1 Introduction
The oxindole framework has generated considerable interest as a core constituent of many natural products and biologically active molecules. In particular, spirooxindoles are important heterocyclic compounds due to their various biological and pharmacological properties. They are widely used as antimicrobial [1], antitumor [2], and therapeutic agents [3]. On the other hand, furan derivatives have exhibited attractive biological activities, such as antitumor [4], antimicrobial [5,6], antispasmodic [7] and cytotoxic properties [8]. Also, synthesis and pharmacological application of various spirooxindole–furan derivatives have been reported [9–11].
The interrupted Feist–Bénary reaction (IFB) is a base-catalyzed condensation of α-haloketones with 1,3-dicarbonyl compounds, which form highly substituted dihydrofuran derivatives [12,13]. Recently a modified Feist–Bénary reaction for the synthesis of bisspirooxindole-fused dihydrofurans has been reported [14]. Also a new variant of this reaction has been reported, including α-tosyloxyacetophenones as electrophiles instead of α-haloketones. It has been shown that phenacyl bromides are poor reactants in the Feist–Bénary reaction, but replacing Br with the tosyloxy group has led to good results [15].
In order to expand the scope of the IFB reaction, and in continuation of our previous works for the synthesis of novel heterocyclic compounds [16–19], we report herein an efficient procedure for the synthesis of new spirooxindole–furan derivatives via the Feist–Bénary reaction of N-phenacyl pyridinium salts as a reactive electrophile with 1,3-dicarbonyl compounds and isatin derivatives (Scheme 1).
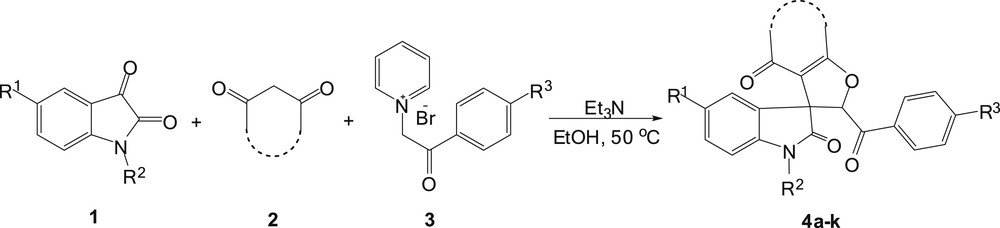
Preparation of novel spirooxindole–furan derivatives.
2 Results and discussion
In order to optimize the conditions, we studied the reaction of N-phenacyl pyridinium bromide, dimedone and isatin as the model reaction under various conditions (Table 1). First, the reaction was examined in the presence of different bases. As shown, using triethylamine gave better yields than the other bases (Table 1, entry 5). Then, the effect of various solvents and different temperatures were examined. Using EtOH, the reaction afforded the corresponding product with a good yield (85%) after 10 h at 50 °C (Table 1, entry 5). We also evaluated the amount of triethylamine required for the reaction. It was found that the use of 1 eq of NEt3 is sufficient to promote the reaction. In the presence of more than this amount of the base, neither the yield nor the reaction time was improved.
Optimization of the reaction conditions.a
| Entry | Base (eq) | Solvent | Temp. (°C) | Time (h) | Yields (%) |
| 1 | – | EtOH | 50 | 24 | – |
| 2 | K2CO3 (1) | EtOH | 50 | 15 | 45 |
| 3 | DBU (1) | EtOH | 50 | 15 | 60 |
| 4 | DABCO (1) | EtOH | 50 | 18 | 65 |
| 5 | NEt3 (1) | EtOH | 50 | 10 | 85 |
| 6 | NEt3 (0.25) | EtOH | 50 | 24 | 30 |
| 7 | NEt3 (0.5) | EtOH | 50 | 24 | 55 |
| 8 | NEt3 (2) | EtOH | 50 | 10 | 85 |
| 9 | NEt3 (1) | THF | 50 | 15 | 50 |
| 10 | NEt3 (1) | H2O | 50 | 12 | 45 |
| 11 | NEt3 (1) | DCM | 50 | 14 | 72 |
| 12 | NEt3 (1) | – | 50 | 24 | 60 |
| 13 | NEt3 (1) | EtOH | 25 | 24 | 40 |
| 14 | NEt3 (1) | EtOH | Reflux | 10 | 80 |
a Reaction conditions: N-phenacyl pyridinium bromide (1 mmol), dimedone (1 mmol), isatin (1 mmol), different conditions, stirring.
