1 Introduction
Renal lithiasis is a consequence of biocrystallization [1–5] of different chemical compounds in the urinary tract corresponding to various pathologies encompassing genetic disorders [4], infection [5], acquired metabolic diseases and most often metabolic risk factors related to dietary habits [6]. Among these relations with pathologies, the prevalence of calcium oxalate (CaOx) stones is associated with significant changes in the dietary habits in industrialized countries [7,8]. In contrast, the presence of silica in kidney stones is quite rare and often related to the administration of drugs [9–13]. None of the large series of stone analyses reported in the literature has identified silica among the chemical phases present in kidney stones.
Recently, a set of 100 consecutive kidney stones coming from the CHU of Ougadougou (Burkina Faso) has been analyzed following the classical analysis procedure based on morphologic examination combined with Fourier-transform infrared (FTIR) analysis [14]. In a significant number of these kidney stones (48%) [15], the presence of amorphous silica has been observed. Silica was present in the nucleus of 42 stones (42%) and was especially frequent in the core of stones from women (72.7% of cases). Moreover, while the content of silica is usually low, silica was the major component of 18% of the stones. Such unusual prevalence of silica in kidney stones has motivated an investigation through physical techniques in order to describe precisely the status of Si atoms.
We and other authors have already demonstrated in different studies the advantages of using physical methods to precisely characterize kidney stones and more generally pathological calcifications [16–18]. Among them, we can quote scanning and transmission electron microscopy (SEM, TEM) [19,20], in situ atomic force microscopy [21], X-ray and neutron scattering [22–24], atomic absorption spectrometry [25], infra-red and Raman vibrational spectroscopies [26–30], micro-computed tomography [31,32], fluorescence induced by X-ray or neutrons [33–35], Nuclear Magnetic Resonance [36–39] as well as techniques specific to synchrotron radiation [40–45].
In this paper, we gathered structural information on silica present in a set of selected kidney stones in order to assess its biological origin. For this purpose, we initially determined stone composition through FTIR experiments, confirmed the amorphous state of silica through X-ray scattering, underlined the presence of different elements through X-ray fluorescence and collected images of different kidney stones at the mesoscopic scale by SEM. Finally, valuable structural information regarding the silica phase was gathered through solid state NMR. This spectroscopic technique, which is a local probe in nature, can bring invaluable structural information regarding the environment of Si [46].
2 Materials and methods
2.1 Patients
We investigated 100 kidney stones collected after open surgery (n = 99) or spontaneous expulsion (n = 1) from the University Hospital of Ouagadougou (Burkina Faso). The patients were 85 adults (64 men aged 47.0 ± 18.5y and 22 women aged 39.1 ± 15.3y) and 14 children (10 boys aged 9.8 ± 4.7y and four girls aged 11.4 ± 5.5y). All participants, and legal tutors for minor children, gave their verbal consent for use of the material. Ethical approval for the study was obtained from the ethics committee of the Tenon Hospital.
2.2 FTIR (FTIR) spectroscopy
All the samples have been characterized by Fourier transform infrared (FTIR) spectroscopy. To do so, an FTIR spectrometer, Vector 22 (Bruker Optics, Champs-sur-Marne, France), was used according to the analytical procedure previously described [47,48]. Data were collected in the absorption mode between 4000 and 400 cm−1 with a resolution of 4 cm−1.
2.3 Scanning electron microscopy (SEM)
A Zeiss SUPRA55-VP SEM was used for the observation of the microstructure [49]. This field-effect “gun” microscope (FE-SEM) operates at 0.5–30 kV. High-resolution observations were obtained by two secondary electron detectors: an in-lens SE detector and an Everhart-Thornley SE detector. To maintain the integrity of the samples, for both SEMs, measurements were taken without the usual deposits of carbon at the surface of the sample.
2.4 X-ray diffraction (XRD)
Phase identification and crystallinity of the dental mineral part were evaluated by X-ray diffraction. Experiments were carried out with a molybdenum rotating anode X-ray generator (RIGAKU RUH2R) coupled with multilayer W/Si optics delivering a focalized and monochromated (λ = 0.711 Å) X-ray beam of 800 μm × 1 mm size onto the sample. X-ray images were recorded with a MAR345 (@MAR Research) detector placed at of a distance of 200 mm from the sample. The acquisition time of each measurement was 30 mn. Diffraction diagrams were obtained by processing radial intensity integration of each image. Then positions of the diffraction peaks were compared with the reference files from the JCPDS database.
