1 Introduction
The indole nucleus has been found as an essential element in medicinal and agricultural chemistry with significant biological activities [1–3]. 3-Substituted indoles are prominent structural motifs found in numerous natural products or therapeutic agents with diverse pharmaceutical activities [4–6]. The development of simple procedures for the synthesis of compounds with biological benefits is the driving force for the discovery and design of new bioactive compounds. Three-component reactions of indoles, aldehydes, and malononitrile were used as a simple procedure to produce 3-substituted indole derivatives [7–13]. Ultrasound irradiation has been also considered as a clean and useful protocol in organic synthesis during the last three decades. In comparison with silent methods, the procedure is more convenient. A large number of organic reactions can be carried out in higher yields, a shorter reaction time, and milder conditions under ultrasound irradiation [14–17]. In continuation of our previous work on the development of new and simple synthetic methodologies [18,19], initially, (indolylmethyl)malononitriles were synthesized by the reaction of aldehydes, malononitrile, and indole in the presence of Zn(OAc)2/NaOAc in EtOH at 60 °C under silent and ultrasound irradiation conditions (Scheme 1).
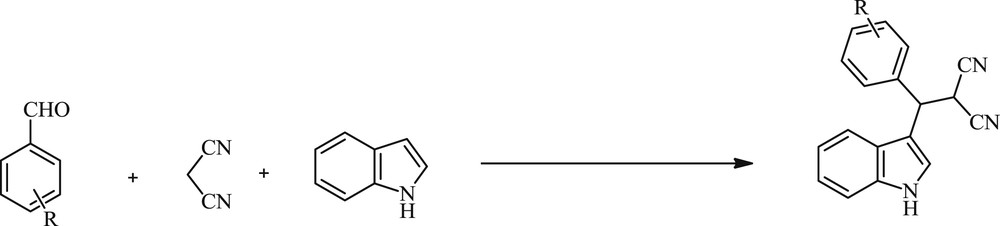
Synthesis of (indolylmethyl)malononitriles catalyzed by Zn(OAc)2/NaOAc.
It is desirable to develop an efficient and cost-effective method for the oxidative elimination of the synthesized (indolylmethyl)malononitriles to produce indoles containing highly polarized double bonds. Therefore, oxohalogen derivatives have been employed as versatile reagents in organic synthesis activities owing to their mild oxidative and halogenating properties [20–25]. In the present study, among these derivatives, Ca(OCl)2 was selected as an oxidant preferred over other available oxidizing agents because it is cheap, operationally safe, environmentally friendly, and easy to handle and work up. In the next step, oxidative elimination of (indolylmethyl)malononitrile derivatives was investigated in the presence of Ca(OCl)2 at room temperature (Scheme 2).
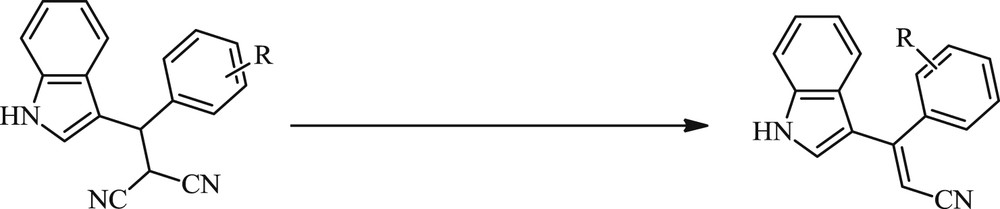
Oxidative elimination of (indolylmethyl)malononitrile in the presence of Ca(OCl)2.
