 n-alkenes and with those of authentic standards available in the authors' laboratory. Identification was confirmed by comparison of their mass spectral fragmentation patterns with those stored in the MS database (National Institute of Standards and Technology and Wiley libraries) and with mass spectra literature data.
2.7.2 Gas chromatography-FID quantification
The relative percentage of the components was electronically calculated from GC-FID peak areas. A Hewlett–Packard 6890 GC-FID system was used for gas chromatography analysis, fitted with a fused-silica-capillary column with a non-polar stationary phase HP5MS™ (30 m × 0.25 mm × 0.25 μm film thickness). The column temperature program was 60 °C for 8 min increased at 2 °C min−1 to 250 °C and held at 250 °C for 15 min. Injection was performed at 250 °C in the split-less mode; 1 μL of sample was injected. A flow rate of 0.3 mL min−1 carrier gas (N2) was used. Flame ionization detection was performed at 320 °C.
2.7.3 Total carotenoid analysis
The total content of carotenoids was measured spectrophotometrically (Biochrom Libra S22 UV/Vis Spectrophotometer) in a 1 cm optical path-length quartz cell at 450 nm in each extract using n-hexane as a blank. The Beer–Lambert law was used to determine the carotenoid concentration in each extract using a calibration curve prepared using a β-carotene standard. The straight calibration curve of absorbance versus carotenoid concentration (mg L−1) was reliant on the Beer–Lambert law. Finally, the yield of carotenoids in each extract was calculated and expressed as mg (β-carotene) 100 g−1 of dry matter.
2.7.4 Determination of specific carotenoids by HPLC
HPLC analysis was carried out using an HPLC Agilent 1100 equipped with a UV-VIS detector (DAD). Separation was achieved at 25 °C on a C18 column (150 × 3.0 mm and 3 μm ID). The mobile phase consisted of acetonitrile/methanol (with 0.6% ammonium acetate)/dichloromethane (77/20/3, v/v/v) and was pumped at a flow rate of 1.4 mL min−1. The sample injection volume was 10 μL and the quantitative detection was at a wavelength of 464 nm. The calibration curves of carotenoids were established using standard carotenoids (concentration range was 5, 10, 25, 50 and 100 mg L−1). Each calibration point was carried out in triplicate. The identification of major carotenoids in carrot extracts was carried out by comparing the retention times and absorption spectra.
2.7.5 Rapeseed oil profile by GC analysis
Fatty acid methyl esters (FAMEs) were separated, identified and quantified by gas chromatography coupled with flame ionization detector (GC-FID). The samples were prepared from extracted oils from rapeseeds using acid-catalyzed trans-methylation [18]. A volume of 1 mL of methanolic sulfuric acid (5% v/v) was added to a specific amount of extracted oils and an internal standard. The mixture was heated at 85 °C for 90 min and subsequently allowed to reach room temperature. Then 1.5 mL of sodium chloride (0.9%) solution and 1 mL of n-hexane were added. The flask was stoppered and shaken vigorously for 30 s before centrifugation at 4000 rpm for 2 min. An aliquot of the organic layer was transferred into a gas chromatography vial. Analysis was performed using a 7820A GC system (Agilent technologies, Santa Clara, CA, USA) equipped with a BD-EN14103 capillary column (30 m × 0.32 mm × 0.25 μm), a FID detector and an auto-sampler. Helium was used as a carrier gas at 33 cm s−1. The injection volume was 2 μL and the samples were injected in split mode (split ratio: 1:20) at 250 °C. The oven temperature program was operated as follows: initial temperature at 50 °C for 1 min, increased at a rate of 20 °C min−1 to 180 °C and at a rate of 2 °C min−1 from 180 to 230 °C, held isothermally at 230 °C for 10 min. Data were collected with Agilent EZChrom Elite software. FAMEs were identified in comparison with purified FAME standards (Sigma Co., St. Louis, MO, USA). Quantification was performed using internal calibration. The internal standard was glyceryl tripheptadecanoate (Sigma Co., USA).
