1 Introduction
Frankincense is a resin derived from Boswellia genus. The gum is obtained by scraping the bark and/or making deep longitudinal incisions 4–8 cm long with a scalpel-like tool called a mengaff. The milky juice, left on the tree to dry, hardens on exposure to air into globular, pear-, or club-shaped pale yellow or pale amber gum tears [1]. The resin lumps are composed of essential oils (EOs; 5–9%), alcohol-soluble resins (65–85%), and the remaining water-soluble gums [2].
The ancient Egyptians used frankincense as a fumigant and in the embalming process [3]. The oils are currently used as a major ingredient in incense formulations, which are burned in Jewish, Roman Catholic, and Greek Orthodox religious rituals and ceremonies. The myriad of pharmacological properties attributed to frankincense includes its use in the treatment of asthma [4], rheumatoid arthritis [5], Crohn's disease [6], osteoarthritis [7]. Frankincense is used in perfumery and aromatherapy. It is also an ingredient sometimes used in skincare.
Boswellia products come in the form of resins, extracts, and EOs. The EO, which has a woody, spicy, and haunting smell, is usually obtained through steam distillation or hydrodistillation (HD) [8]. More than 300 volatiles in frankincense have been reported in the literature. A broad diversity has been found in the qualitative and quantitative composition of the volatiles with respect to different varieties of Boswellia [1,9]. Also, the abundance of EO is highly dependent on distillation process parameters (time and temperature) [9]. For instance, typical duration of distillation may vary between 6 h and up to 12 h [10]. Besides being an energy consumption technique, key labile constituents could be lost during this process at elevated temperatures for prolonged periods, whereas other compounds might be formed as artifacts.
The current environmental trends are to reduce energy and limit the carbon footprint. Novel techniques such as microwave (MW) HD are developed as an alternative to conventional processes for EO extraction [11]. This technique brings a number of advantages to EO extraction, thanks to their reduced equipment size, ability to control a process via mild increments in heating, all of which contribute to reducing qualitative degradation and an environmental impact. Many aromatic plants and spices such as Xylopia aromatica (Lamarck), Lippia alba (Mill.), Zataria multiflora Boiss., and so forth were submitted to this process for EO extraction [12–14]. For instance, Golmakani and Rezaei [15] found that microwave-assisted distillation (MAD) was superior in terms of saving energy and extraction time (3.2 times) than HD for the recovery of EOs from Thymus vulgaris L.
The literature analysis clearly shows a considerable effort to analyze the variability in the composition of Frankincense EOs related to the varietal factors. However, to the best of our knowledge, there is no study that has investigated the impact of the extraction process on the qualitative and quantitative aspects of EO extraction. The aim of this study was to compare and optimize the extraction kinetics, energy consumption, and profitability of the conventional and novel extraction techniques. Special attention will be given to the composition and antioxidant activity of Frankincense EO.
2 Material and methods
2.1 Raw material
Boswellia serrata oleo gum resin used in this study is presented in the form of amber to orange-brown lumps. They were purchased from Salus in Erbis (Rome, Italy) in 200 g bags and were stored at room temperature in a box protected from light.
2.1.1 Solvent for GC–MS analysis
Solvent and reagent for DPPH test: DPPH (1,1-diphényl-2-picrylhydrazil) was purchased from Sigma–Aldrich. Given the contradictory results about a possible antioxidant activity of B. serrata EO, natural lavender EO (Sigma–Aldrich), which has known antioxidant activity, has been used as a reference.
2.2 Extraction procedure
The experimental procedure is described in Fig. 1. Oleo gum resin lumps bags were assembled in one lot. The size of the lumps was reduced using an IKA MF10 basic microfine grinder (IKA, Germany) at 3000 rpm and a 4 mm sieve. The obtained powder was sieved using an RETSCH AS 200 sieve shaker set to a 2 min cycle. Only the fractions between 1 and 2 mm (94% of the sieved powder) were used for the essential extraction step.

