1 Introduction
As assessed by several authors [1–3], renal dysfunction and injury secondary to medications are common, and can present as a subtle injury and/or overt renal failure. Renal injury associated with drugs may involve several parts of the kidney: glomeruli, tubules, interstitium and blood vessels. Drug-induced acute renal failure (ARF) was reported to account for 20% of all ARF cases [4]. Drug-induced nephrolithiasis accounts for 1–2% of all renal calculi [5]. Regarding children, C. Glanzmann et al. [6] have noticed that some drugs are associated with acute renal dysfunction in pediatric intensive care, especially some critical medication groups, such as betalactam antibiotics, glucocorticoids, opioids, and non-steroid anti-inflammatory drugs.
Among the different manifestations of drug-induced nephrotoxicity, we can quote acid–base abnormalities, electrolyte imbalances, urine sediment abnormalities, proteinuria, pyuria, hematuria, and, most commonly, a decline in the glomerular filtration rate [2]. Different mechanisms of drug-induced acute kidney injury (AKI) can be described such as prerenal AKI, glomerulonephritis, tubulopathies, interstitial nephritis, vascular nephropathies, crystalluria, etc.
Numerous drugs including foscarnet, atazanavir, tenofovir [7], vancomycin [8], gentamicin [9], taxol [10], cisplatin [11], and nucleotides [12] were reported as nephrotoxic drugs. Among drugs that are able to induce crystals or stones, we must distinguish between (1) poorly soluble drugs with high urine excretion that favors crystallization of the drug or its metabolites in urine and (2) drugs that provoke urinary crystals and calculi as a consequence of their metabolic effects [4,13–15]. In the two cases, the chemical complexity of this particular etiology i.e. the fact that drugs as well as their metabolites may be involved calls for the use of physicochemical techniques such as Fourier transform infrared microspectroscopy (μFTIR) to identify accurately the substances accumulated in the tissue [16,17].
The aim of this contribution is to assess the chemical nature of drugs and their metabolites as well as their localization in the kidney (presence in the glomerulus or in the tubule). To attain this goal, a set of kidney biopsies has been selected in order to consider nephrotoxicity by foscarnet and atazanavir. The chemical characterization of abnormal deposits will be performed through vibrational spectroscopies such μFTIR. The morphology of the deposits at the micrometer scale will be described through Field Emission Scanning Electron Microscopy (FE-SEM).
2 Materials and methods
2.1 Samples
685 kidney biopsies were investigated. The biological samples came from various hospitals from France, Canada, Belgium, Switzerland, Italy, and North Africa. All biopsies were examined in Tenon Hospital (Paris–France). Five microns slices of the biopsies were deposited either on CaF2 plates or on low-e microscope slides (MirrIR, Kevley Technologies, Tienta Sciences, Indianapolis) in order to perform μFTIR experiments. In the case of Raman observation, the samples were deposited on classical glass supports used at the hospital. For tissue embedded in paraffin, the paraffin was chemically removed in order to improve the crystal detection under the microscope. Each sample was only named by a study number, without indication of the name of the patient or potential identification data.
2.2 Materials
As already described in a previous publication [18], a part of the experiments (n=24 biopsies) were carried out at SOLEIL-Synchrotron (Saint-Aubin, Gif-sur-Yvette, France) on the SMIS beamline. The synchrotron radiation-μFTIR mappings were collected in reflection mode using an Infrared microscope (Thermo/Nicolet Nic-Plan) coupled to a μFTIR spectrometer (Thermo Nicolet MAGNA-IR 550). The IR microscope is equipped with a motorized sample stage (precision 1 mm) and a liquid nitrogen cooled mercury cadmium telluride (MCT – 250 mm) detector. Most of the analysis and maps presented here were achieved with a projected area on the sample of 6 × 6 μm2 and a step size of 6 μm, and each spectrum was acquired after 64 accumulations at 8 cm−1 spectral resolution. Data acquisition and processing were performed using OMNIC software (Version 7.4, Thermo-Scientific). The compounds were identified by comparing them to reference spectra [19].
