1 Introduction
Copper as the third most abundant transition metal in the human body is vital for both environmental and biological systems [1,2]. Copper(I) complexes have attracted much attention because of their rich coordination chemistry and numerous catalytic and technological applications [3–9]. The copper(I) complexes of diimine ligands exhibit structural diversity and rich redox and photophysical properties [10–13] and have found important applications in bioinorganic chemistry [14,15] and for the preparation of functional materials [16–18].
Benzothiazole and its derivatives exhibit a wide range of biological activities [19] and have potential clinical applications, as antitumor [20], anticoagulant [21], antiallergic [22], anti-inflammatory [23], antimicrobial [24], and enzyme inhibitor agents [25]. The electro-optical characteristics of these materials as dopant in organic light-emitting diode devices have also been investigated [26].
In continuation of our studies on copper complexes of 2-substituted benzothiazoles [27,28], herein we report the synthesis and spectral characterization of three Cu(I) complexes of 2-(2-quinolyl)benzothiazole (qbtz) with diverse structures, namely [Cu(qbtz)(μ-I)]2 (1), [Cu3(qbtz)2(μ-CN)3] (2), and [Cu(qbtz)(μ-SCN)] (3). The X-ray crystal structures of these complexes are reported and their cyclic voltammetric behavior is also discussed.
2 Experimental section
2.1 Materials and methods
All solvents and chemicals were of commercial reagent grade and used as received from Aldrich and Merck. Infrared (IR) spectra as KBr pellets were collected on a FT-IR JASCO 680 plus spectrometer in the range 4000–400 cm−1. Ultraviolet–visible (UV–vis) absorption spectra were recorded on a JASCO V-570 spectrophotometer. Elemental analyses were performed using a Perkin Elmer 2400II CHNSO Elemental Analyzer. Electrochemical measurements were carried out at room temperature with an SAMA 500 Research Analyzer using a three-electrode system, a glassy carbon working electrode (Metrohm 6.1204.110 with 2.0 ± 0.1 mm diameter), a platinum disk auxiliary electrode, and an Ag wire as reference electrode. Cyclic voltammogram measurements were performed in DMF with tetrabutylammonium hexafluoridophosphate as the supporting electrolyte. The solutions were deoxygenated by purging with Ar for 5 min. All electrochemical potentials were calibrated versus an internal Fc+/0 (E0 = 0.45 V vs saturated calomel electrode (SCE)) couple under the same conditions [29].
2.2 Synthesis of the ligand qbtz
The ligand qbtz was prepared according to a novel procedure reported elsewhere [28] by heating a mixture of quinaldic acid and 2-aminothiophenol with triphenylphosphite in the presence of the inexpensive ionic liquid tetrabutylammonium bromide at 120 °C. The reaction was completed in 15 min. The viscous slurry obtained was treated with methanol and the resulting solid was filtered and washed with cold methanol and dried in vacuum. Yield: 87%.
2.3 Synthesis of [Cu(qbtz)(μ-I)]2(1)
To a solution of CuI (28.6 mg, 0.15 mmol) in acetonitrile (45 mL) was added dropwise and slowly a solution of the ligand (39.3 mg, 0.15 mmol) in acetonitrile (45 mL). The resulting yellow solution was left undisturbed at room temperature and brown crystals suitable for X-ray crystallography were obtained by slow vaporization of the solvent after 10 days. The crystals were filtered off, washed with cold acetonitrile, and dried in vacuum. Yield: 76%. Anal. Calcd for C32H20I2Cu2N4S2: C, 42.44; H, 2.23; N, 6.19; S, 7.08. Found: C, 42.52; H, 2.25; N, 6.15; S, 7.32%. FT-IR (KBr, cm−1) νmax: 1584 (CNbenzothiazole), 1504 (CNquinoline). UV–vis: λmax(nm) (ɛ, L mol−1 cm−1) (DMF): 422 (380), 348 (47,180), 336 (50,300), 320 (44,440), 284 (42,840), 264 (41,380).
2.4 Synthesis of [Cu3(qbtz)2(μ-CN)3] (2)
To a solution of Cu(CH3CN)4ClO4 (98.2 mg, 0.30 mmol) in 90 mL of acetonitrile was added dropwise and slowly a solution of qbtz (78.6 mg, 0.30 mmol) in 90 mL of acetonitrile. To the resulting yellow solution was then added dropwise a solution of KCN (19.5 mg, 0.30 mmol) in acetonitrile (90 mL). The final solution was left undisturbed at room temperature. Orange crystals of the product suitable for X-ray crystallography were obtained after 5 days. The crystals were collected by filtration, washed with cold acetonitrile, and dried in vacuum. Yield: 72%. Anal. Calcd for C35H20Cu3N7S2: C, 52.90; H, 2.54; N, 12.36; S, 8.08. Found: C, 52.82; H, 2.30; N, 12.34; S, 8.40%. FT-IR (KBr, cm−1) νmax: 1591 (CNbenzothiazole), 1510 (CNquinoline), 2104 (CN). UV–vis: λmax(nm) (ɛ, L mol−1 cm−1) (DMF): 418 (358), 350 (46,100), 336 (49,750), 320 (43,420), 284 (42,020), 264 (42,950).