2.5 X-ray fluorescence (XRF)
X-ray fluorescence allows the precise determination of the elemental composition of the sample. Experiments were carried out with a molybdenum rotating anode X-ray generator (RIGAKU RU200) coupled with multilayer W/Si optics delivering a focalized and monochromated (λ = 0.711 Å) X-ray beam of 150 μm × 150 μm size. Fluorescence spectra were measured with an energy dispersive detector (SDD detector @Ketek), with a time acquisition of 1500s each. XRF analysis was performed with PyMca software [50].
2.6 NMR spectroscopy
29Si and 27Al SPE (Single Pulse Experiment) MAS NMR experiments were performed on a Bruker 300 AVANCE III spectrometers using 7 mm Bruker MAS probes (samples were packed in zirconia rotors). All experimental parameters including pulse flip angles, recycling delays, number of scans, {1H} decoupling schemes are presented in the caption of the corresponding figures. Chemical shifts were referenced towards TMS for 29Si and Al(NO3)3, 1 mol L−1 in HNO3, 1 mol L−1 for 27Al. All spectra were fitted by using the DMFit software available free on the web [51,52].
3 Results
The starting point of this contribution is given by a set of photographs collected on kidney stones containing various proportions of silica. In Fig. 1, both of these kidney stones display a concentric structure, either as well organized layers with radiating crystals (Fig. 1a), or as more diffuse layers (Fig. 1b and c). In Fig. 1a, the white core is mainly made of opaline silica (OPA) while the dark-brown inner and surrounding layers are pure calcium oxalate monohydrate. In Fig. 1b, white layers are mainly composed of OPA with various proportions of whewellite (calcium oxalate monohydrate, COM) and proteins while brown layers are mainly made of COM with minor proportions of OPA. By contrast with the stone shown in Fig. 1a, where OPA was only found in the nucleus, the stone of Fig. 1b contained OPA in the core and inner or peripheral layers as well. Moreover, the core contained also small proportions of ammonium hydrogen urate, which suggests that the precipitation of OPA occurred in alkaline (or poorly acidic) urine. Finally, in Fig. 1c, a stone mostly composed of OPA (81%) mixed with proteins (13%) and COM (6%) revealed diffuse irregular layers (see Fig. 2).

Photographs of various stones containing OPA. (a) Cross-section of the stone T67317, made of a mixture of COM (90%), OPA (7%) and proteins (3%). The white core is mainly composed of OPA; (b) cross-section of the stone T67309. The OPA content was higher than in the sample T67317 and was distributed through the core and the various layers, mixed with COM. The global stone composition was COM (72%) + OPA (15%) + proteins (10%) + ammonium hydrogen urate (3%); (c) and (d) cross-section of the stone T67974 mainly made of OPA (81%), mixed with proteins (13%) and COM (6%). Peripheral layers are shown in figure (c) and the stone core is shown in figure (d). The stone core contained essentially OPA with small proportions of proteins and traces of COM. (white bar: 5 mm). Masquer
Photographs of various stones containing OPA. (a) Cross-section of the stone T67317, made of a mixture of COM (90%), OPA (7%) and proteins (3%). The white core is mainly composed of OPA; (b) cross-section of the stone T67309. The OPA ... Lire la suite

(a,b) SEM photographs of a kidney stone containing 58% of OPA and 25% of COM: parts of the sample which contain mainly COM (a) or mainly silica (b); (c) SEM photograph of a kidney stone containing 82% of OPA; (d) SEM photographs of a kidney stone containing 20% of OPA. (white bar: 10 μm).
These observations at the macroscopic and mesoscopic (under micron) scales have been completed by FTIR experiments [15]. Among the different locations of the stones within the urinary tract, there was no difference between stone containing, or not, OPA. For example, stones that contained OPA were removed from the upper urinary tract in 69% of cases versus 63.5% of cases in the absence of OPA within the stone. Of interest, we found differences regarding the gender of the patient: OPA-containing stones were significantly more frequent in females than in males (69.2% vs 40.5%, p = 0.02). The content of OPA ranged from only 2% up to 82% of the stone mass. For 17% of the stones (12.2% for men, 30.8% for women) silica was the major component. Regarding the nucleus of the stones, silica was the major component for 39% of the stones (32.4% for men, 57.7% for women) which indicate that OPA was often present at the nucleation process (see Fig. 3).