2 Results and discussion
For the preparation of (indolylmethyl)malononitrile derivatives, the one-pot three-component reaction of benzaldehyde, malononitrile, and indole was investigated as a model reaction. To find the best catalyst, several Lewis acids such as ZrOCl2, CuCl, FeCl3 and Zn(OAc)2 were used. The highest yield was achieved with Zn(OAc)2. The formation of the product was more facile and proceeded in a shorter time when the reaction was performed in the presence of Zn(OAc)2/NaOAc. The results show that the use of 15/10 mol % of Zn(OAc)2/NaOAc in EtOH is sufficient. Greater amounts of the catalyst had no significant influence on the reaction yield. To find the optimum temperature, the reaction was conducted with 15/10 mol % of Zn(OAc)2/NaOAc at room temperature, at 60 °C, and at reflux temperature, which resulted in the isolation of the product in a trace amount, 85% and 25% yields, respectively. In addition, CH2Cl2, MeCN and AcOEt were also tested as solvents. In all these cases, the product was formed in lower yields. Solvent-free conditions at different temperatures did not accelerate the reaction. Thus, the reaction was optimally conducted in the presence of 15/10 mol % of Zn(OAc)2/NaOAc at 60 °C in EtOH. To study the effect of ultrasound, the reactions were carried out under silent and ultrasonic irradiation conditions. Ultrasound irradiation accelerated such reactions. The results are summarized in Table 1.
Optimization study for the one-pot synthesis of (indolylmethyl)malononitrile.a
| Entry | Catalyst (mol %) | Solvent | T (°C) | Time (h) | Yield (%) |
| 1 | ZrOCl2·8H2O (10) | EtOH | 60 | 9 | 30 |
| 2 | CuCl (10) | EtOH | 60 | 9 | 45 |
| 3 | FeCl3·6H2O (10) | EtOH | 60 | 9 | 20 |
| 4 | Zn(OAc)2(10) | EtOH | 60 | 9 | 60 |
| 5 | Zn(OAc)2 (15) | EtOH | 60 | 9 | 75 |
| 6 | Zn(OAc)2 (20) | EtOH | 60 | 9 | 75 |
| 7 | Zn(OAc)2/NaOAc (15/5) | EtOH | 60 | 7 | 80 |
| 8 | Zn(OAc)2/NaOAc (15/10) | EtOH | 60 | 7 | 85 |
| 9 | Zn(OAc)2/NaOAc (15/15) | EtOH | 60 | 7 | 85 |
| 10 | Zn(OAc)2/NaOAc (15/10) | H2O | 60 | 9 | 70 |
| 11 | Zn(OAc)2/NaOAc (10/15) | EtOH:H2O (1:1) | 60 | 9 | 72 |
| 12 | Zn(OAc)2/NaOAc (15/10) | EtOH | reflux | 9 | 25 |
| 13 | Zn(OAc)2/NaOAc (15/10) | EtOH | rt | 9 | 5 |
| 14 | NaOAc (10) | EtOH | 60 | 9 | 30 |
| 15b | Zn(OAc)2/NaOAc (15/10) | EtOH | 60 | 20 min | 88 |
a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), and indole(1 mmol).
b Sonication.
(Indolylmethyl)malononitrile derivatives were synthesized under the optimized conditions. It can be observed that the process tolerates both electron-donating and electron-withdrawing substituents in the substrates. In all the cases, the reactions proceeded efficiently in EtOH under mild conditions to afford the corresponding products in high yields. All the products were characterized by IR, 1H and 13C NMR spectra, and elemental analysis.
The (indolylphenylmethyl)malononitrile was subjected to oxidation using Ca(OCl)2 to produce indolylphenylacrylonitrile with an excellent yield. The initial studies were carried out by treating (indolylphenylmethyl)malononitrile with oxidant agents such as CoCl2/KClO3, CaCl2/KClO3 ZrOCl2, NaOCl and Ca(OCl)2 in different solvents at reflux temperature. The progress of the reaction was monitored by thin-layer chromatography (TLC). The formation of the product was more facile and proceeded in a shorter time when the reaction was performed in the presence of Ca(OCl)2 under solvent-free conditions at room temperature (Table 3).