3 Results and discussion
3.1 Extraction and composition of the essential oils
Citrus essential oils containing d-limonene are generally extracted by hydrodistillation where a Clevenger apparatus represents the most common system that has been used for decades to extract and evaluate essential oils in herbs and seeds. Although the hydrodistillation step requires several hours, it is interesting to note that it allows extracting components at a lower temperature than their boiling point (azeotropic distillation), which eliminates degradation risks at high temperatures. In this essence, the introduction of electromagnetic energy as a heating source has helped the improvement of the Clevenger system [19]. This technique has been applied with success for the extraction of essential oils from orange peels. This approach has permitted the reduction of processing time from 3 h (conventional conduction heating system) to 30 min in the microwave-assisted system. In addition, the new design is solvent free, requires low capital investments and consumes lower energy compared to the conventional one. In this context, SFME was the method used to perform the extraction of essential oil from orange peels (Citrus sinensis L. Osbeck).
Table 1 listed the retention time and total content of chemical composition of essential oil obtained from fresh orange peel analyzed by GC-FID and GC-MS. The yield of essential oil was 1.54% after 12 min of extraction. This yield was higher than the achieved by Badee et al. [8] who obtained 0.2% of orange peel essential oil by applying the cold-pressing method. The essential oil consists of more than 98% terpenes and a small fraction of oxygenated components responsible for its distinct smell. The GC analysis shows that the terpene fraction is composed mostly of d-limonene (94.88%) and the oxygenated aroma fractions are composed mainly of linalool. These results are in agreement with a previous work [20], which showed that the terpene fraction is composed mostly of d-limonene at 96.30%.
Chemical compositions of orange peel essential oils obtained by SFME.
| No. | Compoundsa | RIb | RIc | SFME (%) |
| Monoterpenes | 98.23 | |||
| 1 | α-Pinene | 926 | 1023 | 0.45 |
| 2 | Sabinene | 961 | 1121 | 0.53 |
| 3 | β-Pinene | 974 | 1109 | 0.04 |
| 4 | β-Myrcene | 988 | 1165 | 1.59 |
| 5 | α-Phellandrene | 1001 | 1268 | 0.28 |
| 6 | δ-3-Carene | 1014 | 1290 | 0.24 |
| 7 | d-Limonene | 1030 | 1206 | 94.88 |
| 8 | γ-Terpinene | 1103 | 1285 | 0.22 |
| Oxygenated monoterpenes | 0.43 | |||
| 9 | Linalool | 1125 | 1538 | 0.29 |
| 10 | Citronellal | 1167 | 1478 | 0.01 |
| 11 | Terpin-4-ol | 1191 | 1590 | 0.01 |
| 12 | α-Terpineol | 1203 | 1677 | 0.02 |
| 13 | Nerol | 1237 | 1781 | 0.02 |
| 14 | Neral | 1268 | 1670 | 0.01 |
| 15 | Geranial | 1284 | 1714 | 0.06 |
| 16 | Perilla alcohol | 1313 | 1991 | 0.01 |
| Sesquiterpenes | 0.16 | |||
| 17 | α−Copaene | 1357 | 1491 | 0.01 |
| 18 | β-Elemene | 1373 | 1583 | 0.01 |
| 19 | (E)-Caryophellene | 1391 | 1594 | 0.01 |
| 20 | trans-α-Bergamotene | 1437 | 1577 | 0.01 |
| 21 | α-Humulene | 1450 | 1657 | 0.02 |
| 22 | (E)-β-Farnesene | 1453 | 1650 | 0.01 |
| 23 | Valencene | 1488 | 1705 | 0.09 |
| Oxygenated sesquiterpenes | 0.04 | |||
| 24 | Caryophellene oxide | 1570 | 1977 | 0.02 |
| 25 | β-Sinensal | 1701 | 2341 | 0.01 |
| 26 | Nootkatone | 1799 | 2250 | 0.01 |
| Other oxygenated compounds | 0.41 | |||
| 27 | n-Nonanal | 1126 | 1400 | 0.15 |
| 28 | Decanal | 1211 | 1497 | 0.18 |
| 29 | Undecanal | 1318 | 1592 | 0.06 |
| 30 | α-Terpinyl acetate | 1335 | 1684 | 0.01 |
| 31 | Citronellyl Acetate | 1342 | 1645 | 0.01 |
| Total oxygenated compounds (%) | 0.88 | |||
| Total non-oxygenated compounds (%) | 98.39 | |||
| Extraction time (min) | 12 | |||
| Yield (%) | 1.54 |
a Essential oil compounds sorted by chemical families and percentages calculated by GC-FID on a non-polar HP5MS™ capillary column.
b Retention indices relative to C5–C28 n-alkanes calculated on a non-polar HP5MS™ capillary column.
c Retention indices relative to C5–C28 n-alkanes calculated on a polar Carbowax™-PEG capillary column.