2.5 Synthesis of [Cu(qbtz)(μ-SCN)] (3)
Complex 3 was prepared by a procedure similar to that used for complex 2, except that KSCN was used instead of KCN. Brown crystals of the product suitable for X-ray crystallography were obtained after 1 week. The crystals were collected by filtration, washed with cold acetonitrile, and dried in vacuum. Yield: 79%. Anal. Calcd for C34H20Cu2N6S4: C, 53.18; H, 2.63; N, 10.94; S, 16.70. Found: C, 52.86; H, 2.58; N, 10.88; S, 16.56%. FT-IR (KBr, cm−1) νmax: 1588 (CNbenzothiazole), 1508(CNquinoline), 2094 (SCN). UV–vis: λmax(nm) (ɛ, L mol−1 cm−1) (DMF): 422 (376), 348 (76,300), 336 (81,260), 320 (70,980), 284 (68,260), 262 (59,530).
2.6 X-ray crystallography
X-ray data of the compounds 1, 2, and 3 were collected at T = 100 K on a Bruker Kappa APEX-II CCD diffractometer with graphite monochromated Mo Kα (λ = 0.71073 Å) radiation. Cell refinement and data reduction were performed with program SAINT [30]. Correction for absorption was carried out by the multiscan method and program SADABS [30]. The crystal structures were solved with direct methods using the program SHELXS97, and structure refinement on F2 was carried out with the program SHELXL97 [31]. Crystal data together with other relevant information on the structure determination are summarized in Table 1. All crystal structures had in common an orientation disorder of the qbtz ligand by which the benzothiazole and the quinoline fragment switch positions so that the two sides of the N,N′-diphenylethane-1,2-diimine fragment of qbtz practically coincide and only the sulfur of the thiazole ring and a CHCH group of the quinoline ring give separate split positions. In solid-state structures qbtz mimics thereby 2,2′-biquinoline. The proportions of major to minor qbtz orientation varied between 0.677(5) and 0.323(5) in compound 1 and 0.800(4) and 0.200(4) in compound 3. This disorder made it necessary to apply stabilizing restraints for the most affected sites S1/S1′, C9/C9′, and C10/C10′ (unprimed/primed sites for major and minor orientation of qbtz). Further information on this aspect is provided in the deposited Crystallographic Information Files. Minor sites (S1′, C9′, C10′, and their hydrogen atoms) are not shown in the structural drawings (Figs. 1–3). The cyanide-based compound 2 showed the usual orientation ambiguity of bridging cyanides, and the cyanido groups were all treated as a superposition of pairs of CN groups with opposed orientations and occupancy factors adding up to one.
Crystallographic parameters, data collection, and refinement details for 1–3.
| Empirical formula | C32H20I2Cu2N4S2 (1) | C35H20Cu3N7S2 (2) | C34H20Cu2N6S4 (3) |
| Formula weight | 905.52 | 793.32 | 767.88 |
| Temperature (K) | 100(2) | 100(2) | 100(2) |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | Pī | Pī | P21/n |
| a (Å) | 7.9721(5) | 12.4075(14) | 10.6396(5) |
| b (Å) | 9.3475(5) | 12.6342(14) | 15.3656(8) |
| c (Å) | 10.8772(6) | 12.7497(14) | 18.6910(9) |
| α (°) | 72.934(3) | 114.597(2) | 90 |
| β (°) | 77.373(3) | 101.217(3) | 98.1187(9) |
| γ (°) | 74.257(3) | 109.392(3) | 90 |
| v (Å3) | 737.25(7) | 1579.9(3) | 3025.1(3) |
| z | 1 | 2 | 4 |
| Dcalc (Mg/m3) | 2.040 | 1.668 | 1.686 |
| μ (mm−1) | 3.71 | 2.17 | 1.72 |
| Crystal size (mm) | 0.22 × 0.12 × 0.09 | 0.45 × 0.35 × 0.10 | 0.42 × 0.40 × 0.25 |
| F(000) | 436 | 796 | 1552 |
| θ range (°) | 2.3–30.0 | 1.9–26.4 | 2.4–30.0 |
| Absorption correction | Multiscan | Multiscan | Multiscan |
| Reflections collected | 6178 | 19,374 | 55,478 |
| Rint | 0.023 | 0.044 | 0.023 |
| Data/restraints/parameters | 3838/64/206 | 6181/153/447 | 8820/147/465 |
| Goodness-of-fit on F2 | 1.04 | 1.08 | 1.187 |
| Final R indices [I > 2σ(I)]a | R1 = 0.033, wR2 = 0.083 | R1 = 0.062, wR2 = 0.115 | R1 = 0.030, wR2 = 0.0664 |
| Largest diff. peak and hole (e Å−3) | 1.39 and −0.55 | 1.29 and −0.99 | 0.58 and −0.48 |
a

View of the molecular structure of [Cu(qbtz)(μ-I)]2 (1) with 50% probability ellipsoids. Hydrogen atoms and additional sites for the orientation disordered qbtz ligand are omitted for clarity.