Scheme of the experimental setup and the quantitative and qualitative analyses of the extraction of Frankincense EO.
B. serrata EO was recovered using two extraction processes: HD and MAD). HD device was equipped with a heater (Electrothermal, UK). The oleo gum resin powder (mRM = 40 g) was introduced into a 1 L flask. Distilled water (solid/liquid ratio, 1:5 w/w) was added to the resin. The flask was connected to a Clevenger (Milestone, Italy) and a spiral double-jacketed condenser to ensure the condensation of the heated water/EO mixture. The same glassware was used for the MAD extraction process. Heating in the case of MAD extraction was provided by a Milestone ETHOS X oven (Milestone, Italy) equipped with two 950 W magnetrons and an infrared temperature sensor. The system additionally uses a rotating diffuser that evenly distributes the MWs throughout the cavity. Applied power, cycle time, and temperature limit were controlled via ETHOS X compact terminal.
The cooling system was a Smart H150-2100 chiller (LabTech, MA). The temperature and pressure of cooling water were set to 8 °C and 2.3 bar, respectively. The nominal power (PN,condensation) of the chiller was 600 W. The condenser was connected to the chiller that ensures the cooling water at a constant temperature of 8 °C.
The extraction cycle was set to 180 min for both processes. MAD was tested at two power densities: 1 and 2 W/g of solid/liquid mixture. All electric devices were connected to wattmeters, so it was possible to record and compare the energy consumption of each extraction process as a function of time. During the experiments, an approximate volume between 8 and 10 mL of distilled water and EO mixture were collected in small tubes and the time of the volume recovery was recorded. This procedure is repeated throughout the extraction cycle. Then the volume of EO was measured and extraction kinetics comparison was made. All EO samples were dried with anhydrous sodium sulfate and were stored in amber glass tubes for chemical analysis.
2.3 Chemical analysis
2.3.1 GC–MS analysis of EO
GC–MS analyses were carried out using a Thermo Scientific Focus gas chromatographic system with a Thermo Scientific Al 3000 auto-injector, coupled with an ITQ 700 Series GC-Ion Trap Mass Spectrometer (Thermo Fisher Scientific). GC separation was performed on a fused silica capillary column TG-5MS (Thermo Fisher Scientific), stationary phase (5% diphenyl-95% dimethyl-polysiloxane phase).
A volume of 1 μL of each sample was injected in a splitting ratio of 1:100. Injector temperature was set at 250 °C. Molecular components were eluted using helium at a constant flow of 1 mL/min. The following temperature program was used: initial temperature 60 °C for 8 min, 60–250 °C at 4 °C/min.
Mass spectra were recorded in electron impact mode with an electron ionization voltage of 70 eV, an ionization time of 25,000 μs, and a mass range of 50–650 m/z. Ion trap and interface transfer line were, respectively, at 250 and 300 °C.
ThermoXcalibur 2.2 software (Thermo Fisher Scientific) was used for instrumental control and data acquisitions. Mass spectra peak assignment was based on a comparison with NIST database (NIST MS Search 2.0).
2.3.2 DPPH radical scavenging of EO
The radical scavenging activity of extract was evaluated by a modified version of the method, proposed by Brand-Williams et al. [16], converted into micromethod. Each extract was diluted in pure methanol to prepare samples ranging from 20 to 0.625 μg/mL, and then 50 μL of each sample was pipetted into 96-well plates in triplicate and 50 μL of DPPH solution (0.5 mM in methanol) was added in each well. Plates were placed in the dark for 40 min at room temperature and then the absorbance was measured at 510 nm. The results were plotted as the percentage of remaining DPPH (%I DPPH) against the concentration (μg/mL) of the added samples (Eq. 1).
| (1) |
Results are expressed as inhibitory concentration (IC50), which corresponds to volume of EO, required to quench 50% of the initial DPPH radicals under the given experimental conditions.