The deterpenation process of orange peel oil by means of a thin film evaporator was performed in order to recover pure d-limonene. According to Leenaerts [21], the thin layer technique allows a very short residence time under reduced pressure, a significant heat-transfer surface as well as a mixing potential that match heat and mass transfer requirements. The oil deterpenation was achieved after 30 min and the optimal process conditions were the same reported by Zeboudj et al. [15] (temperature = 65.4 °C, flux = 0.036 kg h−1 and pressure = 100 mmHg). After that time, the deterpenation achieved a recovery of 99% of d-limonene. Next, d-limonene was used as agro-synthon to obtain oxidized compounds with industrial interest and also it was used as bio-based solvent as a substitute of n-hexane for extracting of natural products (carotenoids from carrots, rapeseed oil and aromas from caraway seeds).
3.2 Oxidation of d-limonene
Recent research trends have been focused on the incorporation of the Fe ions or Fe oxides into porous supports to improve the reaction processes. The major advantage of the use of heterogeneous catalytic material is its easy recovery by means of simple separation operation and re-use in the next runs. So, the oxidation of d-limonene was performed using three different iron catalysts, one with the iron incorporated into an anionic lamellar compound (Fe-LDH) and two with the metal supported into porous supports (Fe-SBA 15 and Fe-F16SM). Layered double hydroxides (LDHs) and their derived mixed oxides (Fe-LDHs) have received growing attention in research and engineering because of the possibility of varying a large number of synthesis parameters and in that way, modify and tailor various properties [22]. Further, the commercial mesoporous material iron such as iron-loaded SBA-15 (Fe/SBA-15s) and iron folded sheet mesoporous materials (Fe-F16SM) has attracted more and more attention as materials with catalytic application in oxidation processes.
The oxidation results showed a maximum conversion yield of 94.08% after 4 h with the three catalysts. Table 2 presents the results of the selective oxidation of d-limonene. This compound was oxidized quite quantitatively using Fe-LDH and the major compound obtained was α-terpinolene, followed by 1,3,8-p-menthatriene. Regarding of Fe-SBA 15, the oxidation reaction led to the formation of eight compounds where the major compounds were 3-methyl-cyclopentanone, 6-methyl-heptan-2-one and cis-linalool oxide. Some amount of d-limonene remained. The use of Fe-F16SM as a catalyst in the oxidation reaction gave five oxidized compounds where cis-linalool oxide and epoxy-linalool were the major compounds. These results are in agreement with previous research studies [13,23] where it is mentioned that the main products formed were oxygenated derivatives such as epoxides, which are of considerable commercial value.
Selectivity conversion of d-limonene into oxidized compounds using Fe-LDH, Fe-SBA 15 and Fe-FSM 16.
| Compound | Selectivity (%) | ||
| Fe-LDH | Fe-SBA 15 | Fe-F16 SM | |
| cis-Linalool oxide | 3.90 | 17.20 | 34.90 |
| α-Terpinolene | 57.98 | 5.30 | — |
| 1,3,8-p-menthatriene | 15.40 | 3.70 | — |
| 1-Methylene-4-isopropylenecyclohexane | 7.00 | — | — |
| 3,7-Dimethyl-oct-2-enal | 9.80 | — | 13.10 |
| Epoxy linalool | — | 6.90 | 22.25 |
| d-Limonene | — | 9.80 | — |
| 3-Methyl-cyclopentanone | — | 23.90 | — |
| Limonene epoxyde | — | 7.50 | — |
| 6-Methyl-heptan-2-one | — | 22.80 | 6.40 |
| Acetic acid | — | — | 11.60 |
| Total oxidation | 94.08 | 87.30 | 88.25 |
The three catalysts enhance the catalytic behavior and facilitate d-limonene/iron contact. The oxidation mechanism, as explained by Herney-Ramirez and Madeira [24], occurred at a quasi-quantitative yield due to active sites of iron; the large pore size of the macroporous support where iron is embedded enhances the absorption of d-limonene and increase iron dispersion on internal and external sides. The applied oxidation process is based on Fenton's reagent (Fe II/H2O2), which has proved to be a promising and attractive treatment. The advantages of this reagent over other oxidizing treatment methods include also high efficiency, simplicity in destroying the contaminants (eventually leaving no residues), and no need for special equipment. Besides, the operating conditions are usually mild (atmospheric pressure).