After optimization of the model reaction, a variety of spirooxindole–furan derivatives were synthesized with the three-component reaction of isatin derivatives with 1,3-dicarbonyl compounds and pyridinium salts according to Scheme 1. The target compounds were obtained in good yields with high purity (Table 2). The structure of products was confirmed by IR, 1H and 13C NMR spectroscopy and mass spectrometry.
Preparation of spirooxindole–furan derivatives.a
| Entry | R1 | R2 | R3 | 1,3-dicarbonyl compound | Product | Time (h) | Mp (°C) | Yield (%) |
| 1 | H | H | H | 4a | 10 | 275–277 | 85 | |
| 2 | H | CH3CH2 | H | 4b | 12 | 188–190 | 73 | |
| 3 | H | CH2CO2Et | H | 4c | 13 | 165–167 | 70 | |
| 4 | H | H | H | 4d | 12 | 242–244 | 76 | |
| 5 | Cl | H | H | 4e | 10 | 260–263 | 80 | |
| 6 | Br | H | H | 4f | 10 | 274–276 | 78 | |
| 7 | Cl | CH2CO2Et | H | 4g | 11 | 158–160 | 74 | |
| 8 | Cl | PhCH2 | H | 4h | 11 | 203–205 | 82 | |
| 9 | H | H | Cl | 4i | 10 | 248–249 | 80 | |
| 10 | H | H | H | 4j | 12 | 245–247 | 70 | |
| 11 | H | H | Br | 4k | 10 | 235–237 | 76 |
a Reaction conditions: N-phenacyl pyridinium bromide derivatives (1 mmol), dimedone (1 mmol), isatin derivatives (1 mmol), triethylamine (1 mmol), EtOH, 50 °C, stirring.
The mass spectrum of 4a displayed the molecular ion (M+) peak at m/z 387, which was consistent with the product's structure. The 1H NMR spectrum of 4a in CDCl3 exhibited two singlets at 1.15 and 1.22 ppm due to the two methyl groups of dimedone, two doublets at 2.14 and 2.31 ppm (2JHH = 16.4 Hz), two doublets 2.65 and 2.68 ppm (2JHH = 18.0 Hz) for diastereotopic protons of two CH2 groups, and one singlet at 6.48 ppm for CH group of furan ring. The aromatic protons appeared as one doublet at 6.45 ppm (3JHH = 7.6 Hz) and four multiplets at around 6.59–7.44 ppm. The proton of NH in oxindole moiety was observed as one broad singlet at 8.10 ppm. The proton-decoupled 13C NMR spectrum of 4a showed 22 distinct resonances, in agreement with the proposed structure. The spiro carbon displayed a signal at 58.9 ppm.
A plausible mechanism for the formation of products 4a–k is given in Scheme 2. Initially, Knoevenagel condensation between isatin derivatives and CH-acid of 1,3-dicarbonyl compounds in the presence of triethylamine affords adduct 5. Also the pyridinium salt is deprotonated by triethylamine to give pyridinium ylide 6. In the second step, a Michael addition of the pyridinium ylide 6 to adduct 5 produces dipolar intermediate 7. Finally, the attack of enolate moiety in 7 to the electrophilic carbon bearing the leaving pyridyl group leads to spirooxindole fused furan derivative.