2.4 Statistical analysis
Each extraction was repeated at least two times. Means and standard deviations were calculated. Means were compared using Analysis of Variance (ANOVA) followed by Least Significant Difference (LSD) testing at the P < 0.05 level to follow differences between treatments.
3 Results and discussion
3.1 Extraction kinetics of HD and MAD
A first experiment was carried out to determine the maximum quantity of EO in the oleo gum resin using HD process. In this experiment, the solid/liquid ratio was kept constant by recycling the water from the distillate mixture. The extraction was carried until the volume of recovered EO seemed to be stable. The quantity of EO (EOmax) under these conditions was 1.08 mL (density = 0.894). It represented only 2.4% of the oleo gum resin, which is lower than the yield commonly claimed (between 5% and 10%) [1].
The kinetics of B. serrata EO extraction indicate similar tendencies between both processes (Fig. 2). The lag time between the beginning of extraction and the recovery of the first droplets of EO dropped from 25.0 min for HD to 6.7 min for MAD 2 W/g. It seemed that no significant difference could be observed in the yield or in the kinetics between HD and MAD 1 W/g. When the power density of the MW process was doubled, the maximum yield was reached in 47 min suggesting a significant reduction in the extraction cycle by 3.76-folds compared to the HD process.
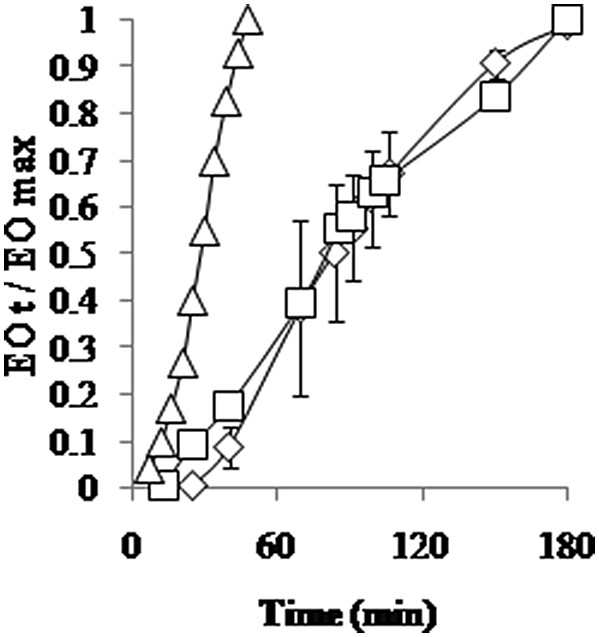
Evolution of B. serrata EO yield during an extraction cycle. The extraction yield is defined as the ratio of EO volume at time t (EOt) over the maximum volume in the resin (EOmax). (◊), HD; (□), MAD 1 W/g; and (Δ) MAD 2 W/g.
3.2 Energy consumption analysis
The electrical consumption of the various processes was monitored, and the value of energy consumption at the end of an extraction cycle is presented in Fig. 3. The energy efficiency of MW depends on the choice of process parameters. If we compare MAD and HD processes, we can notice that at 1 W/g, the treatment is not energy efficient. Although by increasing the power density to 2 W/g, the significant reduction in the extraction cycle time will naturally reduce energy consumption to the point of being more efficient than the reference process (−9.6%).