The efficiency of this reagent results from the fact that the in situ generated hydroxyl radicals (HO•) are a highly reactive species (E° = + 2.8 V), which attack the majority of organic molecules, causing their oxidation to other products and ultimately to CO2 and H2O except for simple organic compounds. The mechanism of Fenton's oxidation basically involves the following steps. First, a mixture of H2O2 and ferrous ion in acid solution generates hydroxyl radicals. Then, the hydroxyl radicals formed can oxidize organic compounds by abstraction of protons, producing organic radicals R•, which are highly reactive and can be further oxidized. Finally, because iron (II) acts as a catalyst, it also has to be restored. The oxidation process of many compounds, among them, those derived from terpenic compounds, is very important because they are used in the production of fragrances, perfumes and food additives [13]. For this reason, the oxidation of d-limonene, which is the major constituent of several citrus oils, is an important methodology for the manufacture of valuable organic compounds.
3.3 Analysis using computational methods
The study of the extraction of bioactive compounds using d-limonene as a green solvent compared to n-hexane was performed by a theoretical procedure, using two computational predictive methods (HSPs and COSMO-RS), technical properties of the solvents and via experimentation. The comparison was made in terms of the amount of compounds in each extract and taking into account the technical parameters of the solvents used.
3.3.1 Hansen solubility parameters (HSPs)
The solubility in the d-limonene of each compound used in the theoretical study was compared with n-hexane, which is one of the most used solvents for extraction in the industry and was used as the reference. The software allows the assessment of the relative energy difference (RED), which estimates the capacity of a solvent to dissolve solutes. Table 3 shows the RED calculated for the two solvents with carotenoids from carrots, triacylglycerides and diacylglycerides from rapeseed oil and aromas from caraway seeds. RED values <1 represent good solubility, which means that n-hexane is not the best solvent, from a theoretical perspective, for the extraction of the carotenoids, triacylglycerides and main monoterpenes from caraway seeds evaluated in this study. According to the HSP results, the RED values of d-limonene are lower than those of n-hexane values in all cases. It means that d-limonene is a better solvent to the extraction of all the compounds evaluated in this work.
HSP values of solutes and solvents and relative energy difference (RED) of solvents for the extraction of carotenoids from carrots, triacylglycerides (TAGs) and diacylglycerides (DAGs) from rapeseed oil and aromas from caraway seeds.
3.3.2 COSMO-RS calculations
The COSMO-RS simulation was also conducted in order to determine the potential of d-limonene, and n-hexane for the extraction of the different compounds of interest in this study. The software integrates a quantum chemistry approach that permits the calculation of various properties such as the relative solubility of a compound in several solvents.
Table 4 shows the solubility of various compounds (carotenoids from carrots, triacylglycerides and diacylglycerides from rapeseed oil and aromas from caraway seeds) in d-limonene and n-hexane. The results are expressed in log10(x-solub) (best solubility is set to 0) and percentage of probability of solubility for a better understanding of the results. d-Limonene showed a higher probability of solubility for all the compounds analyzed in this study compared with n-hexane. According to the rule “like dissolves like”, d-limonene and n-hexane show high probability of solubility (60–100%) for β-carotene and α-carotene. Lycopene is more soluble in d-limonene than n-hexane. However, those solvents presented low (0–20%) solubility for lutein that can be due to the hydroxyl groups of lutein that give a high polarity to the molecule. Regarding the TAGs from rapeseed oil, the solvents showed a high probability of solubility for the major TAGs from this vegetable oil. However, n-hexane showed a medium probability of solubility (20–60%) for the TAG-1. Additionally, the probability of solubility of the three major DAGs was low in the two solvents. This may be due to the high polarity of the compounds compared with that of the TAGs.