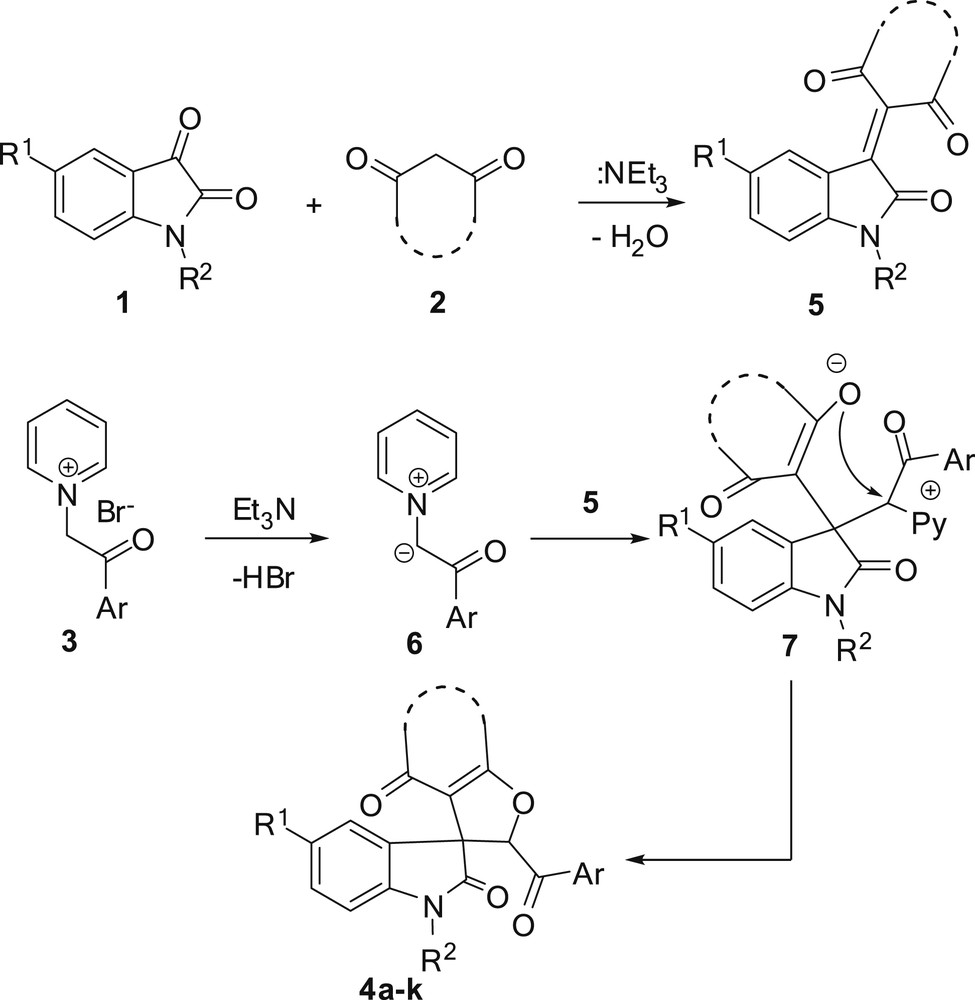
Proposed mechanism for the preparation of spirooxindole–furan derivatives.
3 Experimental
3.1 Materials and techniques
All chemicals and reagents were purchased from Fluka and Merck and used without further purification. Melting points were measured with an Electrothermal 9100 apparatus. NMR spectra were recorded with a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H, 100.6 MHz for 13C) with CDCl3 as solvent. Chemical shifts (δ) are given in parts per million (ppm) relative to TMS, and coupling constants (J) are reported in hertz (Hz). IR spectra were recorded on an FT-IR Bruker vector 22 spectrometer. Mass spectra were recorded on a Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 70 eV.
3.2 General procedure for the synthesis of compounds 4a–k
N-Phenacyl pyridinium bromide derivatives were obtained by the reaction of phenacyl bromide derivatives and pyridine in acetonitrile medium. Triethylamine (1 mmol) was added to a mixture of isatin (1 mmol) and 1,3-dicarbonyl compounds (1 mmol) and N-phenacyl pyridinium bromide in ethanol (5 ml) preheated at 50 °C. The reaction mixture was then stirred at 50 °C for appropriate time. After completion of the reaction, monitored by TLC on SiO2 using EtOAc/hexane (1:1) as the eluent, the solvent was removed under reduced pressure and the product was obtained by recrystallization from diethyl ether.
3.3 Physical and spectral data for compounds 4a–k
3.3.1 2-Benzoyl-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4a)
White powder, mp: 275–277 °C; yield (0.33 g, 85%); IR (KBr) (νmax, cm−1): 3430 (NH), 2939 (H), 1725, 1696 and 1645 (3CO), 1476 (CC), 1257 (O), 1065 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.15 and 1.22 (2s, 6H, 2CH3), 2.14 and 2.31 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.65 and 2.68 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 6.45 (d, 1H, 3JHH = 7.6 Hz, CHOxindole), 6.48 (s, 1H, CHfuran), 6.86–6.87 (m, 2H, 2CHAr), 6.95–7.00 (m, 1H, CHAr), 7.22–7.28 (m, 2H, 2CHAr), 7.40–7.44 (m, 3H, 3CHAr), 8.10 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 27.7 and 29.5 (2CH3), 34.5 (CMe2), 37.7 and 50.8 (2CH2), 58.9 (Cspiro), 91.4 (CHfuran), 109.8 (CHAr), 114.6 (Cq), 122.8 and 124.7 (2CHAr), 126.5 (Cq), 127.5 and 128.5 (4CHAr), 129.1 and 133.7 (2CHAr), 134.5, 139.8 and 177.4 (3Cq), 178.2 (COamide), 192.2 and 192.3 (2COketone); MS, m/z: 387 (M+).