Optimization study for the synthesis of indolylphenylacrylonitrile.a
| Entry | Oxidative agent (g) | Solvent | Temp | Time (h) | Yield (%) |
| 1 | CoCl2/KClO3 (0.0.20/0.10) | H2O | Reflux | 24 | – |
| 2 | CaCl2/KClO3 (0.20/).10) | H2O | Reflux | 24 | – |
| 3 | ZrOCl2·8H2O (0.0.20) | H2O | Reflux | 24 | – |
| 4 | NaOCl (0.20) | H2O | Reflux | 24 | 20 |
| 5 | NaOCl (0.20) | EtOH | Reflux | 24 | 20 |
| 6 | NaOCl (020) | EtOAc | Reflux | 24 | 50 |
| 7 | NaOCl (0.20) | n-Hexane | Reflux | 24 | 75 |
| 8 | Ca(OCl)2 (0.20) | H2O | Reflux | 24 | 50 |
| 9 | Ca(OCl)2 (0.20) | EtOH | Reflux | 24 | 55 |
| 10 | Ca(OCl)2 (0.20) | EtOAc | Reflux | 24 | 70 |
| 11 | Ca(OCl)2 (0.20) | n-Hexane | Reflux | 2.5 | 85 |
| 12 | Ca(OCl)2 (0.20) | – | rt | 20 min | 95 |
| 13 | Ca(OCl)2 (0.10) | – | rt | 20 min | 75 |
| 14 | Ca(OCl)2 (0.17) | – | rt | 20 min | 92 |
a Reaction conditions: 2-((indolyl)(phenyl)methyl)malononitrile (1 mmol), Ca(OCl)2 (0.20 g), Solvent-free, and rt.
To examine the generality of the procedure, the oxidative elimination of various (indolylmethyl)malononitriles and Ca(OCl)2 was checked under optimized reaction conditions. The results are summarized in Table 4.
Oxidative elimination of (indolylmethyl)malononitriles by Ca(OCl)2.a
| Entry | R | Yield (%) | mp (°C) |
| 1 | H | 98 | 73–75 |
| 2 | 3-NO2 | 96 | 105–107 |
| 3 | 4-NO2 | 98 | 120–122 |
| 4 | 4-Br | 93 | 79–81 |
| 5 | 4-Me | 90 | 95–97 |
| 6 | 4-OH | n.r | — |
a Reaction conditions: 2-((indolyl)(aryl)methyl)malononitrile (1 mmol), Ca(OCl)2 (0.20 g), Solvent-free, and rt.
The structure of the indolylacrylonitrile derivatives was investigated by spectroscopic studies such as IR, 1H and 13C NMR and elemental (CHN) analyses. The indolylacrylonitrile derivatives were established on the basis of the chemical shift of olefin singlet for vinyl proton in 1H NMR. Although the mechanism of these reactions has not yet been established experimentally, the formation of the product can be rationalized as outlined in Scheme 3. The Knoevenagel product (1) can be generated in a reaction between benzaldehyde and malononitrile in the presence of Zn(OAc)2/NaOAc. Michael addition of indole on compound 1 can be enhanced by Zn2+ of Zn(OAc)2 upon activation of olefinic compound 1 to form 3-substituted indole 2. Finally, oxidative elimination of 2 in the presence of Ca(OCl)2 resulted in 3-(Indolyl)-3-phenylacrylonitrile (3).
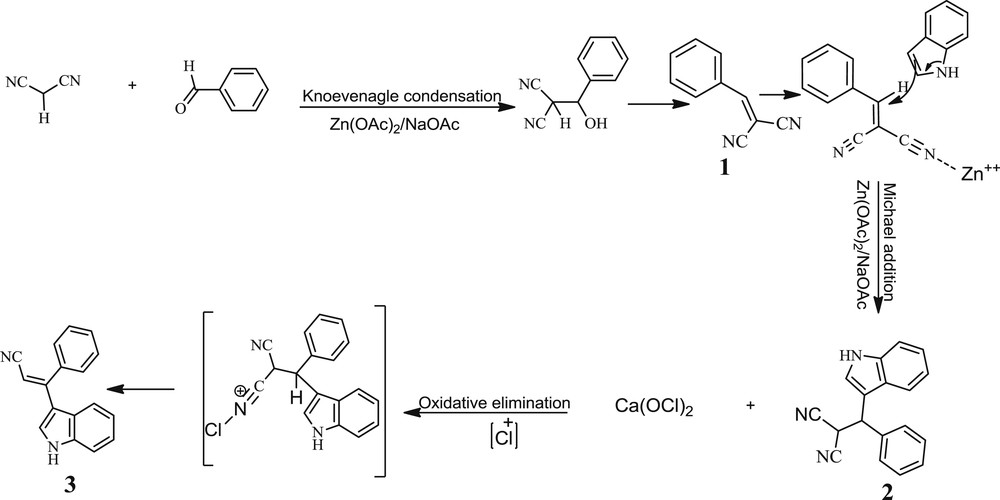
The proposed mechanism for the synthesis of indolylacrylonitrile.