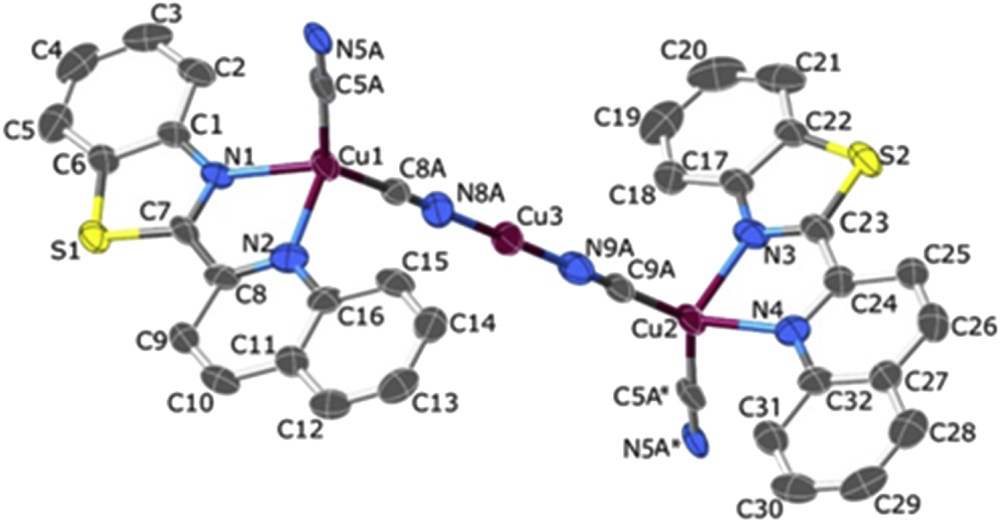
The structure of [Cu3(qbtz)2(μ-CN)3] with 70% probability ellipsoids. Hydrogen atoms and disordered qbtz sites are omitted for clarity. (*) Stands for the symmetry operation (x, 1 + y, 1 + z).

Crystal packing of the [Cu3(qbtz)2(μ-CN)3] complex.
3 Results and discussion
3.1 Description of the crystal structure of [Cu(qbtz)(μ-I)]2 (1)
The molecular structure of complex 1 is shown in Fig. 1. The crystallographic and refinement data for 1 are summarized in Table 1, and selected bond distances and angles are listed in Table 2. Complex 1 crystallizes in the triclinic space group Pī and is a neutral molecular compound consisting of centrosymmetric Cu2(μ-I)2 units with the separation of 2.5855(8) Å between the two Cu(I) centers. This distance is close to that observed in related complexes like bis(μ-iodido)-bis(2,2′-bipyridine)-di-copper(I) (2.579 Å) [32] or bis(μ-iodido)-bis(di-2-pyridylketone)-di-copper(I) (2.547 Å) [33]. The inversion center is within the CuI2Cu bridge. An analysis with the help of the Cambridge Structural Database [34] reveals that the CuCu distances in 76 Cu2(μ-I)2 units with N-bearing coligands vary widely between 2.51 and 3.27 Å showing a median of 2.68 Å, whereas the sum of two copper van der Waals radii is 2.80 Å (see Supplementary data for a table of the 76 structures]. Whether and to what extent a short CuCu separation of 2.5855 Å within a Cu2(μ-I)2 unit like in 1 indicates the presence of a stabilizing cuprophilic interaction remains open to question until suitable quantum-chemical calculations are available [35]. Although a tetrahedral geometry might be expected for a four-coordinated copper(I) center, the coordination sphere around the copper atom in 1 is distorted by the restricting bite angle of the chelating qbtz ligand. Disregarding the Cu…Cu interaction, the copper(I) in this complex is in a pseudotetrahedral environment with a large angular distortion, arising from the low intraligand N2Cu1N1 chelate angle (79.35°) that is much less than the ideal tetrahedral angle of 109.5°. On the contrary, the angles N2Cu1I1 (117.86°) and I1Cu1I1i (120.32°) are much larger than the ideal tetrahedral angle. The bond distances Cu1N1, 2.086(3) Å, and Cu1N2, 2.092(2) Å, are within the expected range and are comparable with those reported for related complexes (2.084(2)–2.131(3) Å for Cu(qbtz)(PPh3)Br/I, [27]). The CuI bond distances (2.5569(4) and 2.6377(4) Å) are similar to those in related dinuclear copper (I) complexes [32–35]. The qbtz ligand is twisted by 10.6° about the bond axis C7C8. In the crystal lattice, the dinuclear complexes are packed approximately perpendicular to (110) by slipped π–π stacking interactions between adjacent qbtz ligands. The π–π stacking parameters are listed in Table 3.
Selected bond lengths (Å) and angles (°) for 1.
| Bond lengths | |||
| Cu1N1 | 2.086(3) | Cu1I1i | 2.6377(4) |
| Cu1N2 | 2.092(2) | Cu1Cu1i | 2.5855(8) |
| Cu1I1 | 2.5569(4) | I1I1i | 4.506(5) |
| Bond angles | |||
| N1Cu1N2 | 79.35(12) | I1Cu1Cu1i | 61.72(2) |
| N1Cu1I1 | 118.12(8) | Cu1iCu1I1i | 58.61(2) |
| N1Cu1I1i | 106.67(7) | Cu1I1Cu1i | 59.67(2) |
| N2Cu1I1 | 117.83(7) | N1Cu1Cu1i | 139.35(9) |
| N2Cu1I1i | 107.04(7) | N2Cu1Cu1i | 139.52(9) |
| I1Cu1I1i | 120.32(2) |
Aromatic interaction parameters (Å and °) for description of π–π interaction in 1–3.