3.3.2 2-Benzoyl-1′-ethyl-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4b)
White powder, mp: 188–190 °C; yield (0.30 g, 73%); IR (KBr) (νmax, cm−1): 2954 (H), 1709, 1644 and 1612 (3CO), 1223 (O), 1064 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.13 (s, 3H, CH3), 1.15 (t, 3H, 3JHH = 7.2 Hz, CH3), 1.20 (s, 3H, CH3), 2.08 and 2.28 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.65 and 2.67 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 3.49–3.77 (m, 2H, CH2), 6.46 (d, 1H, 3JHH = 7.6 Hz, CHOxindole), 6.47 (s, 1H, CHfuran), 6.88–6.90 (m, 2H, 2CHAr), 7.04–7.08 (m, 1H, CHAr), 7.19–7.23 (m, 2H, 2CHAr), 7.31–7.43 (m, 3H, 3CHAr); 13C NMR (100 MHz, CDCl3), δC (ppm): 12.2, 27.7 and 29.5 (3CH3), 34.5 (CMe2), 35.2, 37.7 and 50.8 (3CH2), 58.6 (Cspiro), 91.4 (CHfuran), 108.0 (CHAr), 114.7 (Cq), 122.7 and 124.6 (2CHAr), 126.4 (Cq), 127.4 and 128.4(4CHAr), 129.0 and 133.5 (2CHAr), 134.8, 141.8 and 175.3 (3Cq), 177.6 (COamide), 191.9 and 192.5 (2COketone); MS, m/z: 415 (M+).
3.3.3 Ethyl 2-(2-benzoyl-6,6-dimethyl-2′,4-dioxo-4,5,6,7-tetrahydro-2H-spiro[benzofuran-3,3′-indoline]-1′-yl)acetate (4c)
White powder, mp: 165–167 °C; yield (0.33 g, 70%); IR (KBr) (νmax, cm−1): 3064 (H), 2968 (H), 1751, 1707, 1642 and 1615 (4CO), 1204 (O), 1061 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.16 and 1.20 (2s, 6H, 2CH3), 1.30 (t, 3H, 3JHH = 7.2 Hz, CH3), 2.13 and 2.25 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.64 and 2.70 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 4.22–4.32 (m, 2H, CH2), 4.36 and 4.43 (2d, 2H, 2JHH = 17.2 Hz, AB-system, NCH2), 6.43 (d, 1H, 3JHH = 7.6 Hz, CHOxindole), 6.45 (s, 1H, CHfuran), 6.83–6.91 (m, 2H, 2CHAr), 7.01–7.04 (m, 1H, CHAr), 7.26–7.29 (m, 2H, 2CHAr), 7.41–7.48 (m, 3H, 3CHAr); 13C NMR (100 MHz, CDCl3), δC (ppm): 14.1, 28.0 and 29.1 (3CH3), 34.5 (CMe2), 37.8, 42.0 and 50.9 (3CH2), 58.6 (Cspiro), 61.7 (OCH2), 90.7 (CHfuran), 108.1 (CHAr), 114.4 (Cq), 123.2 and 124.8 (2CHAr), 125.7 (Cq), 127.7 and 128.6 (4CHAr), 129.0 and 133.7 (2CHAr), 134.3 and 141.7 (2Cq), 167.1 (COester), 176.1 (Cq), 178.1 (COamide), 191.7 and 192.0 (2COketone); MS, m/z: 473 (M+).