3 Conclusion
In summary, a remarkable and expedient technique is reported for rapid synthesis of 3-substituted indoles containing highly polarized double bonds from easily accessible starting materials. Ultrasound accelerated a one-pot three-component reaction of an aldehyde, malononitrile, and indole. This method can be of choice for the preparation of a variety of (indolylmethyl)malononitrile derivatives some of which take a long reaction time to be made through silent approaches. It is also shown for the first time how oxidative elimination of (indolylmethyl)malononitrile promoted by calcium hypochlorite is able to yield indolylacrylonitrile, not previously described, in excellent yields, in short reaction times, and under solvent-free conditions.
4 Experimental
All the chemicals were of reagent grades and were used as received without further purification. 1H and 13C NMR spectra were recorded at 25 °C at a 300 MHz or 400 MHz (Bruker Avance) instrument using TMS as the internal standard. The chemical shifts were given in parts per million (ppm). The IR spectroscopy of the samples as KBr pellets was carried out on a Bruker Eqinox 55 FT-IR spectrometer in the 400–4000 cm−1 region. Sonication was performed in a Shanghai Branson-CQX ultrasonic cleaner (with a frequency of 25 kHz and a nominal power of 250 W). A reaction flask was placed in an ultrasonic bath, where the surface of the reactants was slightly lower than the level of the water. The reaction temperature was controlled by adding or removing water from the ultrasonic bath. The melting points were determined by Büchi melting point B-540 B.V.CHI apparatus in open capillaries. They were uncorrected. All the reactions and the purity of the products were monitored using thin-layer chromatography (TLC) on aluminum-backed plates coated with Merck Kieselgel 60 F254 silica gel and by watching the spots under ultraviolet light.
4.1 General procedure for the synthesis of (indolylmethyl)malononitriles
4.1.1 Silent conditions
Aldehyde (1 mmol), malononitrile (1 mmol), indole (1 mmol), Zn(OAc)2/NaOAc (15/10 mol %) and EtOH (5 mL) were placed in a round-bottom flask that was heated at 60 °C. The progress of the reaction was followed by TLC. After the completion of the reaction, the solvent was evaporated, the crude mixture was solidified from a mixture of ethanol and water, and it was filtered. A pure product was obtained by recrystallization in ethanol.
4.1.2 Ultrasound irradiation
A solution of an appropriate aldehyde (1 mmol), malononitrile (1 mmol), indole (1 mmol), and Zn(OAc)2/NaOAc (15/10 mol %) in EtOH (5 mL) was sonicated in a sonic bath for an appropriate period of time until the initial materials were no longer detectable by TLC. After the completion of the reaction, the solvent was evaporated, and the crude mixture was solidified from a mixture of ethanol and water and then filtered. A pure product was obtained by recrystallization in ethanol.
4.2 General procedure for the synthesis of indolylacrylonitrile
2-((Indolyl)(aryl)methyl)malononitrile (1 mmol) and Ca(OCl)2 (0.20 g) were added to a mortar. The mixture was ground using the mortar and pestle at room temperature for 20 min. After the completion of the reaction, to isolate the catalyst, the mixture was dissolved in hot CH2Cl2 and filtered. The solvent of the resulting filtrate was evaporated, and a pure product was obtained by recrystallization from ethanol. The authenticity of the products was established by the data of IR, 1H NMR, 13C NMR and elemental analyses.
4.3 Selected spectroscopic data
4.3.1 2-((Indolyl)(phenyl)methyl)malononitrile
White crystals; mp 82–84 °C. IR (KBr): ῡ = 3346, 3031, 2882, 2258, 1620, 1458, 746 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.29 (s, 1H), 7.48–7.43 (m, 2H), 7.43–7.40 (m, 5H), 7.32 (d, J = 8.1 Hz, 1H), 7.26 (t, J = 7.8 Hz, 1H), 7.10 (t, J = 7.5 Hz, 1H), 4.95 (d, J = 6.3 Hz, 1H), 4.46 (d, J = 6.3 Hz, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 139.2, 136.1, 128.6, 128.0, 127.7, 125.8, 122.7, 121.6, 118.8, 118.5, 114.1, 113.8, 112.3, 111.6, 42.4, 28.6 ppm (Table 2, entry 1).