| Cg(I)–Cg(J) | dCg–Cga | dplane–planeb | Slippage | Symmetry codes |
| Complex 1 | ||||
| Cg(1)…Cg(3) | 3.6514 | 3.4289, 3.3589 | 1 − X, −Y, 1 − Z | |
| Cg(2)…Cg(3) | 3.6622 | 3.3817, 3.4281 | 1 − X, −Y, 1 − Z | |
| Cg(2)…Cg(5) | 3.7511 | 3.3823, 3.5458 | 1 − X, −Y, 1 − Z | |
| Cg(3)…Cg(3) | 3.6816 | 3.3123, 3.3123 | 1.607 | 2 − X, −Y, 1 − Z |
| Cg(3)…Cg(5) | 3.6675 | 3.3094, 3.2972 | 2 − X, −Y, 1 − Z | |
| Cg(4)…Cg(4) | 3.6856 | 3.5995, 3.5995 | 0.792 | 1 − X, −Y, 1 − Z |
| Complex 2 | ||||
| Cg(1)…Cg(2) | 3.5121 | 3.4595, 3.4146 | 1 − X, −Y, −Z | |
| Cg(1)…Cg(6) | 3.6147 | 3.2775, 3.3642 | −1 + X, Y, Z | |
| Cg(2)…Cg(3) | 3.9532 | 3.4287, 3.3919 | 1 − X, −Y, −Z | |
| Cg(2)…Cg(5) | 3.5363 | 3.3998, 3.3651 | X, 1 + Y, Z | |
| Cg(3)…Cg(6) | 3.6418 | 3.3419, 3.3527 | −1 + X, Y, Z | |
| Cg(4)…Cg(5) | 3.6661 | 3.3638, 3.3543 | X, 1 + Y, Z | |
| Complex 3 | ||||
| Cg(1)…Cg(5) | 3.9935 | 3.6305, 3.6220 | 1 + X, Y, Z | |
| Cg(2)…Cg(2) | 3.8780 | 3.4318, 3.4318 | 1.806 | 1 − X, −Y, −Z |
| Cg(2)…Cg(4) | 3.7353 | 3.4343, 3.4274 | 1 − X, −Y, −Z | |
| Cg(3)…Cg(5) | 3.8035 | 3.3509, 3.6356 | 1 + X, Y, Z |
a Centroid–centroid distance.
b Perpendicular distance of Cg(I) on ring J and perpendicular distance of Cg(J) on ring I.
3.2 Description of the crystal structure of [Cu3(qbtz)2(μ-CN)3] (2)
The structure and crystal packing of complex 2 is shown in Figs. 2 and 3. Crystallographic and refinement data for 2 are summarized in Table 1, and selected bond distances and angles are listed in Table 4. Complex 2 was synthesized with the aim of obtaining Cu(CN)(qbtz) with monovalent Cu. The synthesis, however, generated Cu3(CN)3(qbtz)2, that is, with a 3:3:2 ratio of the constituents. Of the three crystallographically independent copper atoms Cu(1) and Cu(2) feature tetrahedral four coordination by each two N atoms from the chelating qbtz ligand and two CN groups (each bridging two Cu atom). The third copper atom, Cu(3), displays a quasi-linear two coordination by two CN groups. This complex crystallizes in the space group Pī and exhibits a one-dimensional chain structure with a …Cu(qbtz)CNCu(qbtz)CNCuCNCu(qbtz)CNCu(qbtz)… architecture practically identical with that of catena-(tris(μ2-cyanido)-bis(2,2′-biquinoline)-tri-copper(I)) [36,37], which crystallizes in the lattice space group C2/m, however. The structure of 2 shows the usual orientational disorder (superposition of quinoline and benzothiazole) for the qbtz ligand (two independent ligands; population parameters for major/minor orientation 0.751/0.249 and 0.705/0.295). The atom coordinating a CN group to a transition metal is not invariably either C or N; moreover, we did not find any literature citation claiming that C must be bonded to the two-coordinate Cu(I) in a continuous Cu(I)CN chain. To assess this situation in 2, we carried out refinements including CN occupancies. These refinements yielded occupancy factors of the N-bonded (to Cu3) variety of 0.48(5), 0.88(5), and 0.85(5) for the cyanido groups N5A–C5A, N8A–C8A, and N9A–C9A, respectively. It must, however, be borne in mind that crystals of 2 were not of the splendid persuasion, and that, therefore, these occupation numbers should not be taken too literally. But in any case, there is a perceptible preference for the N bonding to Cu3, and the exclusively C-bonded CN was clearly disfavored by R values. A confirmation of preferred N bonding may also be found in the very similar CCDC refcode MODLOH (see the detailed discussion in the Supplementary data). The bond distances to the chelating qbtz ligand, Cu1N1 = 2.127(5) Å, Cu1N2 = 2.160(5) Å, Cu2N3 = 2.128(5) Å, and Cu2N4 = 2.151(5) Å, are within the expected range and are in agreement with those reported for related complexes (2.131(3), 2.126(3), and 2.084(2) Å) [27]. The bond angles around the tetrahedral centers Cu1 and Cu2 deviate notably from the ideal value of 109.5°, showing the smallest value of ∼77° for the qbtz intraligand angle and the largest value of ∼130° for the CNCuCN angle, whereas the rest of the angles vary between 104 and 113° (Table 4). The atoms around the Cu3 center are defined by two cyanide ions with bond lengths of Cu3N9A = 1.838(5) Å and Cu3N8A = 1.843(5) Å. The bond angle around the Cu3 center is, N9ACu3N8A = 179.5(3)°, close to the ideal value of 180° for a linear two coordination [36–39]. Slipped π–π stacking interactions are also observed in this complex. The π–π stacking parameters are listed in Table 3.