IR microspectroscopy was performed on an IN10MX microscope (Thermo Scientific) for recording large maps. All spectra were collected in ultrafast mode using a 50 μm×50 μm aperture. The spectra were collected in the 4000–700 cm−1 mid-IR range at a resolution of 8 cm−1 with one spectrum per pixel. Data analysis of IR spectra and chemical images was performed using OMNIC software (Thermo Scientific). Other biopsies were analyzed, in our hospital department in the Service des Explorations Fonctionnelles Multidisciplinaires (Tenon hospital – Paris), with the Spotlight 400 FTIR imaging System in the mid-infrared spectral range to obtain infrared maps of tissue slides at high spatial resolution, down to 10 microns. When required, an optional ATR imaging accessory was used, improving the spatial resolution by a factor four, down to 3 microns at 1000 cm−1.
3 Results and discussion
A very limited proportion of patients receiving drugs develops crystalluria and, sometimes, acute renal failure due to tubular obstruction by drug crystals. This suggests that the formation of drug-induced crystal involves an interplay of risk factors specific either of the drug itself (high dose, high urinary excretion, low solubility product, long-term treatment without supervision, size and crystal forms) (Fig. 1) and of the patient (inadequate diuresis, urinary stasis, antacids, uricosurics, anti inflammatory drugs, urinary pH, and urinary tract infection) [20].
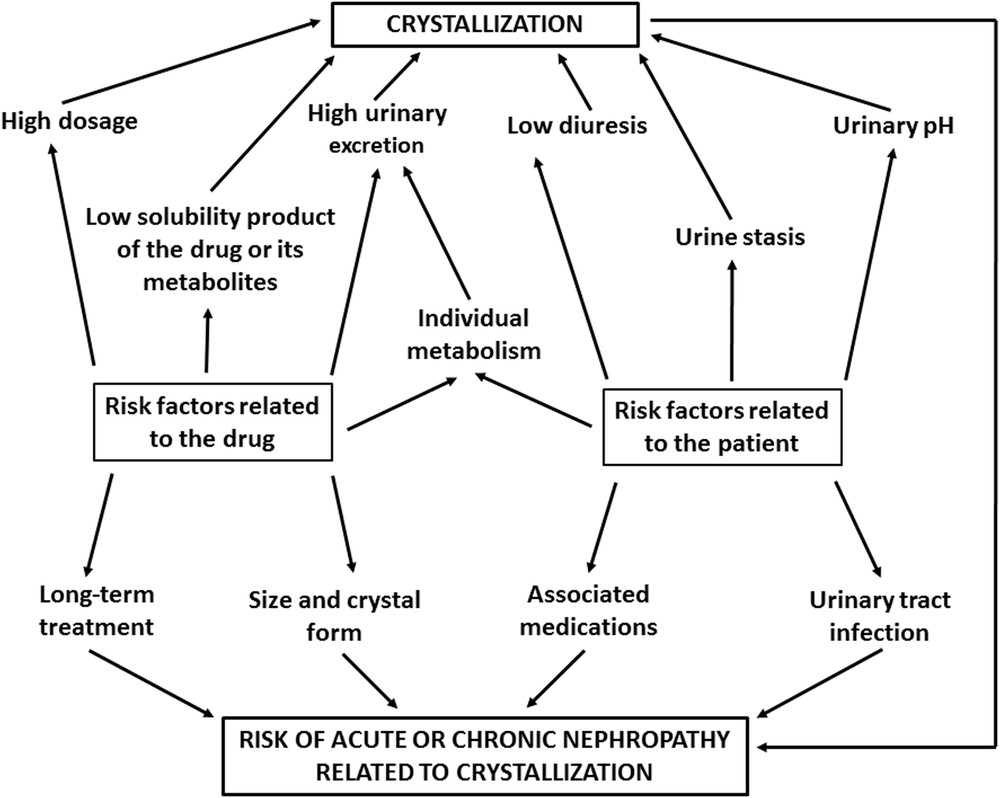
Risk factors of crystallization related to the drug and/or the patient.
Drugs, which may crystallize in urine are not so numerous [21] and belong to various pharmacological groups: antibacterial drugs, protease inhibitors, analgesics, antihypertensive agents, antacids, and some other drugs. We found a number of these drugs in renal deposits. Note that hypercalciuria and nephrolithiasis may result from calcium supplements, especially when associated with vitamin D [22].