COSMO-RS relative solubility (log10(x-solub)) and probability of solubility of carotenoids from carrots, triacylglycerides (TAGs) and diacylglycerides (DAGs) from rapeseed oil and aromas from caraway seeds using d-limonene and n-hexane as solvents.
Concerning the aromas from caraway seeds, the probability of solubility of monoterpenes (sabinene, β-myrcene, δ-3-carene, d-limonene and α-terpinene) and sesquiterpenes (β-caryophyllene and β-caryophyllene oxide) in d-limonene is high and similar to that of n-hexane (60–100%). This is due to the fact that the polarity of these compounds is similar to the solvents evaluated. Moreover, most of the oxygenated monoterpenes, compounds more polar than the abovementioned ones, showed a higher probability of solubility in the d-limonene than in n-hexane.
3.4 Total carotenoid yield and composition of the extracts
Carotenoids are one of the major groups of natural pigments that find widespread utilization in the food industry. Furthermore, they are also used for medical, cosmetic, and biotechnological purposes [25]. For this reason, the extraction of these bioactive compounds was performed in this study.
Carotenoids were extracted from carrots by solid–liquid extraction performed by maceration using d-limonene and n-hexane as solvents at room temperature for 1 h. To carry out a better comparison of the amount of carotenoids obtained by the two solvents, we determined the maximum content of carotenoids in the carrots by extracting with n-hexane at boiling point for three extraction cycles (until no color was observed in the solvent). As is presented in Table 5, the maximum carotenoid content in the carrots used in this study was 31.0 mg 100 g−1 of dry vegetable matter (DM). d-Limonene was able to extract 94.8% of the maximum carotenoid content in carrots while n-hexane only showed 78.1% of this value. The extraction of carotenoids depends on the solvent used, its polarity, and the solubility of them in the extraction solvents. Therefore, we determined that the solubility of β-carotene in d-limonene is almost 1.5 fold that in n-hexane. So, this could explain why the bio-based solvent achieved greatest yield.
Total carotenoid yield (CY) compared to maximum carotenoid content in carrots (%), solubility of β-carotene and percentage of HPLC separated carotenoids from carrots extracted with d-limonene and n-hexane.a
| Solvent | Carotenoid yield (CY) (mg 100 g−1 DM) | CY compared to maximum carotenoid content in carrots (%) | HPLC identified carotenoids (%) | |||
| α-Carotene | β-Carotene | Lycopene | Lutein | |||
| n-Hexaneb | 31.0 ± 2.1 | 100.0 | ||||
| n-Hexane | 24.2 ± 2.0 | 78.1 ± 4.1 | 34.7 ± 1.8 | 63.7 ± 1.4 | tr | 1.6 ± 0.2 |
| d-Limonene | 29.4 ± 2.7 | 94.8 ± 1.7 | 32.5 ± 1.1 | 61.7 ± 0.9 | 5.5 ± 1.3 | 0.34 ± 0.1 |
a Values are mean ± SD (n = 3). DM, dry vegetable matter. tr, traces.
b Maximum carotenoid content in carrot (refluxing in n-hexane for 2 h, three cycles).
Moreover, HPLC analysis of the extracts showed similar relative percentages of α-carotene, β-carotene and lutein for both solvents and it did not reveal any peaks that would indicate isomerization or degradation. Those results are according to the literature where it is mentioned that β-carotene constitutes the main part of carrot carotenoids (60–80%), the fraction of α-carotene is 10–40%, and the fraction of lutein does not exceed 1–5% [26]. Nevertheless, d-limonene was capable of extracting 5.5% of lycopene and the extract of n-hexane did not show this compound. However, there are variations in the composition of carotenoids in vegetables due to factors such as variety, stage of maturity, geographic origin, farming practices and climate or season [27]. Considering the results, d-limonene can be considered as a potential alternative solvent to n-hexane for the extraction of carotenoids from carrots.
3.5 Extraction and composition of rapeseed oil
The rapeseed oil production process involves several steps including preparation of seeds, mechanical pressing and solvent extraction of the press cake [28]. The press cake extraction is performed in countercurrent extractors generally using n-hexane. Despite the advantage of using this petroleum-based solvent in extraction, questions may arise due to its negative influence on human health and environment. So, reducing the amount of hexane used in oil processing while keeping the same extraction performance has become desirable and is a key issue for industries for economic and ecological reasons. In this context, we carried out the extraction of rapeseed oil using an alternative bio-based green solvent in comparison to n-hexane.
