1 Introduction
Alkaloids are versatile natural products with various bioactivities, among which isatin (indole-2,3-dione, a kind of endogenous indole alkaloid) present in mammalian tissues and fluids has drawn much attention from the fields of chemistry and biology [1]. It has been identified as a selective inhibitor of monoamine oxidase B [2] and is an interesting compound involved in nerve protection. Besides, isatin derivatives have been reported with various activities [3–5]. The 3-hydroxyindole moiety has been found in many alkaloid natural products, such as welwitindolinone C, 3-hydroxyglucoisatisin, dioxibrassinine, and donaxaridine [6], and it has also been found in many biologically active molecules such as convolutamydines [7] and convolutamydines A and B, which show potent antitumor activity against HL-60 cell line. It is an important structural motif in proteasome inhibitors [8]. With an active carboxyl group, isatin can easily transform to 3-hydroxyindole by an addition reaction [5,9]. We have reported the synthesis and bioactivity of several kinds of isatin derivatives [5,10,11]; to explore the bioactivity of the derivatives, a series of new compounds, 3-ethyl-3-hydroxy-indole-2-ones, were synthesized by an addition reaction, and then we studied the cytoprotectivity of H2O2-induced apoptosis of PC12 cells and the antitumor activity against A549 and P388 cell lines.
2 Experimental section
2.1 Materials
The agents and solvents of analytical reagent grade were purchased from Xi'an Chemical Co, Ltd and were used without any further purification. NMR characterization was performed using a Bruker DRX-400 spectrometer, 400 MHz for 1H and 100 MHz for 13C. Mass spectra were recorded using a Micromass Platform spectrometer with electron impact mode at 75 eV.
2.2 Synthesis of 3-ethyl-3-hydroxy-indole-2-ones
The addition reaction of Et2Zn and common aldehyde ketone has been used for long time, but it was used for the first time in the addition of N-substituted isatin and Et2Zn. In this study, 1 mmol Et2Zn solution and 10 mL toluene were added in a dried flask protected under nitrogen atmosphere. The flask was placed into an ice-water bath cooled to 0 °C. Then 1 mmol N-substituted isatin was added to the flask, and the resulting solution was stirred at 0 °C for 4 h. After the mixture was warmed to room temperature, the reaction was quenched by adding a 10 mL saturated NH4Cl aqueous solution. The aqueous layer was extracted with AcOEt (3 × 10 mL). The AcOEt solution was dried over anhydrous Na2SO4, and concentrated by vacuum. The target product was obtained by purification with silica gel flash column chromatography (hexane:AcOEt = 10:1 to 4:1). The reaction is summarized in Scheme 1.
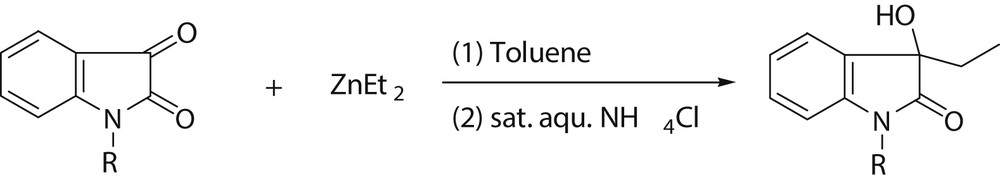
The synthesis of 3-ethyl-3-hydroxy-indole-2-ones.
2.3 Biological activity screening
The synthesized compounds were screened for their biological activities on protective activity of H2O2-induced apoptosis of PC12 cells and antitumor activity against A549 and P388 cell lines by the reported methods [5,12].
3 Results and discussion
3.1 Chemistry
The structure, yield, NMR, and MS data of the synthesized 3-ethyl-3-hydroxy-indole-2-ones compounds in this study are summarized in Table 1. From the results, it was found that all the reactions provide high yields except the reaction of Et2Zn and N-2-oxo-2-ethoxy-ethyl isatin, which gave a yield of 65%. The reason may be due to the carboxyl group on the N-substitutent, which can react with Et2Zn.