Selected bond lengths (Å) and angles (°) for 2.
| Bond lengths | |||
| Cu1C8A | 1.912(5) | Cu2C9A | 1.902(5) |
| Cu1N5A | 1.943(5) | Cu2N5A | 1.946(5) |
| Cu1N1 | 2.127(5) | Cu2N3 | 2.128(5) |
| Cu1N2 | 2.160(5) | Cu2N4 | 2.151(5) |
| Cu3N9A | 1.838(5) | Cu3N8A | 1.843(5) |
| Bond angles | |||
| N1Cu1N2 | 77.28(19) | N3Cu2N4 | 77.3(2) |
| N1Cu1N5A | 104.03(19) | N3Cu2C5A | 105.8(2) |
| N1Cu1C8A | 115.2(2) | N3Cu2C9A | 113.1(2) |
| N2Cu1N5A | 107.5(2) | N4Cu2C5A | 104.27(19) |
| N2Cu1C8A | 109.9(2) | N4Cu2C9A | 111.6(2) |
| C5BCu1C8A | 130.2(2) | C5ACu2C9A | 131.4(2) |
| N8ACu3N9A | 179.5(3) | ||
| Cu1N5AC5A(i) | 176.3(5) | Cu2C5AN5A(i) | 174.8(5) |
| Cu1C8AN8A | 177.8(5) | Cu2C9AN9A | 178.5(5) |
| Cu3N8AC8A | 178.5(5) | Cu3N9AC9A | 178.8(6) |
3.3 Description of the crystal structure of [Cu(qbtz)(μ-SCN)] (3)
The structure of complex 3 is shown in Fig. 4. The crystallographic and refinement data for 3 are summarized in Table 1, and selected bond distances and angles are listed in Table 5. This compound crystallizes in the monoclinic space group P21/n. The asymmetric unit of the structure contains two formula units of Cu(SCN)(qbtz). Each of the two independent copper atoms, Cu1 and Cu2, is coordinated by two N atoms of a qbtz ligand and by a N atom and a S atom of two different bridging SCN groups. Both CuN3S coordination figures are distinctly distorted tetrahedral showing notable differences between Cu1 and Cu2 in bond lengths and bond angles as well. Main difference is that the bond angle SCuNSCN for Cu1 is 99.20°, whereas for Cu2 it is 121.63° (compare also N5S4 = 3.229 Å with N6S3i = 3.676 Å, cf. Fig. 3). The CuNqbtz mean bond length of 3 is 2.097 Å, comparable to the corresponding value of <CuNqbtz> = 2.089 Å for 1, but shorter than <CuNqbtz> = 2.142 Å for 2. The mean bond distance <CuNSCN> = 1.924 Å is close to the <CuN,C> bond distance for tetrahedral Cu in 2. CuS bond distances are in agreement with those reported for related complexes [27]. In the crystal lattice, complex 3 forms continuous zigzag chains along [ı0ī] with π–π stacking interactions between the qbtz fragments of adjacent chains (Fig. 5). The π–π stacking parameters are listed in Table 3. Such zigzag chains are present also in the crystal structure of the 2,2′-biquinoline analogue of compound 3, [Cu(biq)(μ-SCN)] [40], but there is only one kind of Cu that lies together with SCN on a crystallographic mirror plane bisecting the chelating biquinoline ligand (orthorhombic space group Pnma). The bond lengths in [Cu(biq)(μ-SCN)] [40], CuNbiq = 2.107 Å, CuNSCN = 2.931 Å, and CuSSCN = 2.286 Å, are similar to 3.
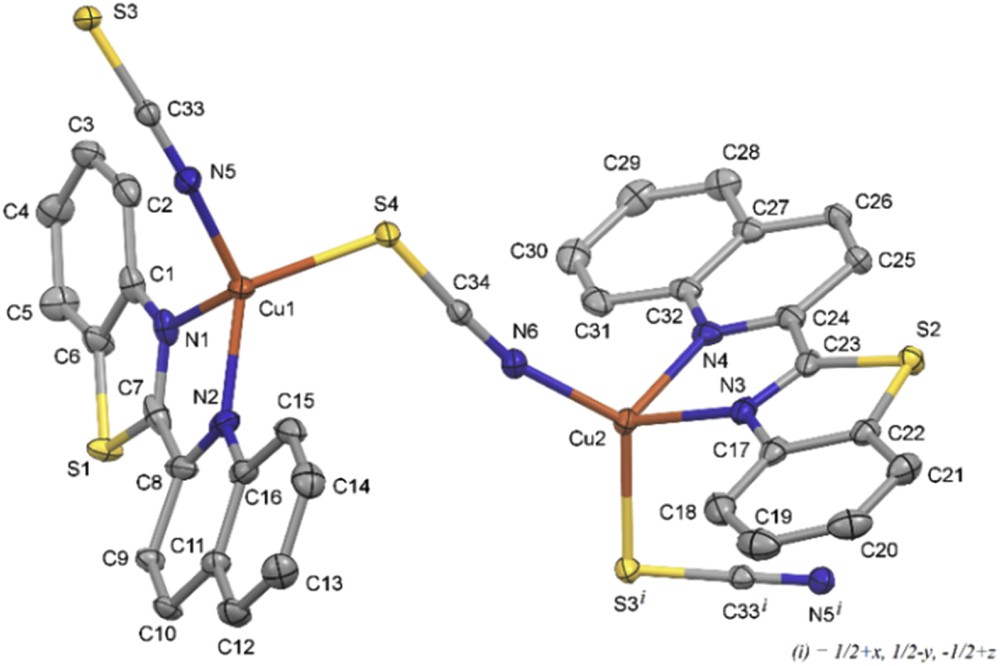
View of the structure of [Cu(qbtz)(μ-SCN)] with 50% probability ellipsoids. Hydrogen atoms and disordered qbtz sites are omitted for clarity. The zigzag chain of this structure continues with Cu atoms attached to S3 and N5i.