3.3.4 2-Benzoyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4d)
White powder, mp: 242–244 °C; yield (0.27 g, 76%); IR (KBr) (νmax, cm−1): 3307 (NH), 2929 (H), 1724, 1702 and 1641 (3CO), 1225 (O), 1070 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 2.07–2.27 (m, 2H, CH2), 2.29–2.41 (m, 2H, CH2), 2.71–2.91 (m, 2H, CH2), 6.45 (d, 1H, 3JHH = 8.4 Hz, CHOxindole), 6.46 (s, 1H, CHfuran), 6.86–6.88 (m, 2H, CHAr), 6.96–6.99 (m, 1H, CHAr), 7.23–7.28 (m, 2H, 2CHAr), 7.39–7.44 (m, 3H, 3CHAr), 7.81 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 21.5, 24.1 and 36.5 (3CH2), 59.0 (Cspiro), 91.2 (CHfuran), 109.6 (CHAr), 116.0 (Cq), 122.8 and 124.9 (2CHAr), 126.5 (Cq), 127.5 and 128.5 (4CHAr), 129.1 and 133.7 (2CHAr), 134.6, 139.6 and 177.4 (3Cq), 179.2 (COamide), 192.3 and 192.7 (2COketone); MS, m/z: 359 (M+).
3.3.5 2-Benzoyl-5′-chloro-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4e)
White powder, mp: 260–263 °C; yield (0.34 g, 80%); IR (KBr) (νmax, cm−1): 3291 (NH), 2958 (H), 1727, 1696 and 1644 (3CO), 1223 (O), 1065 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.17 and 1.23 (2s, 6H, 2CH3), 2.17 and 2.29 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.63 and 2.72 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 6.39 (d, 1H, 3JHH = 8.4 Hz, CHOxindole), 6.46 (s, 1H, CHfuran), 6.85 (d, 1H, 4JHH = 2.0 Hz, CHAr), 6.97 (dd, 1H, 3JHH = 8.4 Hz, 4JHH = 2.0 Hz, CHAr), 7.28 (t, 2H, 3JHH = 8.4 Hz, 2CHAr), 7.44–7.47 (m, 3H, 3CHAr), 7.93 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 28.0 and 29.2 (2CH3), 34.5 (CMe2), 37.7 and 50.8 (2CH2), 58.9 (Cspiro), 91.2 (CHfuran), 110.6 (CHAr), 114.3 (Cq), 125.1 and 127.6 (4CHAr), 128.2 and 128.3 (2Cq), 128.7, 129.1 and 133.9 (3CHAr), 134.5, 138.3 and 176.9 (3Cq), 178.6 (COamide), 191.9 and 192.3 (2COketone); MS, m/z: 423 and 421 (M+).
3.3.6 2-Benzoyl-5′-bromo-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4f)
White powder, mp: 274–276 °C; yield (0.36 g, 78%); IR (KBr) (νmax, cm−1): 3300 (NH), 2954 (H), 1728, 1696 and 1648 (3CO), 1220 (O), 1065 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.18 and 1.23 (2s, 6H, 2CH3), 2.18 and 2.30 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.65 and 2.71 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 6.32 (d, 1H, 3JHH = 8.4 Hz, CHOxindole), 6.46 (s, 1H, CHfuran), 6.98 (d, 1H, 4JHH = 2.0 Hz, CHAr), 7.10 (dd, 1H, 3JHH = 8.4 Hz, 4JHH = 2.0 Hz, CHAr), 7.28 (t, 2H, 3JHH = 8.4 Hz, 2CHAr), 7.43–7.47 (m, 3H, 3CHAr), 8.10 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 28.1 and 29.2 (2CH3), 34.6 (CMe2), 37.7 and 50.8 (2CH2), 58.8 (Cspiro), 91.2 (CHfuran), 111.2 (CHAr), 114.2 and 115.3 (2Cq), 127.6 and 127.8 (4CHAr), 128.6 (Cq), 128.7, 132.0 and 133.9 (3CHAr), 134.5, 138.9 and 176.8 (3Cq), 178.8 (COamide), 191.9 and 192.3 (2COketone); MS, m/z: 467 and 465 (M+).