The structure, yield, NMR, and MS of the synthesized 3-ethyl-3-hydroxy-indole-2-ones.
| Compound structure and the number | Yield (%) | NMR and MS |
| 89 | 1H NMR (CDCl3, 400 MHz), δ: 7.38 (1H, d, J = 7.6 Hz), 7.34 (1H, t, J = 7.6 Hz), 7.11 (1H, t, J = 7.6 Hz), 6.85 (1H, d, J = 7.6 Hz), 3.20 (3H, s), 3.04 (1H, s), 1.99–2.07 (1H, m), 0.75 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.3, 143.5, 129.7, 129.5, 128.7, 123.8, 123.1, 108.3, 31.4, 26.1, 7.5; MS (ESI) m/z: 214 (M+) | |
| 92 | 1H NMR (CDCl3, 400 MHz), δ: 7.39 (1H, d, J = 7.2 Hz), 7.32 (1H, t, J = 7.6 Hz), 7.10 (1H, t, J = 7.6 Hz), 6.86 (1H, d, J = 7.6 Hz), 3.75–3.85 (1H, m), 3.63–3.71 (1H, m), 3.45 (1H, br), 2.00–2.03 (2H, m), 1.26 (3H, t, J = 7.2 Hz) 0.72 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.2, 143.6, 129.8, 129.5, 128.4, 124.0, 122.9, 108.4, 34.6, 31.7, 12.6, 7.6; MS (ESI) m/z: 228 (M+) | |
| 93 | 1H NMR (CDCl3, 400 MHz), δ: 7.44 (1H, d, J = 7.2 Hz), 7.32 (1H, t, J = 7.6 Hz), 7.10 (1H, t, J = 7.6 Hz), 6.85 (1H, d, J = 7.6 Hz), 3.76–3.80 (1H, m), 3.58–3.62 (1H, m), 3.21 (1H, br), 2.00–2.04 (2H, m), 1.70–1.73 (2H, m), 1.32–1.38 (2H, m), 0.91 (3H, t, J = 7.2 Hz), 0.73 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.1, 143.3, 129.7, 129.0, 123.9, 122.8, 108.5, 39.8, 31.6, 26.9, 22.4, 13.1, 7.6; MS (ESI) m/z: 256 (M+) | |
| 88 | 1H NMR (CDCl3, 400 MHz), δ: 7.38 (1H, d, J = 7.6 Hz), 7.32 (1H, t, J = 7.6 Hz), 7.10 (1H, t, J = 7.6 Hz), 6.86 (1H, d, J = 8.0 Hz), 3.72–3.80 (1H, m), 3.57–3.65 (1H, m), 3.13 (1H, s), 1.90–2.18 (2H, m), 1.60–1.66 (2H, m), 1.34–1.45 (4H, m), 0.95 (3H, t, J = 7.6 Hz), 0.73 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.1, 143.1, 129.9, 129.5, 123.9, 122.8, 108.6, 40.0, 31.7, 31.4, 27.3, 26.6, 22.5, 13.9, 7.6; MS (ESI) m/z: 284 (M+) | |
| 85 | 1H NMR (CDCl3, 400 MHz), δ: 7.40 (1H, d, J = 7.6 Hz), 7.31 (1H, td, J = 7.6, 1.2 Hz), 7.10 (1H, t, J = 7.6 Hz), 6.84 (1H, d, J = 8.0 Hz), 5.79–5.85 (1H, m), 5.20–5.26 (2H, m), 4.25 (1H, dd, J = 15.6, 7.6 Hz), 4.20 (1H, dd, J = 15.6, 7.6 Hz), 3.11 (1H, s), 2.03 (2H, q, J = 7.6 Hz), 0.76 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.1, 142.8, 131.2, 129.7, 129.5, 123.9, 123.1, 117.7, 109.3, 42.3, 31.7, 7.7; MS (ESI) m/z: 240 (M + Na+) | |
| 65 | 1H NMR (CDCl3, 400 MHz), δ: 7.39 (1H, d, J = 7.6 Hz), 7.31 (1H, t, J = 7.16 Hz), 7.13 (1H, t, J = 7.6 Hz), 6.72 (1H, d, J = 7.6 Hz), 4.62 (1H, d, J = 17.6 Hz), 4.18–4.27 (3H, m), 3.0 (1H, s), 2.04 (2H, q, J = 7.2 Hz), 1.25 (3H, t, J = 7.2 Hz), 0.77 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.1, 167.5, 142.2, 129.6, 129.5, 124.0, 123.4, 108.3, 61.8, 41.3, 31.7, 14.1, 7.5; MS (ESI) m/z: 286 (M + Na+) | |
| 87 | 1H NMR (CDCl3, 400 MHz), δ: 7.39 (1H, d, J = 7.6 Hz), 7.19–7.30 (6H, m), 7.07 (1H, d, J = 8.0 Hz), 5.02 (1H, d, J = 15.6 Hz), 4.76 (1H, d, J = 15.6 Hz), 3.06 (1H, s), 2.03–3.01 (2H, m), 0.79 (3H, t, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz), δ: 178.4, 142.7, 129.7, 129.5, 128.8, 128.6, 127.7, 127.6, 126.7, 123.9, 123.1, 109.4, 43.8, 31.7, 7.7; MS (ESI) m/z: 290 (M+) |
Nakazaki et al. [13] have reported the reaction between alkyllithium and N-phenyl isatin catalyzed by LiBr with a yield of 68–98%. It should be noted that the reaction was carried out at −78 °C, and use of cosolvents was required. Kumar et al. [14] investigated the reaction between trialkylaluminum without any catalyst at 70 °C and found the reaction is very rapid and yields are high. But more trialkylaluminum was needed than the reaction molar ratio, isatin:organoaluminum = 1:2.