Selected bond lengths (Å) and angles (°) for 3.
| Bond lengths | |||
| Cu1N5 | 1.9377(15) | Cu2N6 | 1.9111(15) |
| Cu1N2 | 2.0463(15) | Cu2N3 | 2.0759(15) |
| Cu1N1 | 2.1298(15) | Cu2N4 | 2.1357(15) |
| Cu1S4 | 2.2910(5) | Cu2S3i | 2.2945(5) |
| S3C33 | 1.658(2) | S4C34 | 1.649(2) |
| C33N5 | 1.160(2) | C34N6 | 1.155(2) |
| Bond angles | |||
| N1Cu1N2 | 79.28(7) | N3Cu2N4 | 78.62(6) |
| N1Cu1N5 | 110.31(7) | N3Cu2N6 | 120.67(6) |
| N1Cu1S4 | 115.56(4) | N3Cu2S3i | 103.71(4) |
| N2Cu1N5 | 132.78(6) | N4Cu2N6 | 111.13(6) |
| N2Cu1S4 | 118.58(5) | N4Cu2S3i | 113.32(4) |
| N5Cu1S4 | 99.20(5) | N6Cu2S3i | 121.63(5) |
| Cu1N5C33 | 161.76(16) | Cu2N6C34 | 164.78(15) |
| Cu1S4C34 | 113.81(6) | Cu2S3iC33i | 94.17(6) |
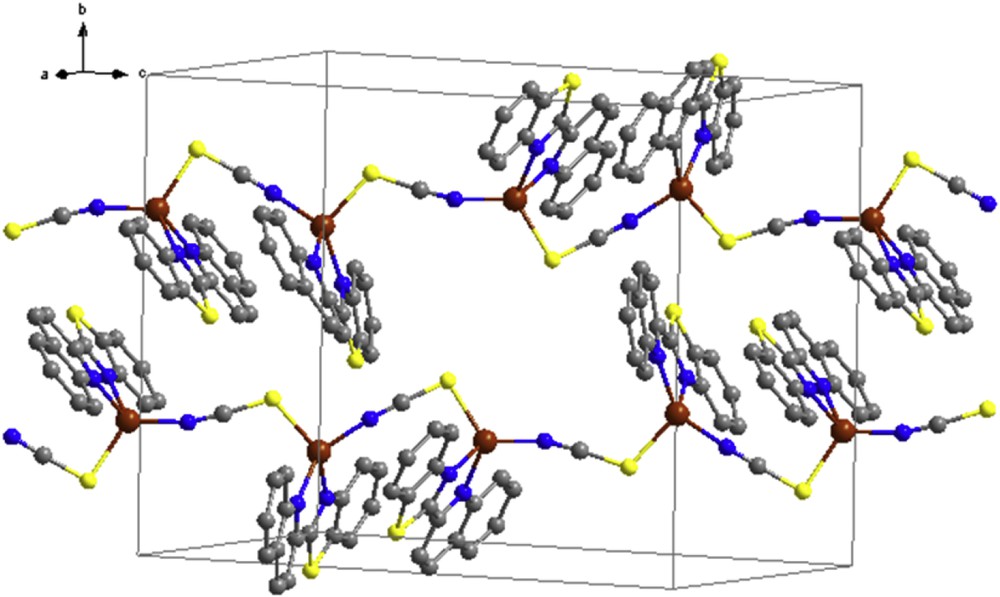
Crystal packing of the [Cu(qbtz)(μ-SCN)] complex.
3.4 Spectral characterization
The FT-IR spectral data of the three complexes of copper are listed in Section 2. The IR spectrum of the free ligand shows two bands at 1558 and 1593 cm−1 characteristic of the CNquinoline and CNbenzothiazole [27]. In the spectra of complexes the ν(CN) is generally shifted to lower frequencies relative to the free ligand, indicating a decrease in the CN bond order because of back bonding from the metal center to the π∗ orbital of the ligand and increasing the resonance in the planar coordinated benzothiazole. The CNquinoline and CNbenzothiazole stretching vibrations appear at 1508 and 1587 cm−1 for complex 1, 1509 and 1590 cm−1 for complex 2, and 1508 and 1588 cm−1 for complex 3. The strong absorption band at 2104 cm−1 in complex 2 is assigned to the coordinated cyanido ligand [41]. The strong absorption band at 2094 cm−1 in complex 3 is assigned to the thiocyanato CN stretching frequencies indicating the coordination of SCN− anion with a 1,3-μ-SCN bridging modes [41].
The UV–vis spectra of these compounds were recorded in DMF solution in the 200–800 nm region and the data are presented in Section 2. The electronic absorption spectra of complexes 1–3 show intense bands in the 260–441 nm region, indicating the presence of intraligand (π→π*) and charge transfer transitions. Copper(I) with d10 configuration is diamagnetic and no d-d electronic transition is expected for its complexes.
3.5 Electrochemical studies
The electrochemical behavior of the qtbz ligand and copper complexes has been studied by cyclic voltammetry in DMF solution at a scan rate of 100 mVs−1, with 0.1 M [N(n-Bu)4]PF6 as the supporting electrolyte at a glassy carbon working electrode. The approximate concentrations of the compounds were 5 × 10−4 M. Ferrocene (Fc) was used as the internal standard, and all redox potentials are referenced to the Fc+/0 couple [29].
The voltammogram of [Cu(qbtz)(μ-I)]2 in DMF solution is shown in Fig. 6. The anodic wave at 0.391 V and the corresponding cathodic wave observed at 0.114 V (ΔE = 277 mV) are attributed to the CuI/II couple undergoing an irreversible redox process and is in agreement with the values reported for related Cu(I) complexes [42].