At the hospital, the classical analysis procedure starts by observation through optical microscope underlining the presence of birefringent or non-refringent deposits. This criterion is not sufficient to ensure the precise identification of the compound. Several drugs are present as birefringent crystals, namely triamterene, N-acetylsulfadiazine, ciprofloxacine, indinavir, and atazanavir while others are non-refringent, namely foscarnet or vancomycine. To identify precisely the chemical nature of the drugs, μFTIR experiments have to be performed. In Table 1, all the drugs we identified in kidney tissue over the past thirty years are summarized.
Drug-induced crystals identified in kidney biopsies.
| Drug | Nb of cases | Molecule(s) identified in kidney biopsies |
| Triamterene | 1 | Hydroxy-4′-triamterene sulfate |
| Triamterene | 2 | Triamterene + metabolites |
| Piridoxilate | 1 | Calcium oxalate monohydrate |
| Ciprofloxacine | 1 | Ciprofloxacine, magnesium salt |
| Sulfadiazine | 1 | N-acetylsulfadiazine |
| Vancomycine | 1 | Vancomycine |
| Naftidrofuryl oxalate | 2 | Calcium oxalate monohydrate |
| Indinavir sulfate | 2 | Indinavir monohydrate |
| Atazanavir sulfate | 2 | Atazanavir |
| Foscarnet | 7 | Foscarnet, calcium and/or sodium salts, carbapatite |
In this contribution, we focus on foscarnet (trisodium salt of phosphonoformic acid) and atazanavir.
3.1 Foscarnet
Foscarnet is a pyrophosphate analog of chemical formula CH3O5P (Fig. 2) that exhibits a broad activity against both DNA and RNA viruses and is considered standard treatment against cytomegalovirus.
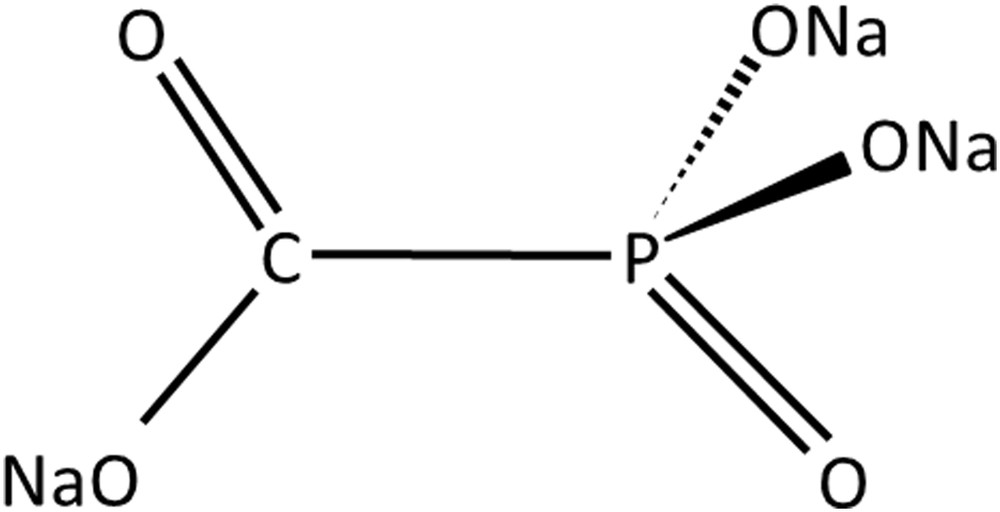
Chemical formula of foscarnet.
More precisely, this drug achieves its antiviral effect via the inhibition of viral polymerases by preventing pyrophosphate exchange [23]. The half life of the drug is between 3 and 7 h. Foscarnet is theoretically non-metabolized and excreted unchanged by the kidney. Recently, D. Boutolleau et al. [24] have described the emergence of cytomegalovirus resistance to foscarnet in a 54-year-old man receiving foscarnet salvage therapy because of multidrug-resistant HIV-1 infection. One of the major drawbacks of this drug is related to crystal deposition. Even if such pathological calcification has been observed in different organs, special attention has to be paid to its presence in kidney [25].