The mechanism is described in Scheme 2. In this reaction, the Zn2+ coordinates with the O atom of the carboxyl group in isatin, by which the carboxyl group is activated. Then the CH3CH2− attacks the C atom of the activated carboxyl group, by a classical addition reaction process, and a zinc salt intermediate is formed accordingly. Through a similar process, the zinc salt activates another molecule of isatin and initiates another addition reaction, which results in a bi-oxindole zinc salt. Finally, the target product, 3-ethyl-3-hydroxy-indole-2-one, was produced by the hydroxylation process in a saturated aqueous solution of NH4Cl.
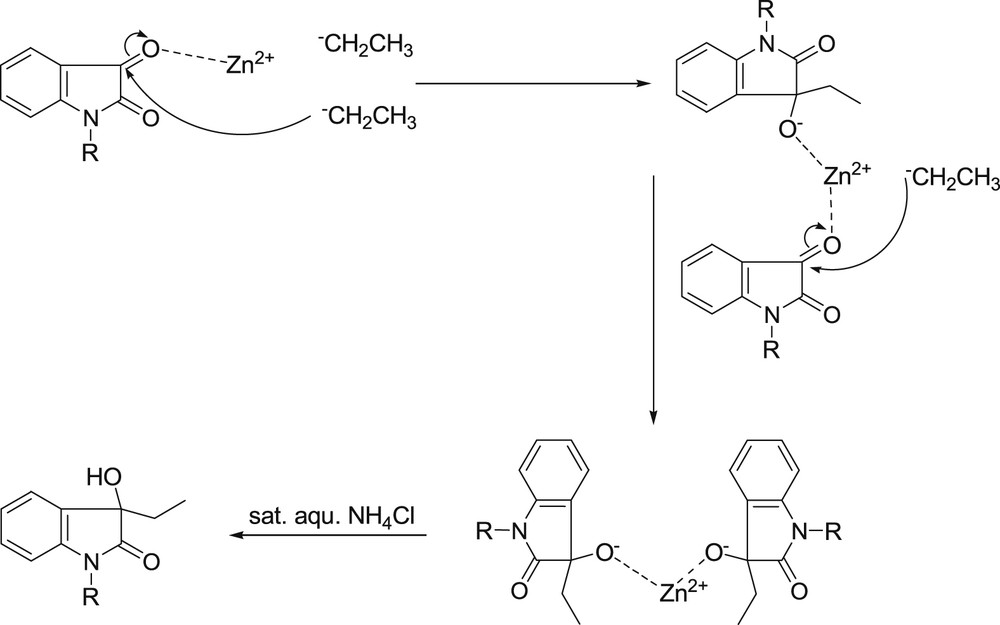
The reaction between Et2Zn and N-substituted isatin.