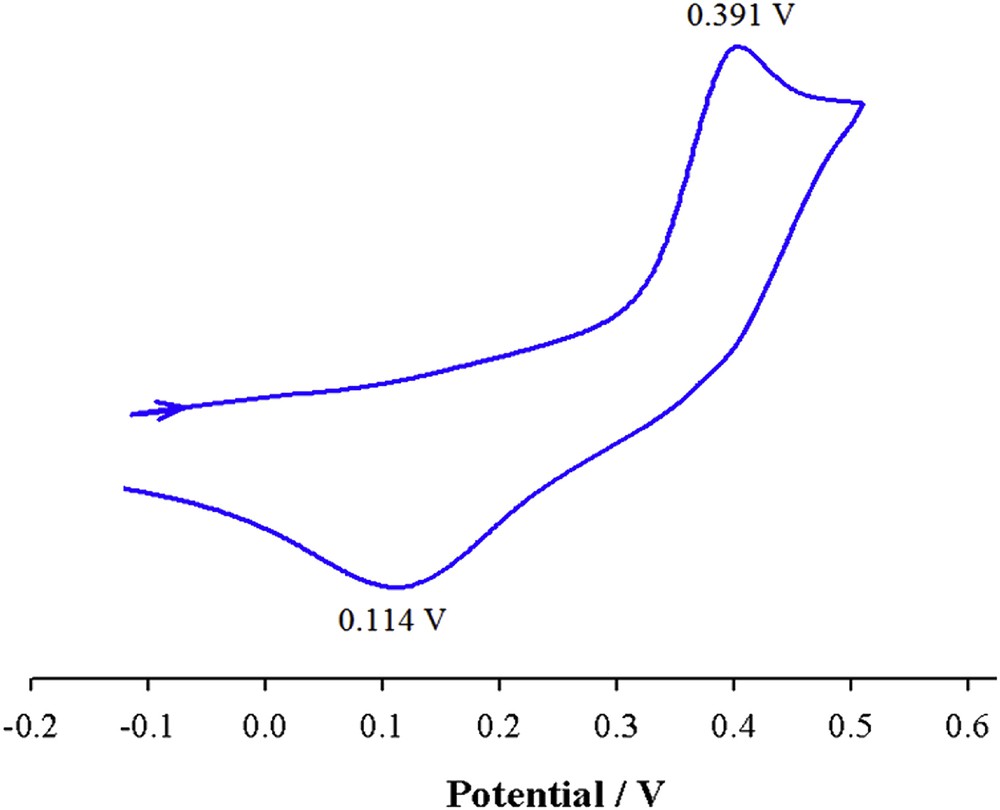
Cyclic voltammogram of [Cu(qbtz)(μ-I)]2 in DMF solution at 298 K (scan rate = 100 mV s−1, c = 5 × 10−4 M).
The cyclic voltammogram of [Cu3(qbtz)2(μ-CN)3] in DMF solution (Fig. 7) shows anodic waves at 0.512 and 0.562 V and the corresponding cathodic waves at 0.325 and 0.253 V and are attributed to the CuI/II irreversible redox processes (ΔE = 187 and 309 mV). These observations correlate well with the existence of two types of copper centers in this complex.
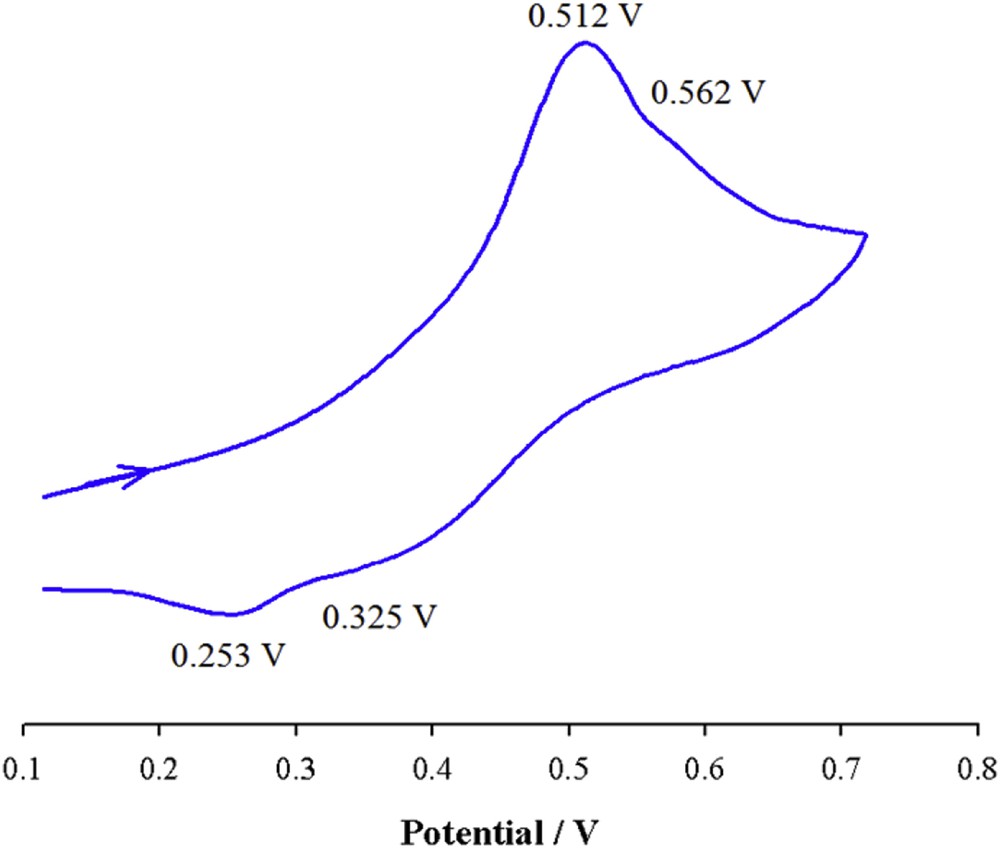
Cyclic voltammogram of [Cu3(qbtz)2(μ-CN)3] in DMF solution at 298 K (scan rate = 100 mV s−1, c = 5 × 10−4 M).