The extraction of oil from rapeseed was carried out by solid–liquid extraction performed by maceration. After the extraction process, the composition of the extracts was determined by GC-FID after transmethylation of fatty acids. Table 6 shows that the lipid profile of oil extracted with d-limonene is comparable to the one extracted with n-hexane and no differences in selectivity between both solvents were observed. The main fatty acids in extracted oils were oleic (C18:1), linoleic (C18:2), linolenic (C18:3) and palmitic (C16:0), which represent more than 90% of the total fatty acids in extracted oils. Other fatty acids such as stearic (C18:0), arachidic (C20:0), behenic (C22:0) and dihomo-γ-linolenic (C20:3) acids were also quantified with a less predominant proportion. Moreover, the extraction yield of d-limonene (42.7 g 100 g−1 DM) was similar to that of n-hexane (43.2 g 100 g−1 DM). These results were similar to those described by Sicaire et al. [29] obtained with n-hexane, although n-hexane is commonly used for extraction of vegetable oils due to its good extractive ability and selectivity. Furthermore, the solubility of the rapeseed oil in the two solvents was evaluated and high solubility of the vegetable oil compounds was observed in both solvents.
Fatty acid composition of rapeseed oil extracted with d-limonene and n-hexane.
| Fatty acid composition | Solvent | |
| d-Limonene | n-Hexane | |
| Saturated | ||
| C16:0 | 4.75 ± 0.24 | 4.80 ± 0.34 |
| C18:0 | 1.50 ± 0.31 | 1.55 ± 0.42 |
| C20:0 | 0.93 ± 0.25 | 0.97 ± 0.18 |
| C22:0 | 1.25 ± 0.29 | 1.29 ± 0.17 |
| Mono-unsaturated | ||
| C18:1n-9 | 60.57 ± 0.47 | 60.14 ± 0.52 |
| Poly-unsaturated | ||
| C18:2n-6 | 19.57 ± 0.42 | 19.65 ± 0.28 |
| C18:3n-3 | 7.51 ± 0.32 | 7.63 ± 0.15 |
| C20:3n-3 | 3.92 ± 0.65 | 3.97 ± 0.23 |
| ∑SFAs | 8.43 | 8.61 |
| ∑MUFAs | 60.57 | 60.14 |
| ∑PUFAs | 31.00 | 31.25 |
3.6 Extraction of aromas from caraway seeds
Aromas are complex mixtures of volatile substances generally present at low concentrations [30]. These compounds are useful in daily life as food additives, flavours, fragrances, pharmaceuticals, colors, or directly in medicine. Before such substances can be used or analysed, they have to be extracted from the matrix. n-Hexane has been used widely as an extraction solvent for these compounds. Here, we evaluated the potential of d-limonene as an alternative agro-solvent to extract aromas from caraway seeds compared to n-hexane.
The extracts were analysed by GC-MS to compare the performance of the solvents in terms of the extraction of aromas and selectivity between the two major compounds (d-limonene and carvone). The characterization of the essential oils showed that the aroma of caraway seeds is mainly constituted by monoterpenes. In our experiment, the extract of n-hexane showed that the main component was carvone (64%) and the second main constituent was d-limonene (34%). These two compounds represent 98% of total composition. These results are in agreement with the results reported by Filly et al. [30], who found that these two monoterpenes are the main compounds in extracts obtained using various solvents. The remaining fractions, such as aldehydes, oxygenated monoterpenes, sesquiterpenes and oxygenated sesquiterpenes, formed a minor chemical group of compounds that were just observed as traces. The GC-MS analysis of the extract of d-limonene showed that the d-limonene used as solvent and the d-limonene extracted in the essential oils had the same retention time and identical mass spectra. Thus, in this extract, only carvone was identified.
In addition, the solubility of the main compounds of the caraway seed aromas in either the bio-based solvent or n-hexane was tested and the high solubility of these aromas was observed in the two solvents. In accordance with the results, the alternative green solvent is a promising solvent to extract aroma from vegetable sources with results similar to n-hexane. Consequently, it is a real advantage to reduce the ecological impacts of the process in the aroma industry.