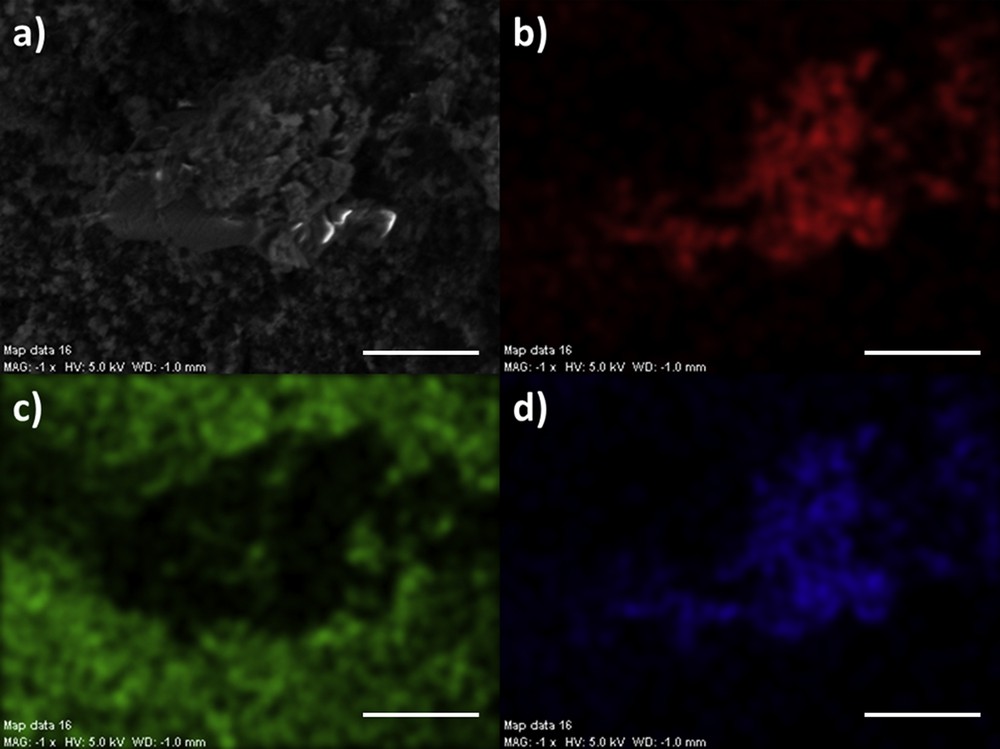
Spatial repartition of different elements measured through EDX of a kidney stone containing 65% of OPA and 25% of COM (a) SEM photograph, (b) Ca distribution, (c) Si distribution, (d) C distribution. (white bar: 10 μm).
The chemical composition as measured by FTIR spectroscopy has been completed by X-ray fluorescence in order to obtain the elementary composition. In Fig. 5, typical X-ray fluorescence spectra have been plotted. The different contributions of Si (Kα = 1.730 KeV), S (Kα = 2.31 KeV), Ar (Kα = 2.96 KeV), Ca (Kα = 3691 eV, Kβ = 4012 eV) are visible. The presence of Ar is related to the experimental conditions. The presence of a small amount of Al (Kα = 1.49 KeV) is also pointed through a feature in the fluorescence signal of Si. Interestingly, the fact that X-ray fluorescence shows the presence of Al and Si seems to indicate that aluminosilicate phases are present in these kidney stones.

Typical X-ray fluorescence spectrum collected for selected samples. We can see clearly the contributions of Si (Kα = 1.73 keV), S (Kα = 2.31 keV), Ar (Kα = 2.96 keV), Ca (Kα = 3.69 keV, Kβ = 4.01 keV).
Fig. 6 shows powder XRD patterns measured for different kidney stones. On the scattering patterns, we can see clearly a set of well-defined X-ray diffraction peaks (at 2θ° = 14.75°, 24.18°, 29.99°) corresponding to the whewellite in line with its crystallographic structure (monoclinic P21/c; a = 6.316 Å, b = 14.541 Å, c = 10.116 Å) [53]. Moreover, an additional broad component is associated with silica. Such large peak indicates an amorphous structure for the silica. The intensity of this large diffraction peak is related to the content of silica as measured by FTIR spectroscopy (see Fig. 4).