The voltammogram of [Cu(qbtz)(μ-SCN)] is shown in Fig. 8. The anodic wave observed at 0.410 and the corresponding cathodic wave at 0.223 V (ΔE = 187 mV) are attributed to the irreversible redox processes of CuI/II couple, which is in agreement with similar reports on Cu(I) complexes [43].
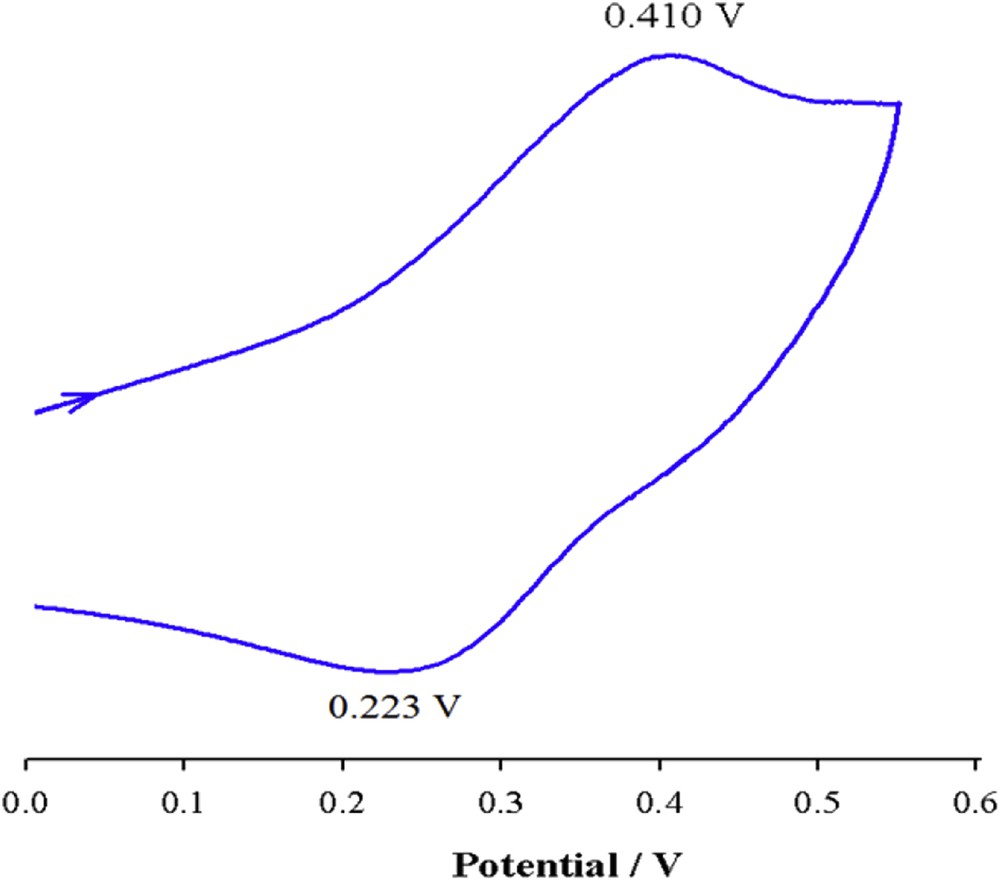
Cyclic voltammogram of [Cu(qbtz)(μ-SCN)] in DMF at 298 K (scan rate = 100 mV s−1, c = 5 × 10−4 M).
The oxidation potential of CuI/II, Epa, in [Cu3(qbtz)2(μ-CN)3] is more positive than those of 1 and 3 complexes. This is presumably because of the stronger π-acceptor character of the CN− relative to I− and SCN− ligands in the corresponding copper complexes (I− < SCN− < CN−), which make the central metal ion harder to oxidize. The dissociation of the halide or pseudohalide ligands leading to the monomeric solvent-coordinated species may be the source of weak reduction peaks that overlap with the CuI/II redox process.
4 Conclusions
This article describes the synthesis of three new multinuclear copper complexes with the bidentate ligand 2-(2-quinolyl)benzothiazole and the ancillary ligands I−, CN−, and SCN−. Fully characterized, the single crystal X-ray structure analysis of these complexes shows that complex 1 is a dinuclear neutral molecular compound consisting of centrosymmetric Cu2(μ-I)2 units. For cyanide, the synthesis generated Cu3(CN)3(qbtz)2. Two of the three crystallographically independent Cu atoms feature tetrahedral four coordination and the third copper atom features a quasi-linear two coordination by two CN groups. This complex exhibits a one-dimensional chain structure. In complex 3 with a zigzag chain structure, the copper ion adopts a distorted tetrahedral coordination with two N atoms of the qbtz ligand and two end-on bridging thiocyanato ligands. These results indicate that the ancillary ligands contribute substantially to the structural diversity of the complexes. The CuI/CuII redox process is also governed by the nature of the ancillary ligands and their modes of coordination.
Acknowledgments
Partial support of this work by the Isfahan University of Technology Research Council is gratefully acknowledged. The X-ray center of the Vienna University of Technology is acknowledged for providing access to the single-crystal diffractometer.
Appendix A Supplementary data
The following is the supplementary data related to this article:
Crystallographic data for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 1430253 for (1), No. 1430254 for (2), and No. 1430255 for (3). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.