3.3.7 Ethyl 2-(2-benzoyl-5′-chloro-6,6-dimethyl-2′,4-dioxo-4,5,6,7-tetrahydro-2H-spiro[benzofuran-3,3′-indoline]-1′-yl)acetate (4g)
White powder, mp: 158–160 °C; yield (0.38 g, 74%); IR (KBr) (νmax, cm−1): 3034 (H), 2925 (H), 1725 and 1645 (2CO), 1215 (O), 1069 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.18 and 1.21 (2s, 6H, 2CH3), 1.31 (t, 3H, 3JHH = 7.2 Hz, CH3), 2.20 and 2.24 (2d, 2H, 2JHH = 13.2 Hz, AB-system, CH2), 2.63 and 2.72 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 4.22–4.27 (m, 2H, CH2), 4.31 and 4.46 (2d, 2H, 2JHH = 17.6 Hz, AB-system, NCH2), 6.37 (d, 1H, 3JHH = 8.4 Hz, CHOxindole), 6.44 (s, 1H, CHfuran), 6.86 (d, 1H, 4JHH = 2.0 Hz, CHAr), 7.01 (dd, 1H, 3JHH = 8.4 Hz, 4JHH = 2.4 Hz, CHAr), 7.28 (t, 2H, 3JHH = 8.4 Hz, 2CHAr), 7.48–7.52 (m, 3H, 3CHAr); 13C NMR (100 MHz, CDCl3), δC (ppm): 14.1, 28.3 and 28.9 (3CH3), 34.6 (CMe2), 37.8, 42.1 and 50.8 (3CH2), 58.1 (Cspiro), 61.9 (OCH2), 90.4 (CHfuran), 109.1 (CHAr), 114.0 (Cq), 125.2 and 127.8 (4CHAr), 128.3 (Cq), 128.8 and 129.0 (2CHAr), 133.5 (Cq), 133.9 (CHAr), 134.2 and 140.7 (2Cq), 166.9 (COester), 175.7 (Cq), 178.5 (COamide), 191.6 and 191.8 (2COketone); MS, m/z: 509 and 507 (M+).
3.3.8 2-Benzoyl-1′-benzyl-5′-chloro-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4h)
White powder, mp: 203–205 °C; yield (0.42 g, 82%); IR (KBr) (νmax, cm−1): 3090 (H), 2959 (H), 1713 and 1644 (2CO), 1229 (O), 1074 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.18 and 1.24 (2s, 6H, 2CH3), 2.18 and 2.33 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.67 and 2.71 (2d, 2H, 2JHH = 18.0 Hz, AB-system, CH2), 4.28 and 5.18 (2d, 2H, 2JHH = 16.0 Hz, AB-system, NCH2), 6.12 (d, 1H, 3JHH = 8.8 Hz, CHOxindole), 6.51 (s, 1H, CHfuran), 6.88–6.90 (m, 2H, 2CHAr), 7.20–7.24 (m, 2H, 2CHAr), 7.29–7.39 (m, 7H, 7CHAr), 7.44–7.48 (m, 1H, CHAr); 13C NMR (100 MHz, CDCl3), δC (ppm): 28.0 and 29.3 (2CH3), 34.5 (CMe2), 37.8, 44.5 and 50.8 (3CH2), 58.6 (Cspiro), 91.5 (CHfuran), 110.1 (CHAr), 114.3 (Cq), 124.9 and 126.9 (4CHAr), 127.5 and 127.7 (2CHAr), 127.8 and 128.4 (2Cq), 128.5 and 128.9 (4CHAr), 129.0 and 133.7 (2CHAr), 134.5, 134.7, 140.5 and 175.7 (4Cq), 178.3 (COamide), 192.0 and 192.1 (2COketone); MS, m/z: 513 and 511 (M+).
3.3.9 2-(4-Chlorobenzoyl)-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4i)
White powder, mp: 248–249 °C; yield (0.34 g, 80%); IR (KBr) (νmax, cm−1): 3291 (NH), 2960 (H), 1735, 1690 and 1639 (3CO), 1224 (O), 1062 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.15 and 1.22 (2s, 6H, 2CH3), 2.15 and 2.33 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.66 and 2.68 (2d, 2H, 2JHH = 14.4 Hz, AB-system, CH2), 6.42 (s, 1H, CHfuran), 6.49 (d, 1H, 3JHH = 8.0 Hz, CHOxindole), 6.83–6.86 (m, 2H, 2CHAr), 6.97–7.01 (m, 1H, CHAr), 7.22 (d, 2H, 3JHH = 8.8 Hz, 2CHAr), 7.36 (d, 2H, 3JHH = 8.8 Hz, 2CHAr), 8.70 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 27.7 and 29.4 (2CH3), 34.5 (CMe2), 37.7 and 50.8 (2CH2), 59.0 (Cspiro), 91.3 (CHfuran), 110.2 (CHAr), 114.6 (Cq), 122.8 and 124.6 (4CHAr), 126.4 (Cq), 128.8, 128.9 and 129.2 (3CHAr), 132.8, 140.0, 140.2 and 177.5 (4Cq), 178.3 (COamide), 191.1 and 192.4 (2COketone); MS, m/z: 423 and 421 (M+).