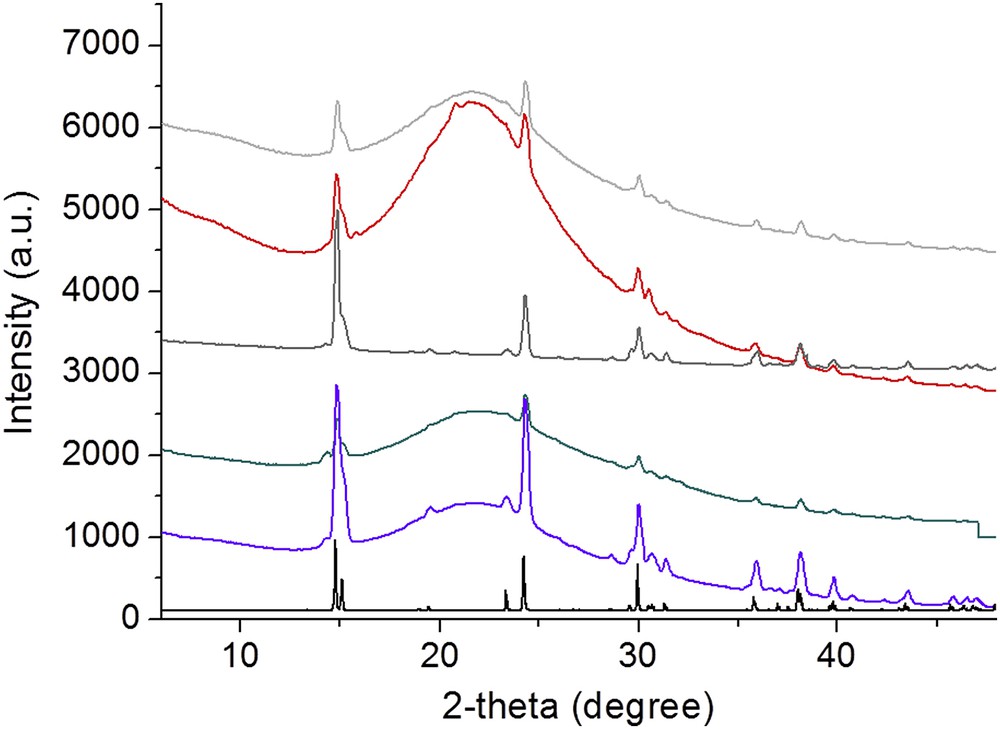
X-ray scattering diagram of selected samples. A broad X-ray scattering peak corresponding to silica superimposed to whewellite peaks (pure whewellite: black solid line).

Typical OPA FTIR spectrum collected for a kidney stone containing a high content of silica. The black arrow indicates a very small proportion of COM.
These stones were further investigated by 29Si and 27Al solid state NMR (under MAS, Magic Angle Spinning). A typical 29Si MAS spectrum (decoupled from protons) is presented in Fig. 7. Such a spectrum can be fully interpreted by taking into account Q2, Q3 and Q4 units (Q2: Si(OH)2(OSi)2, Q3: Si(OH)(OSi)3, Q4: Si(OSi)4 [54]. It is comparable to those obtained for natural opals [55]. Q2 species correspond to a very minor contribution.

29Si SPE (single pulse experiment) MAS NMR spectrum of the kidney stone. B0 = 7 T, 7 mm MAS probe, νrot. = 5 kHz, t90°(29Si) = 5.0 μs, pulse angle = 30°, {1H} SPINAL-64 high power decoupling, recycling delay: 600 s, number of scans: 248 (≈41 h). In black: experimental spectrum. In red: deconvolution of the exp. spectrum in Q2, Q3 and Q4 units using DMFit [51,52] (see text).
The 27Al MAS NMR spectra are presented in Fig. 8. Considering the long acquisition time (several hours) for such a sensitive NMR nucleus, it is clearly demonstrated here that traces of aluminum are actually detected. One major signal (with maximum intensity at δ ≈ 55 ppm) is observed. It corresponds essentially to 4-fold coordinated species but 5-fold coordinated nuclei cannot be excluded. A test spectrum corresponding to an empty rotor is presented in the inset in Fig. 8. It demonstrates unambiguously that the signal centered ≈55 ppm is characteristic of the sample. The much less intense signal at ≈0 ppm can be attributed partly to the rotor background [56]. It has to be mentioned here that a similar 27Al MAS spectrum was obtained for tabasheer, an opal of plant origin (about 1–2wt % in aluminum) [57]. In this particular case, it is assumed that most of the Al atoms are part of the silicate network.