Different investigations have been dedicated to the nephrotoxicity of foscarnet [26,27]. G. Deray et al. have shown that foscarnet is a highly nephrotoxic drug which induces acute tubular necrosis [28]. More recently, A. Wallin and A. Ryrfeldt have suggested that the toxic effects of foscarnet may be due to its ability to form poorly soluble complexes with divalent cations [29]. A metabolic consequence is hypocalcemia and its clinical consequences such as tetany [30]. Another adverse effect is the crystallization of foscarnet salts. Indeed, while trisodium foscarnet is water soluble, the molecule is able to chelate metallic ions such as Ca2+, Mg2+, Fe2+ and Zn2+ and then to form precipitates [31]. The first case of foscarnet-induced nephropathy was described in 1990 [32].
The kidney was the most affected organ, but neurologic and systemic injuries also were observed. Histologic analysis of autopsied organs revealed precipitation of crystals accompanied by granulomatous inflammatory reaction in numerous tissues, including the kidney, pulmonary parenchyma and esophageal and cardiac mucosa [25].
In a previous publication [33], we have shown that even in the very same biopsy different chemical phases can be identified. Thus, we start our study by μFTIR experiments in order to localize and identify the foscarnet deposits. Foscarnet deposits are identified through the presence of IR absorption bands at 970 cm−1 (Fig. 2). In a previous work, it was reported that foscarnet deposits may be present as different salts containing calcium and/or sodium ions [31]. Drugs have their own metabolism with a urinary excretion of the unchanged drug. Due to its particular chemical formula, foscarnet could be metabolized to form inorganic phosphate within the cells as suspected by the accumulation of apatite in the cells of the proximal tubule [18] in a patient treated with that drug (Fig. 3).
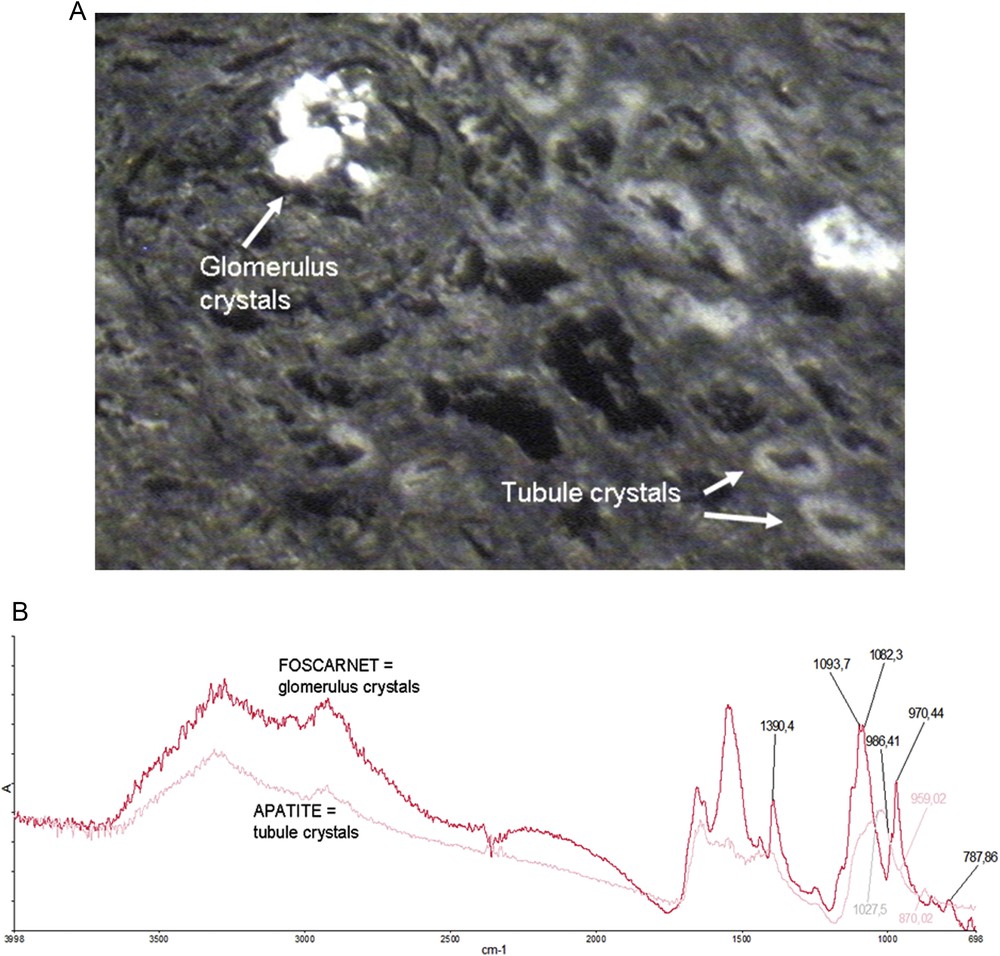
Foscarnet IR spectra display typical absorption bands at 1390, 1082 and 970 cm−1. A: Optical image. B: Typical IR spectra of foscarnet and apatite in a protein tissue (renal biopsy).