3.2 Biological activity
PC12 cell line derives from rat adrenal medulla, which has been used to get detailed information about diseases related to brain, such as hypoxia and Parkinson's disease. In our study, PC12 cell has been used to evaluate the neuroprotection activity of oxindole compounds [11]. In this article, the neuroprotection of the 3-ethyl-3-hydroxy-indole-2-ones in vitro screening results was evaluated, and the results are shown in Table 2. From the result, it was found that compounds DNO-2, DNO-3, and DNO-5 show some protection activity on the H2O2-induced apoptosis of PC12 cells with the higher activity than that of VE ((±)-α-tocopherol), with the percentage of 34.3%, 32.2%, and 28.8% at 2 μM, respectively. Also they are almost noncytotoxic against the PC12 cell at the concentration less than 2–20 μM, whereas DNO-1, DNO-2, DNO-6, and DNO-7 show high cytotoxicity at 200 μM. Concerning the structure of these compounds, the 3-ethyl-3-hydroxy-indole-2-ones with N-alkyl groups show high neuroprotection activity, and they are not cytotoxic against PC12 cell at the concentration less than 2–20 μM. The unsaturated CC bond in the N-substituent group can enhance the protection activity, whereas the ester group and phenyl group can decrease the activity and lead to high cytotoxicity against PC12 cell.
Inhibitory and protective effects of 3-ethyl-3-hydroxy-indole-2-ones on PC12 cell.
| Compound | Inhibitory effecta (%) | Protective effectb (%) | ||||
| 200 μM | 20 μM | 2 μM | 200 μM | 20 μM | 2 μM | |
| DBO-1 | 23.6 | 0 | 0 | – | 25.1 | 15.4 |
| DBO-2 | 33.9 | 0 | 0 | – | 36.5 | 34.3 |
| DBO-3 | 0 | 0 | 0 | 0 | 37.7 | 32.2 |
| DBO-4 | 0 | 0 | 0 | 49.0 | 8.4 | 2.7 |
| DBO-5 | 0 | 0 | 5.7 | 0 | 53.9 | 28.8 |
| DBO-6 | 37.6 | 16.5 | 9.3 | – | 0 | 1.8 |
| DBO-7 | 84.4 | 11.9 | 5.9 | – | 0 | 11.9 |
| VE | 0.0 | 22.5 |
a Inhibition against PC12 cell growth.
b Protective effect on the H2O2-induced apoptosis of PC12 cells.
Many isatin derivatives have been reported displaying high antitumor activity [15,16]. In this article, the antitumor activity of these compounds against A549 and P388 cell lines was screened, and the results are summarized in Table 3. From this table, we can find that almost all compounds show inhibition against both cancer cells at the concentration of 100 μM. DBO-3, DBO-4, DBO-5, DBO-6, and DBO-7 show high activity against A459 cell line, with the inhibition percentage more than 50% at 100 μM. Concerning the structures of these compounds, the long chains contribute to the high activity against the A459 cell line, and the highest activity of DNO-6 may be due to the ester group in the N-substituent group.
In vitro cytotoxicity against A549 and P388 cell lines.
| Compound | P388 (%) | A459 (%) | ||
| 100 μM | 10 μM | 100 μM | 10 μM | |
| DBO-1 | 38.9 | 20.5 | 40.5 | 8.2 |
| DBO-2 | 36.8 | 19.0 | 47.3 | 9.8 |
| DBO-3 | 32.6 | 16.7 | 55.8 | 13.9 |
| DBO-4 | 20.0 | 6.7 | 64.1 | 14.1 |
| DBO-5 | 13.6 | 13.7 | 62.0 | 14.4 |
| DBO-6 | 35.4 | 17.1 | 70.2 | 12.6 |
| DBO-7 | 22.8 | 21.3 | 54.8 | 9.5 |
4 Conclusions
A series of 3-ethyl-3-hydroxy-indole-2-ones were synthesized through the autocatalytic addition reaction of Et2Zn and N-substituted isatin and characterized by NMR and MS. The antitumor and neuroprotection activities of these compounds were evaluated and showed protection activity on the apoptosis of PC12 cells induced by H2O2, which are more effective than that of (±)-α-tocopherol Vitamin E (VE) at 2 and 20 μM. Moreover, these compounds also showed antitumor activity against A549 and P388 cell lines.
Acknowledgments
This research was financially supported by the grants from National Science Foundation of China (50874092) and Scientific Research Program Funded by Shaanxi Provincial Education Department (16JS094). The authors would like to thank Professor Allan Prior, Daniel K. Inouye College of Pharmacy, University of Hawaii at Hilo, for the language revision.