Zn(OAc)2/NaOAc promoted one-pot synthesis of (indolylmethyl)malononitriles.a
| Entry | R | Time thermal (h)/Sonication (min) | Yield(%) thermal/Sonication | mp (°C) | Ref. |
| 1 | H | 9/15 | 85/88 | 82–84 | [11] |
| 2 | 3-NO2 | 6/15 | 85/90 | 81–83 | [16] |
| 3 | 4-NO2 | 4/11 | 90/85 | 190–192 | [7] |
| 4 | 4-F | 6/15 | 85/80 | 116–118 | [10] |
| 5 | 4-Cl | 6/15 | 87/90 | 78–80 | [11] |
| 6 | 2,4-diCl | 6/15 | 85/90 | 169–171 | [16] |
| 7 | 4-Br | 6/15 | 70/75 | 98–100 | [10] |
| 8 | 4-CN | 9/20 | 80/85 | 165–167 | [9] |
| 9 | 4-OH | 11/25 | 86/90 | 179–181 | |
| 10 | 4-OMe | 11/22 | 55/60 | 94–96 | [9] |
| 11 | 4-Me | 10/20 | 70/75 | 120–122 | [7] |
| 12 | 4-ipr | 10/20 | 85/90 | 125–127 | [9] |
a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), indole(1 mmol), and Zn(OAc)2/NaOAc (15/10 mol %) at 60 °C or u.s./60 °C in EtOH.
4.3.2 2-((Indolyl)(4-nitrophenyl)methyl)malononitrile
Yellow crystals; mp 190–192 °C. IR (KBr): ῡ = 3378, 3054, 2890, 2258,1607, 1528, 1421, 1351, 744 cm−1; 1H NMR (400 MHz,DMSO-d6): δ = 11.33 (s, 1H), 8.26 (d, J = 8.8 Hz, 2H), 7.80 (d, J = 8.8 Hz, 2H), 7.61 (s, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.11 (t, J = 6.8 Hz,1H), 6.97 (t, J = 7.2 Hz, 1H), 5.99 (d, J = 9.2 Hz, 1H), 5.51 (d, J = 9.2 Hz, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 147.0, 146.6, 136.0, 129.4, 125.6, 123.9, 123.1, 121.8, 119.1, 118.3, 113.6, 113.4, 111.7, 111.2, 41.7, 28.0 ppm (Table 2, entry 3).
4.3.3 2-((Indolyl)(4-fluorophenyl)methyl)malononitrile
White crystals; mp 116–118 °C. IR (KBr): ῡ = 3428, 3054, 2883, 2257, 1605, 1426, 751 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 11.25 (s, 1H), 7.57 (m, 3H), 7.45 (d, J = 7.6 Hz,1H), 7.40 (d, J = 7.6 Hz, 1H), 7.22 (m, 2H), 7.10 (t, J = 7.6 Hz, 1H), 6.96 (t, J = 7.2 Hz, 1H), 5.84 (d, J = 9.2 Hz, 1H), 5.27 (d, J = 9.2 Hz, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 160.3, 136.0, 135.4, 130.1, 125.8, 122.6, 121.7, 118.9, 118.4, 115.5, 113.9, 113.7, 112.1, 111.6, 41.5, 28.7 ppm (Table 2, entry 3).