Foscarnet crystals observed by FE-SEM exhibit rectangular shapes and are agglomerated to make abnormal large deposits (Fig. 4). These deposits are present here in tubules and not in glomeruli.
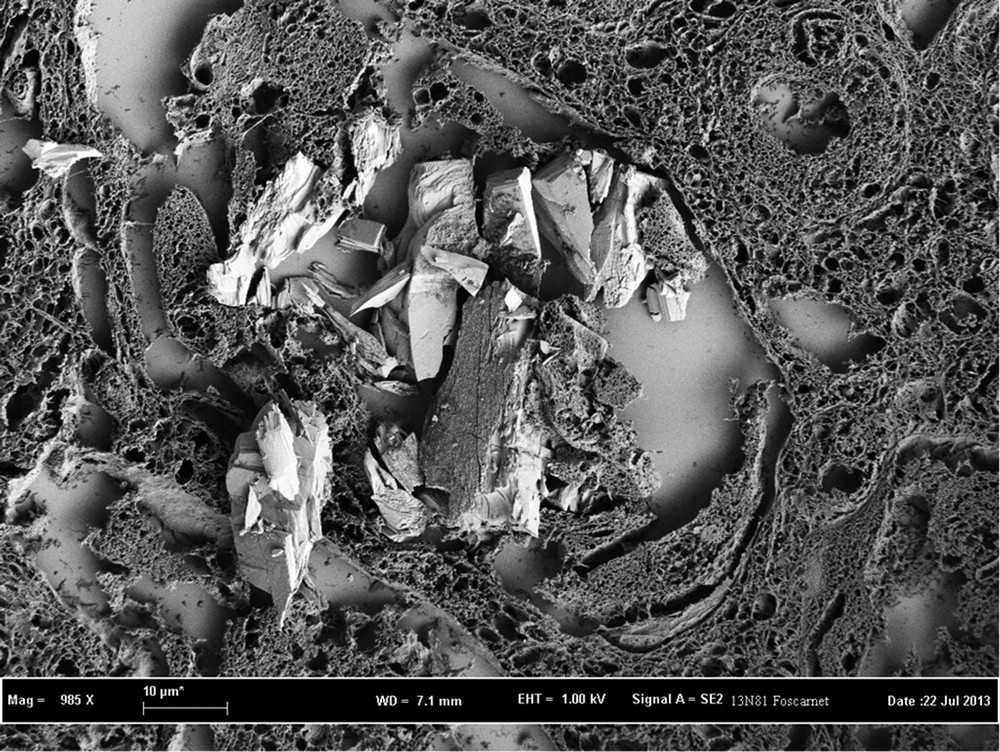
FE-SEM image of foscarnet crystals in tubules (renal biopsy).
3.2 Atazanavir
Several drugs against HIV may be the cause of renal failure in patients under tritherapy. Indinavir was early identified as a cause for kidney dysfunction [34] and nephrolithiasis mainly related to crystallization of the indinavir monohydrate in urine [35] and kidney tubules [36]. Because of its poor solubility in the normal urine pH range, the cause of indinavir crystallization was related to its concentration due to a short half-life (around 90 min) after oral administration. Efavirenz was also reported sometimes as a cause of nephrolithiasis [37,38]. Other molecules commonly used for the treatment of HIV have been reported as nephrotoxic [39–41]. Among them, atazanavir was reported as responsible for acute interstitial nephritis [42,43]. With the withdrawal of atazanavir, the renal impairment was reversible with the selection of another anti-protease.