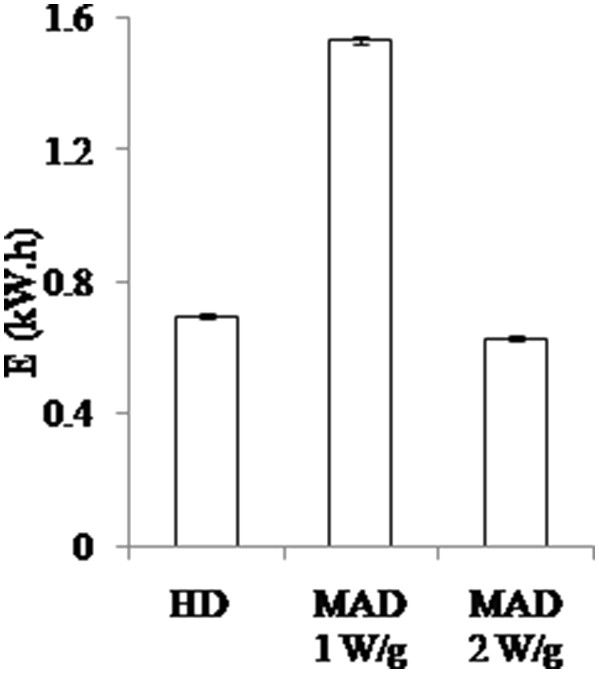
Total energy consumption (kW h) of an extraction cycle (180 min) for HD and MAD at different power densities (1 and 2 W/g).
It is worth noting that the power delivered by MW is constant throughout the extraction cycle, which is not the case of the heating mantle. Indeed, the current is cut as soon as the heating set point is reached. Therefore, these results are valid only in the context of this study and are not representative of other scales and with other heating modes.
3.3 Profitability analysis of HD and MAD
Another way to compare the efficiency of the extraction processes can also be described by a ratio of income over costs (Eq. 2). The income is determined by the product of the selling price (EOprice) and the recovered quantity (EOt) during an extraction cycle of the EO. In this research, in a purely theoretical comparison approach, only the direct costs were considered, specifically the cost of electrical energy and the purchase price of the raw material.
| (2) |
The balance between income and cost corresponds to a profitability value of 1 (Fig. 4). The results from Eq. 2 show that both processes are profitable as the values are higher than 1. MW process is clearly more profitable than HD. Notice that with this equation, it is also possible to determine an optimal cycle time of extraction. If we consider the profitability curve of the HD process, an optimum is reached at 110 min (2.28). Beyond this time, the profitability decreases to almost 2.0.
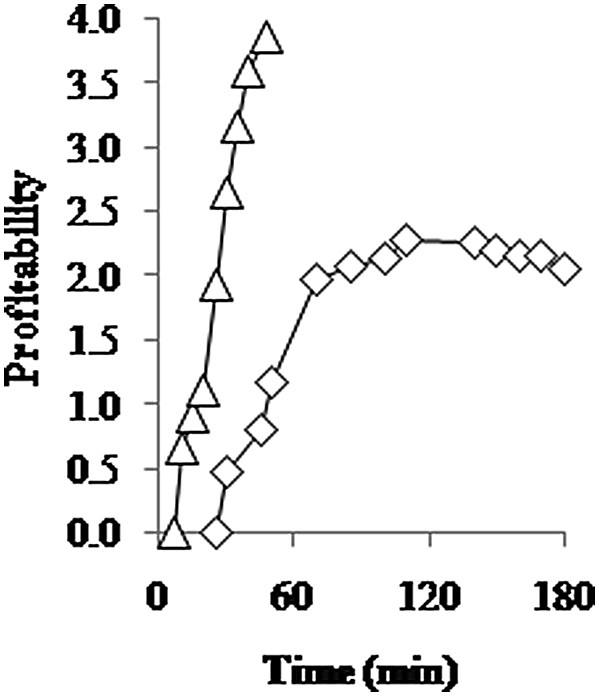
Profitability of HD (◊) and MAD 2 W/g (Δ) during an extraction cycle of B. serrata EO extraction.
3.4 Chemical and antioxidant analysis of EO
GC–MS chromatogram analysis revealed 53 compounds among which 38 were fully identified (Table 1). The mean contents of these compounds in the EO samples were 85.5%, 87.7%, and 91.8% for MAD 1 W/g, HD, and MAD 2 W/g, respectively. The chemical analysis of Frankincense EO showed that most of the identified molecules belong to monoterpene, oxygenated monoterpene, and sesquiterpene families. The main components were characterized as α-thujene, estragole, α-pinene, cembrene and in less proportions, o-cymene, 3-carene, β-bourbonene, and methyl eugenol. All these compounds were reported in the Indian olibanum obtained by steam distillation of B. serrata gum-oleoresin [17].