27Al SPE (single pulse experiment) MAS NMR spectrum of a representative kidney stone (same sample studied by 29Si MAS). B0 = 7 T, 7-mm MAS probe, νrot. = 5 kHz, selective excitation of the central transition checked on YAG garnet, recycling delay: 1 s, number of scans: 54,000 (≈15 h). The characteristic zones for 4-and 6-fold 27Al resonances are indicated as a guideline. Inset: 27Al SPE MAS NMR spectrum of an empty rotor and of the kidney stone sample. Acquisition time: 2 h.
4 Discussion
The influence of silica on the kidney function has been investigated by different authors [56,58]. For example, S. Vupputuri et al. [59] found a positive relationship between occupational silica exposure and CKD.
The presence of silica in kidney stones has been reported in different animals [60,61] but is quite rare in human. In that case, as previously mentioned, the presence of silica is related to drug administration [9,12,13]. Information regarding a possible drug origin for the kidney stones selected in this study was not available for all patients. However, several patients who produced stones containing OPA denied any kind of medication. Moreover, as underlined by Raghuvanshi et al. [62], even if occupational exposure to silica dust has been increasing the possible risk of varieties of pathologies, no case of kidney stones made of silica has been reported.
29Si SPE MAS NMR is a suitable tool of investigation for the characterization of amorphous silicates and aluminosilicates. The amorphous nature of the silica present in the stones is clearly demonstrated by the broad Q2, Q3 and Q4 resonances observed in Fig. 7. The broadening of the lines reflects mainly the distributions of isotropic 29Si chemical shifts, due to variations in bond lengths and bond angles. We stress here that 29Si SPE MAS NMR can be considered as quantitative as soon as full relaxation of the spin system is explicitly taken into account. The T1(29Si) longitudinal relaxation times for the Q2 + Q3 and Q4 units were estimated by saturation-recovery experiments (not shown). The recovery curves were fitted with stretched exponentials following recent work by Stebbins et al. [63,64]. From the fitted curves, the ratio Q4/(Q2 + Q3) is estimated to 86/14. The deconvolution of the experimental 29Si MAS spectrum (Fig. 7) leads to 79/21. In other words, the relaxation delay used here (i.e. 600 s) is still not sufficient to ensure full relaxation of the Q4 units. The detailed study of the 29Si relaxation will be presented in a future work.
The presence of traces of aluminium in urinary stones is demonstrated as well by 27Al SPE MAS NMR. The amount of aluminium in the samples is very limited; consequently, eventual Al impurities in the NMR rotors have to be considered (see the inset in Fig. 8). The Al atoms are mostly 4-fold coordinated (5-fold coordination cannot be strictly ruled out as experiments were performed in a unique magnetic field). The associated 27Al broad resonance is compatible with aluminium species found in aluminosilicates [54].
In the case of 27Al, the broadening of the line can be assigned to both distributions of isotropic 27Al chemical shifts and quadrupolar constants (as I = 5/2 for 27Al). Both contributions could be unambiguously separated by variable field NMR experiments (as second-order quadrupolar effects are inversely proportional to B0) and subsequent fitting of the obtained line shapes. Theoretical models for the distributions are available in DMFit [51,52].
The quantification of the aluminium content by using an internal reference (such as alun, KAl(SO4)2·12H2O) is currently under investigation. It has to be mentioned here that even lower amounts of aluminum could be detected by combining advanced solid state NMR techniques, such as WURST excitation [65] and QCPMG [66] acquisition of the spectra. Indeed, it has been demonstrated that such a combination of specific NMR techniques could increase dramatically the signal/noise ratio of NMR experiments related to quadrupolar nuclei by several orders of magnitude [67].
5 Conclusion
Unusual amounts of silica have been identified through FTIR spectroscopy in a set of 100 urinary stones coming from the Hospital of Ouagadougou (Burkina Faso). X-ray scattering as well as NMR measurements indicate the amorphous character of silica. Moreover, solid state NMR is able to characterize the presence of elemental traces such as aluminium. It is suggested that the origin of aluminium could be a mixture with silicon derivatives in the form of aluminosilicates.
Acknowledgements
Arnaud Dessombz was supported by grants “Once Upon a Tooth” IDEX (grant number: ANR-11-IDEX-0005-02) and ANR Nanoshap (grant number: ANR-09-BLAN-0120-02).



Vous devez vous connecter pour continuer.
S'authentifier