Atazanavir is a multicycle molecule (Fig. 5). It is a potent and safe protease inhibitor with one daily administration (300–400 mg per day).
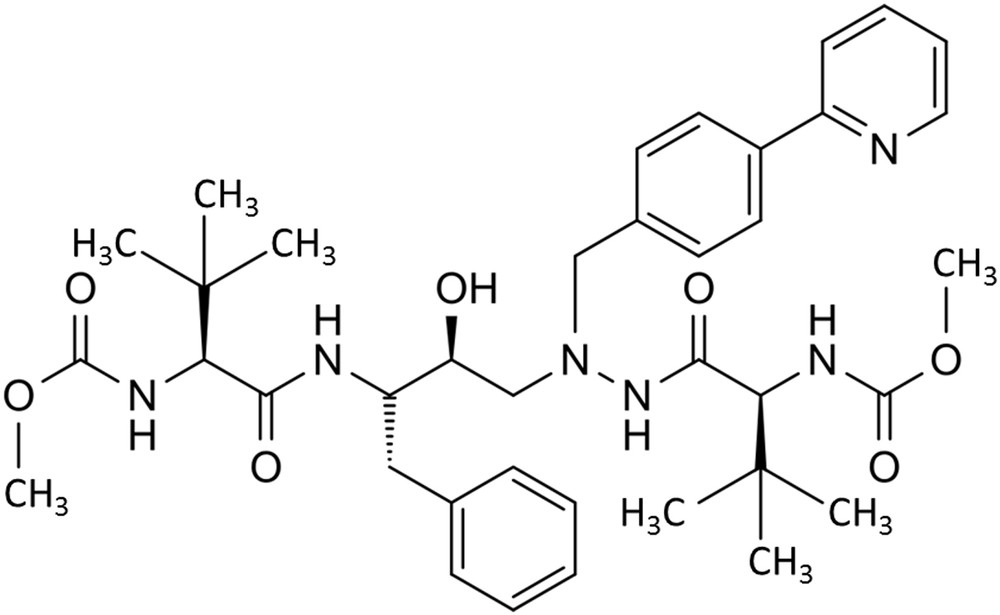
Chemical formula of atazanavir.
Following a single 400-mg dose of 14C-atazanavir, 79% and 13% of the total radioactivity was recovered in the feces and urine, respectively. Atazanavir is oxidized in the liver by the cytochrome P450 enzymes and then converted as glucuronide derivatives [44]. The two main ways for eliminating the drug are the bile ducts and the kidney. The unchanged drug accounts for approximately 20% and 7% of the administered dose in the feces and urine, respectively [45]. Contrary to indinavir, the half-life of atazanavir is around 7 h, thus reducing the excretion peak of the drug in urine and the risk for crystallization. However, atazanavir is now the main substance responsible for drug-induced nephrolithiasis in our country. The first case of kidney stone was published in 2006 [46], followed by other reports [47]. A total of 30 cases were reported by the FDA in the USA in 2007 [48]. In our experience, we identified atazanavir in 90 kidney stones analyzed in our laboratory between 2005 and 2016. Recently, we had the opportunity to identify two other cases of atazanavir-induced nephropathy. In the two biopsies, we were able to identify atazanavir crystals by FTIR microscopy (Fig. 6).