Chemical constituent of the EO of B. serrata recovered from HD and MAD at 1 W/g (MAD 1 W/g) and 2 W/g (MAD 2 W/g)
| Compound | HDa | MAD 1 W/ga | MAD 2 W/ga |
| α-Thujene | 17.2 | 23.9 | 17.6 |
| α-Pinene | 11.5 | 13.5 | 13.5 |
| Thuja-2,4(10)-diene | 0.2 | 0.4 | 0.1 |
| β-Pinene | 0.6 | 0.6 | 0.7 |
| α-Phellandrene | 0.1 | 0.2 | 0.1 |
| (E)-β-Ocimene | 0.2 | 0.2 | 0.3 |
| Terpinolene | 0.1 | 0.2 | 0.1 |
| o-Cymene | 4 | 4.4 | 2.1 |
| 3-Carene | 2.1 | 2.3 | 2 |
| γ-Terpinene | 0.1 | 0.2 | 0.1 |
| α-Thujone | 0.5 | 0.7 | 0.3 |
| Sabinyl acetate | 0.2 | 0.2 | 0.1 |
| (−)-Myrtenol | 0.3 | 0.3 | 0.1 |
| cis-Verbenol | 0.4 | 0.4 | 0.4 |
| cis-Sabinol | 0.5 | 0.6 | 0.2 |
| Thujen-2-one | 0.1 | 0.1 | 0.1 |
| trans-Sabinene hydrate | 0.4 | 0.4 | 0.2 |
| 2-Carene | 0.2 | 0.2 | 0.1 |
| Estragole | 17.3 | 11.6 | 17.6 |
| (−)-Verbenone | 0.2 | 0.2 | 0.1 |
| p-Cumic aldehyde | 0.1 | 0.1 | 0 |
| Bornyl acetate | 0.1 | 0.1 | 0.1 |
| Cyclosativene | 0.1 | 0.1 | 0.1 |
| α-Copaene | 0.7 | 0.6 | 0.6 |
| β-Bourbonene | 1.6 | 1.4 | 1.6 |
| Methyl eugenol | 2.9 | 2 | 2.8 |
| β-Ylangene | 0.2 | 0.2 | 0.2 |
| β-Copaene | 0.2 | 0.2 | 0.2 |
| α-Bergamotene | 0.3 | 0.2 | 0.3 |
| γ-Muurolene | 0.9 | 0.6 | 1.3 |
| α-Muurolene | 0.1 | 0.1 | 0.1 |
| δ-Guaiene | 3.3 | 2.7 | 2.5 |
| Guaia-1(10)11-diene | 0.2 | 0.2 | 0.2 |
| Elemicin | 1 | 0.8 | 0.9 |
| Cadinene | 0.4 | 0.3 | 0.3 |
| α-Gurjunene | 0.3 | 0.3 | 0.3 |
| Verticiol | 1.2 | 2.3 | 0.1 |
| Cembrene | 6.2 | 5.8 | 5 |
a The results are presented in percentage (%). Mean value (n = 4).
The deviation of proportion from the reference treatment (HD) of olibanum oil compounds obtained by MAD is represented in Fig. 5. It clearly shows the impact of the power density on the composition of the EO. Although MW treatment increased the proportions of the main components (α-thujene and α-pinene), the opposite tendency was observed for o-cymene, estragole, methyl eugenol, and δ-guaiene. In general, the same tendency was observed for both power densities except for cembrene. The same observations were made regarding the decrease in estragole proportions and the increase in α-pinene proportions in basil oil extracted by MW [18]. The results suggest that MW treatment enables to obtain EOs of different chemical compositions from reference extraction methods. Furthermore, the composition depends on the treatment parameters.