4.3.4 2-((Indolyl)(4-hydroxyphenyl)methyl)malononitrile
Yellow crystals; mp 179–181 °C. IR (KBr): ῡ = 3353, 2899, 2226, 1566, 1445, 744 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 11.17 (s, 1H), 7.89 (d, J = 8.8 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 8.4 Hz, 2H), 7.19 (s, 1H), 7.09 (t, J = 7.2 Hz, 1H), 6.9 (t, J = 8.5 Hz, 1H), 6.68 (d, J = 8.4 Hz, 2H), 5.70 (d, J = 9.2 Hz, 1H), 5.10 (d, J = 9.2 Hz, 1H), 3.40 (broad, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 160.4, 133.8, 130.0, 129.1, 122.7, 121.5, 120.8118.7, 118.6, 118.2, 116.5, 115.2, 115.0, 114.1, 112.7, 111.3 ppm; Anal. calcd for C18H13N3O: C 75.25; H 4.56; N 14.63%. Found: C 75.1; H 4.6; N 14.4% (Table 2, entry 9).
4.3.5 2-((Indolyl)(p-tolyl)methyl)malononitrile
Pale yellow crystals; mp 120–122 °C. IR (KBr): ῡ = 3350, 3035, 2883, 2225, 1588, 1459, 745 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 11.22 (s, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.47 (d, J = 7.8 Hz, 1H), 7.44 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 7.10–6.96 (m, 2H), 5.82 (d, J = 9.2 Hz, 1H), 5.17 (d, J = 9.2 Hz, 1H), 2.27 (s, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 137.0, 136.2, 130.6, 130.1, 129.1, 127.9, 125.8, 122.6, 121.6, 118.5, 114.1, 113.8, 112.4, 111.6, 42.1, 28.7, 20.5 ppm (Table 2, entry 11).
4.3.6 3-(Indolyl)-3-phenylacrylonitrile
White crystals; mp 73–75 °C. IR (KBr): ῡ = 3400, 3030, 2259, 1621, 1493, 743 cm−1; 1H NMR (300 MHz, CDCl3): δ = 8.40 (s, 1H), 7.73 (s, 1H), 7.62 (m, 2H), 7.48–7.41 (m, 5H), 7.26 (t, J = 7.8 Hz, 1H), 7.14 (t, J = 7.5 Hz, 1H), 5.12 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 168.0, 137.1, 136.3, 134.4, 131.9, 129.8, 128.8, 125.6, 123.1, 121.6, 120.3, 116.3, 115.6, 113.1, 112.1 ppm; Anal. calcd for C17H12N2: C 83.58; H 4.95; N 11.47%. Found: C 83.7; H 4.6; N 11.4% (Table 4, entry 1).
4.3.7 3-(Indolyl)-3-(3-nitrophenyl)acrylonitrile
Yellow crystals; mp 105–107 °C. IR (KBr): ῡ = 3374, 3062, 2251, 1620, 1583, 1527, 1352, 745 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 12.92 (s, 1H), 8.51 (d, J = 8.0 Hz, 1H), 8.42 (s, 1H), 8.35 (s, 1H), 8.04 (d, J = 8.0 Hz, 1H), 7.88 (t, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.6 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 5.88 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ = 164.9, 147.7, 137.8, 137.2, 136.3, 135.4, 130.7, 126.2, 125.1, 124.6, 123.4, 122.0, 120.4, 115.8, 115.3, 113.2, 111.9 ppm; Anal. calcd for C17H11N3O2: C 70.58; H 3.83; N 14.53%. Found: C 70.8; H 3.6; N 14.4% (Table 4, entry 2).
4.3.8 3-(Indolyl)-3-(p-tolyl)acrylonitrile
White crystals; mp 95–97 °C. IR (KBr): ῡ = 3380, 3050, 2240, 1632, 1490, 760 cm−1; 1H NMR (300 MHz, CDCl3): δ = 9.85 (s, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.78 (s, 1H), 7.62 (m, 2H), 7.55 (d, J = 7.5 Hz, 2H), 7.47 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 7.5 Hz, 2H), 5.12 (s, 1H), 2.27 (s, 3H) ppm; 13C NMR (100 MHz, DMSO-d6) δ = 168.0, 137.1, 136.3, 134.4, 131.9, 129.8, 128.8, 125.6, 123.1, 121.6, 120.3, 116.3, 115.6, 113.1, 112.1, 20.5 ppm; Anal. calcd for C18H14N2: C 83.69; H 5.46; N 10.84%. Found: C 83.7; H 5.6; N 11.1% (Table 4, entry 5).
Acknowledgements
The authors thank the Research Council of Yazd University for financial support.