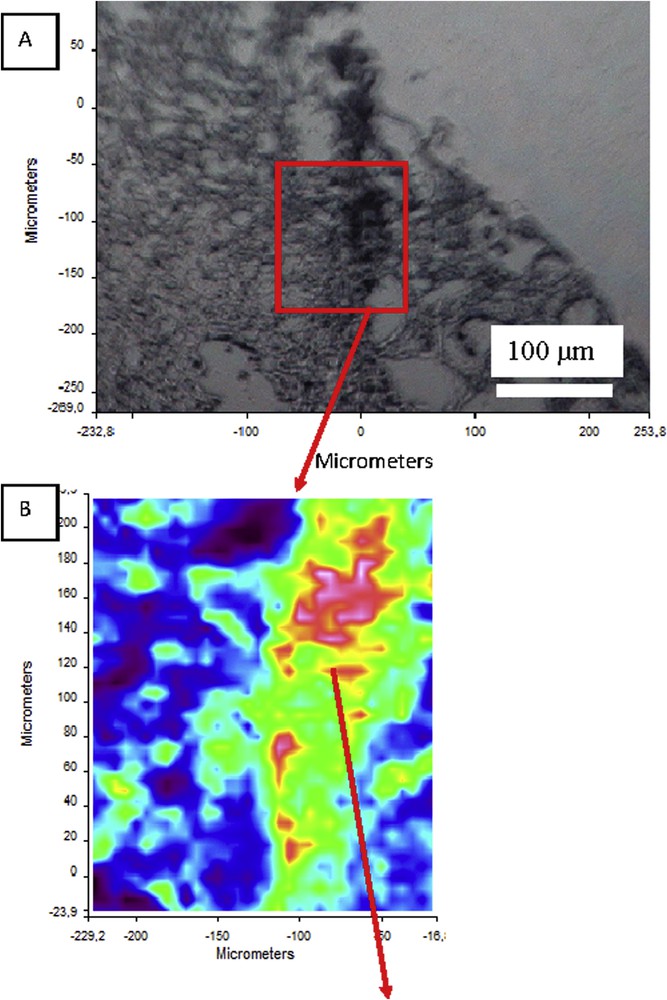
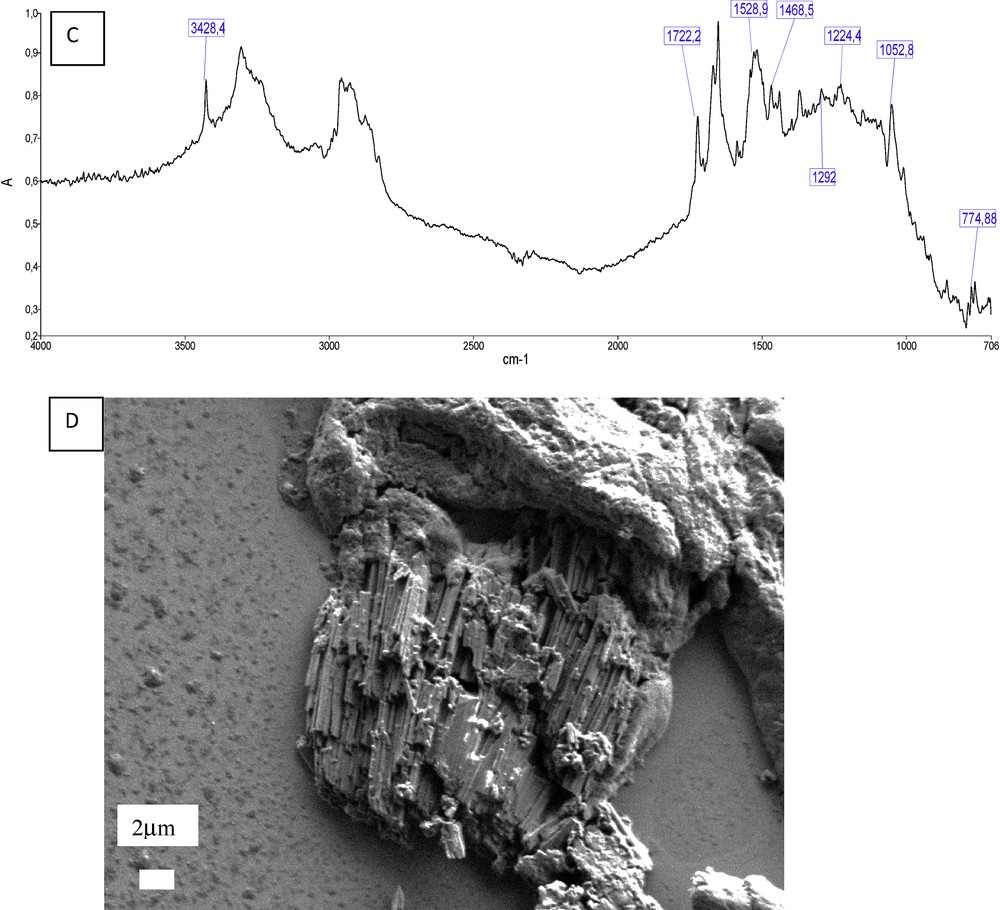
Kidney biopsy containing atazanavir crystals. A: Optical image; B: IR map; C: IR spectrum of atazanavir with the characteristic peaks (3428, 1722, 1529, 1053 cm−1) in a protein matrix (kidney tissue); D: Electronic images of atazanavir crystals obtained by SEM.