3.7 Ecological and energy approaches
Extensive studies have shown the importance and reported the potential of green solvents that could be used instead of the petrochemical solvents in the extraction of bioactive compounds [31]. However, parameters other than solubility, such as toxicity and energy efficiency, should be considered when evaluating the potential of an alternative green solvent. Table 7 shows different parameters of the two solvents used in this study.
Physicochemical properties of solvents. Hansen solubility parameters (HSPs), long-term toxicity (CMR): “no” indicates non-CMR according to the European legislation, acute toxicity according to the Hodge and Sterner Scale (Itox).a,b
| Parameter | d-Limonene | n-Hexane |
| Physicochemical properties | ||
| Molecular weight (g/mol) | 136.23 | 86.2 |
| Viscosity (cP) | 0.923 | 0.31 |
| Log P | 4.45 | 3.94 |
| Flash point (°C) | 48.0 | −23.3 |
| Melting point (°C) | −74.35 | −127.4 |
| Energy efficiency | ||
| Boiling point (°C) | 175.0 | 68.5 |
| ∂Hvap. (kJ/mol) | 39.5 | 28.9 |
| Cp, liquid (kJ/mol*K) | 0.249 | 0.226 |
| Energy evaporation of 1 kg solvent (E) (kWh) | 0.157 | 0.121 |
| E compared to Hexane (%) | +29.75 | — |
| HSPs | ||
| δd (MPa1/2) | 16.7 | 15 |
| δp (MPa1/2) | 1.8 | 0 |
| δh (MPa1/2) | 3.1 | 0 |
| Toxicity | ||
| Resource | Cereal crop | Petroleum |
| CMR | No | 2 |
| Itox | 5 | 6 |
a The values of the solvent parameters in the table were obtained from: https://ilab.acdlabs.com/ilab2/.
b CMR classification: http://www.prc.cnrs-gif.fr/spip.php?article169&lang=fr.
In relation to bioeconomical issues, n-hexane is a solvent obtained from petroleum while d-limonene is a green solvent, which can be obtained from the biomass feedstock. d-Limonene stands as a valuable replacement for traditional solvents, many of which emit poly-aromatic hydrocarbons (PAHs) or fumes from volatile organic compounds (VOCs). Solvents that are commonly replaced with d-limonene solvents include methyl ethyl ketone, acetone, toluene, glycol ethers, and numerous fluorinated and chlorinated solvents. According to the energy efficiency, the energy necessary to evaporate 1 kg of solvent (E) was calculated according to Sicaire et al. [29] using the specific heat, latent heat of vaporization and the boiling point. Here, d-limonene required 29.75% more E to be evaporated than n-hexane. However, the bio-based solvent extracted more carotenes than n-hexane under the same operation conditions, which means that economic cost generated by the extra E can be compensated by the higher carotenoid extraction yield. Besides, despite the fact that the results of the extraction of rapeseed oil and aromas from caraway seeds were similar to those of n-hexane, the use of d-limonene can reduce the environmental impact caused for the use of petroleum-based solvents.
4 Conclusion
The objective of this study was to transform green d-limonene as one primary platform chemical issued from agro-industrial waste. d-Limonene obtained after the deterpenation process of essential oils from orange peels was transformed into high added-value compounds by catalytic oxidation using commercial mesoporous material iron and double lamellar hydroxide iron (Fe-LDH, Fe-SBA 15 and Fe-F16SM). Different oxidized high added-value compounds were obtained with a good selectivity. Additionally, the potential of d-limonene as a green alternative solvent to replace n-hexane in the extraction of bioactive compounds was evaluated by theoretical methods using two simulation tools (HSPs and COSMO-RS) and via experimentation.
Acknowledgements
This work was supported in part thanks to funding from the fellowship given to Edinson Yara Varón (No. TECSPR14-2-0029) from the People Programme (Marie Curie Actions) of the Seventh Framework Programme of the European Union (FP7/2007-2013) under REA grant agreement no. 600388 (TECNIOspring programme), and from the Agency for Business Competitiveness of the Government of Catalonia, ACCIÓ.