3.3.10 6-Benzoyl-1,3-dimethyl-1H-spiro[furo [2,3-d]pyrimidine-5,3′-indoline]-2,2′,4(3H,6H)-trione (4j)
White powder, mp: 245–247 °C; yield (0.28 g, 70%); IR (KBr) (νmax, cm−1): 3430 (NH), 2925 (H), 1709 and 1646 (2CO), 1190 (O), 1111 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 3.23 and 3.58 (2s, 6H, 2NCH3), 6.48 (d, 1H, 3JHH = 7.6 Hz, CHOxindole), 6.60 (s, 1H, CHfuran), 6.90 (td, 1H, 3JHH = 7.6 Hz, 4JHH = 0.8 Hz, CHAr), 6.94 (td, 1H, 3JHH = 8.0 Hz, 4JHH = 0.8 Hz, CHAr), 7.01 (td, 1H, 3JHH = 7.6 Hz, 4JHH = 1.6 Hz, CHAr), 7.25–7.29 (m, 2H, 2CHAr), 7.42–7.47 (m, 3H, 3CHAr), 7.97 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 27.9 and 30.1 (2CH3), 58.8 (Cspiro), 91.1 (CHfuran), 109.9 and 123.2 (2CHAr), 125.4 and 125.6 (2Cq), 127.5 and 128.7 (4CHAr), 129.6 and 134.1 (2CHAr), 139.7 (Cq), 148.0 (CHAr), 151.4 and 158.1 (2Cq), 163.2, 176.9 and 179.8 (3COamide), 190.6 (COketone); MS, m/z: 403 (M+).
3.3.11 2-(4-Bromobenzoyl)-6,6-dimethyl-6,7-dihydro-2H-spiro[benzofuran-3,3′-indoline]-2′,4(5H)-dione (4k)
White powder, mp: 235–237 °C; yield (0.35 g, 76%); IR (KBr) (νmax, cm−1): 3290 (NH), 2958 (H), 1723 and 1650 (2CO), 1475 (CC), 1224 (O), 1066 (O); 1H NMR (400 MHz, CDCl3), δH (ppm): 1.15 and 1.22 (2s, 6H, 2CH3), 2.14 and 2.32 (2d, 2H, 2JHH = 16.4 Hz, AB-system, CH2), 2.64 and 2.69 (2d, 2H, 2JHH = 18.4 Hz, AB-system, CH2), 6.41 (s, 1H, CHfuran), 6.53 (d, 1H, 3JHH = 8.0 Hz, CHOxindole), 6.85–6.91 (m, 2H, 2CHAr), 7.01–7.05 (m, 1H, CHAr), 7.28 (d, 2H, 3JHH = 8.8 Hz, 2CHAr), 7.40 (d, 2H, 3JHH = 8.8 Hz, 2CHAr), 7.85 (br s, 1H, NH); 13C NMR (100 MHz, CDCl3), δC (ppm): 27.7 and 29.4 (2CH3), 34.5 (CMe2), 37.7 and 50.8 (2CH2), 58.9 (Cspiro), 91.2 (CHfuran), 109.8 (CHAr), 113.0 (Cq), 123.0 and 124.8 (4CHAr), 126.4 (Cq), 128.9, 129.0 and 129.3 (3CHAr), 131.9, 133.3, 139.6 and 177.2 (4Cq), 178.0 (COamide), 191.3 and 192.1 (2COketone); MS, m/z: 467 and 465 (M+).
4 Conclusion
In conclusion, we have demonstrated an efficient, clean and step-wise economic procedure for the synthesis of novel spirooxindole–furan derivatives through a new variant of the interrupted Feist–Bénary reaction using N-phenacyl pyridinium salts as electrophiles. The corresponding products have been obtained in good yields under very convenient conditions.
Acknowledgements
This research was supported by the Research Council of the University of Mazandaran, Iran.