FE-SEM images of atazanavir deposits are made of needles or thin rod-shaped crystals aggregated in the tubular lumen (Fig. 6).
Contrary to indinavir crystalluria and nephrolithiasis observed in indinavir-treated patients that are explained by physicochemical properties and pharmacokinetics of the drug, the mechanism of atazanavir nephrotoxicity is unknown. While indinavir crystalluria, which is highly urinary pH-dependent, was found in 20% of 142 patients treated by indinavir [49], atazanavir crystals were found in the urine of only 9% of patients despite relatively high concentrations in the urine compared to other drugs such as lopinavir or ritonavir [50]. A longer exposure to atazanavir treatment was the unique factor identified to explain crystalluria. However the pathogenic mechanism to explain the incidence of renal colic and nephropathy in atazanavir-treated patients is not clearly established [51]. It has been proposed that as what happens in indinavir calculi, the precipitation of atazanavir in renal tubules may be the main cause, as a consequence of the fact that 7% of atazanavir and 20% of indinavir are excreted unchanged in urine unlike the other HIV drugs. Such differences in urine excretion of the unchanged drug could explain the lower occurrence and abundance of atazanavir crystals in the urine of patients by comparison with indinavir-treated patients. Marinescu et al. show atazanavir solubility in urine and the time to crystallization decrease, as urine is alkalized at pH>6 [52]. Among their 98 patients, 9 experienced nephrolithiasis while under atazanavir therapy. Among them, 3 (33%) had atazanavir crystals in their urine. In contrast, none of the other patients had atazanavir crystalluria. Recently, Gervasoni et al. showed that HIV patients treated with atazanavir who experienced nephrolithiasis had an atazanavir plasma concentration four times higher than patients without stones [53]. The study by Hamada et al. revealed significant differences in bilirubin concentrations in patients when they had renal colic, suggesting that individuals with a slow metabolism of atazanavir would have a greater incidence of lithiasis [54].
A multivariate analysis by Rusconi et al. showed a significant association between atazanavir-induced nephrolithiasis and changes in the single-nucleotide polymorphism at positions c211, 339, and 440 in UDP-glucuronosyltransferase 1A-3′ region [55], which suggests a special sensitivity of some patients to kidney side effects induced by atazanavir.
4 Conclusion
At least 1.6% of urolithiasis are drug-induced in France, a frequency likely underestimated. In 2.16% of renal biopsies examined in our laboratory, we found drug crystals or drug-induced deposits. The precise identification of crystal deposits in the renal tissue may be essential for the diagnosis of an unexplained renal failure. Clearly, in foscarnet-induced acute renal failure, several types of crystals and different locations of the crystals may be observed, suggesting different risk factors involved in the drug precipitation in addition to the dosage of the drug. Common histological procedures clearly fail to identify crystal deposits accurately. In most cases, light and polarizing microscopic examination should be completed by physical methods. In our experience, the FTIR microscope and scanning electron microscope are important tools for an accurate etiological diagnosis. Taking into account the risk factors related to the drug or the patient may help in most cases to prevent crystallization of the drug and kidney injury.
Acknowledgements
This work was supported by the Physics and Chemistry Institutes of CNRS and by contracts ANR-09-BLAN-0120-02, ANR-12-BS08-0022, ANR-13-JSV-10010-01, convergence UPMC CVG1205 and CORDDIM-2013-COD130042. The authors are grateful to the Soleil SR Facility for beam time allocation (proposal numbers, SMIS- 20130016, SMIS-20120070, SMIS-20110084, SMIS-20100566, and SMIS-20100039), which allowed them to start this research.