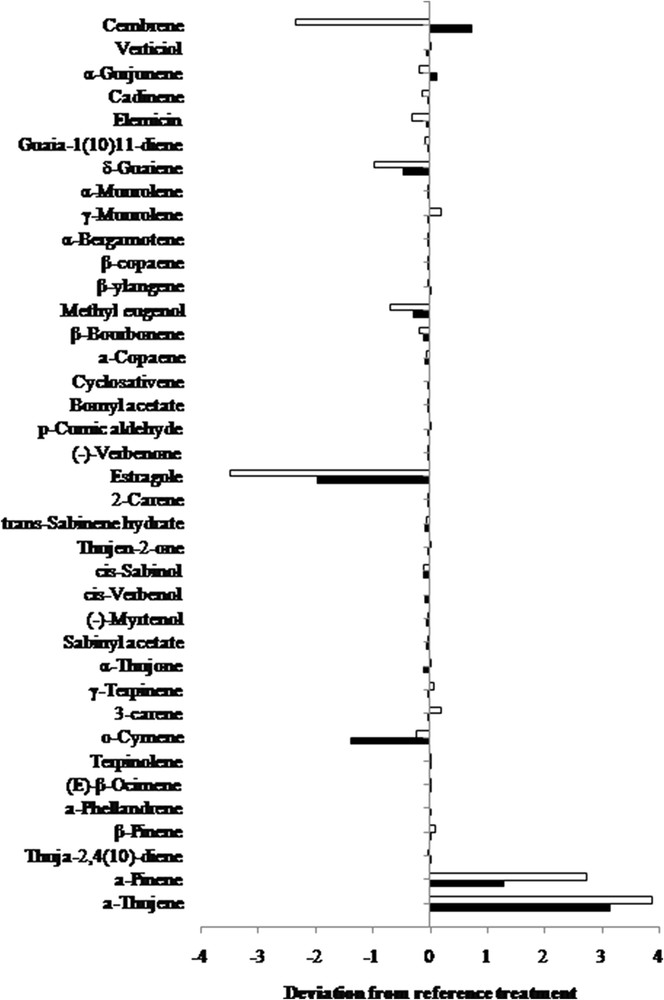
The variations in olibanum oil identified compounds, obtained by the subtraction of proportion of the reference (HD) from MAD at 1 W/g (black bars) and 2 W/g (white bars).
The EOs are analyzed for their antioxidant activity (DPPH test). Lavender EO with known antioxidant activity was used as a reference to detect antioxidant activity in Boswellia EO. The antioxidant activity is defined by the volume of EO in microliters required to inhibit 50% of the DPPH radical.
An EO that requires less volume to achieve 50% inhibition of the DPPH radical would have greater antioxidant potency. For instance, 5.2 μL of lavender EO is required to inhibit 50% of the DPPH radical. The same inhibition rate was achieved with only 3.9 μL of olibanum EO extracted with the reference method of HD suggesting a higher antioxidant potency for olibanum EO. A 26.5% decrease in the EO volume is noted for the MAD at 1 W/g compared to the HD. The opposite trend was noted when treatment at 2 W/g was used. Indeed, the volume required to reach 50% inhibition rate increased by 52.9% compared to the reference extraction, which suggests a possible degradation of the antioxidant activity linked to a thermal degradation of the molecules responsible for this activity due to the high-power heating.
4 Conclusions
MAD is an economically viable method for the extraction of olibanum EO. This technology has been shown to be kinetically advantageous (hence more productive), more energy efficient depending on the conditions of extraction.
The strength of this technology is mainly because the EO produced has a chemical composition different from that of conventional treatment. The results showed that the antioxidant activity of the EO could be improved depending on the treatment parameters of MW. Without being a formal sensory study, we could distinguish clear differences when the oils were smelled.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.


