1 Introduction
The selective oxidation of sulfides to sulfoxides is an important reaction in organic chemistry, because sulfoxides play an important role as versatile and synthetic intermediates for the production of various chemically and biologically active molecules, and also activation of enzymes [1–4]. Therefore, various oxidizing agents (e.g., metal oxidants, organic oxidant, halogens, peroxides, and air) have been proposed for this type of transformation [5–7], but some of these methods have limitations such as the use of stoichiometric toxic reagents, low atom efficiency, tedious work up of products, and the formation of large amounts of toxic waste [8]. Among oxidizing agents, hydrogen peroxide is the most attractive oxidant, because of its environmental implications, lower costs, and is readily available and produces water as the only byproduct [9–11], but major drawback of hydrogen peroxide is its poor oxidizing power; hence extensive studies have been undertaken to develop new catalysts (homogeneous and heterogeneous catalysts) for this reaction [12–14]. Although these methods have partly addressed problem of the stoichiometric reagents, some of these methods require the transition metal catalyst for the selective oxidation of sulfides, which have a number of disadvantages such as preparation of complex catalyst, using toxic transition metal compounds, and removing or recovery of the expensive catalyst and remaining metals in products [15–19].
In recent years, one of the main objectives of modernizations in catalytic processes is the development of new and efficient technologies to improve environmental conditions and industries related to fine chemicals. Porous solids are particularly attractive, because they were reported for potential applications such as catalysts, catalyst supports, and as adsorbents [20–23].
Zeolites are porous crystalline materials that are defined as aluminosilicates with discrete uniform particle with high internal surface area, small pore size, and flexible frameworks [24,25], which are useful catalysts in petroleum cracking, petrochemical industry, reforming processes, and chemical reactions [26–28]. Also, zeolites can have both Brønsted and Lewis acid sites and can be used as a solid acid to activate oxygenated molecules containing carbonyl and hydroxyl groups, that is noncorrosive, and as a stabile, low-cost, and commercially available reagent [29–31].
2 Experimental section
2.1 Materials and physical measurements
Sulfides, solvents (such as ethyl acetate and n-hexane), and H2O2 (30%) were purchased from Merck chemical company and used without further purification. H-ZSM5 is commercially available from Zeolyst International. Melting points were recorded using an Electrothermal 9100 apparatus. 1H NMR spectra were recorded using a Bruker 400 MHz spectrometer in CDCl3 as solvent. Chemical shifts are reported in ppm with Tetramethylsilane (TMS) as an internal standard.
2.2 General procedure for the oxidation of sulfides to sulfoxides using 30% H2O2 in the presence of H-ZSM5 as a catalyst
H-ZSM5 (12 mg) was added to a mixture of sulfide (1 mmol) and 30% H2O2 (2.4 equiv), then the mixture was stirred at room temperature under solvent-free conditions and the progress of the reaction was monitored by Thin-layer chromatography (TLC) (EtOAc/n-hexane, 1/2). After completion of the reaction, EtOAc (5 mL) was added, the catalyst was separated by filtration, and washed with additional EtOAc (5 mL). The organic layer was washed with brine (5 mL) and dried over anhydrous Na2SO4. Finally, the organic solvent was evaporated, and products were obtained in good to high yield. All the products are known and were characterized by comparing their spectral and physical data with those of authentic samples.
2.3 Selected spectra data
2.3.1 Methyl phenyl sulfoxide
1H NMR (400 MHz, CDCl3) δ = 7.70–7.62 (m, 2H), 759–7.48 (m, 3H), 2.72 (s, 3H) ppm; IR (KBr); 3030, 1488, 1451, 1296, 1128, 1029, 696 cm−1.
2.3.2 Didodecyl sulfoxide
1H NMR (400 MHz, CDCl3) δ = 2.75–261 (m, 4H), 1.85–1.75 (br, 4H), 1.45–1.29 (m, 36H), 0.91 (t, J = 4 Hz, 6H) ppm; IR (KBr); 2920, 2849, 1466, 1011.
3 Results and discussion
In continuation of our studies on environmentally benign chemical processes [32–34], we report our results about H-ZSM5 zeolite as a recyclable and effective heterogeneous catalyst for selective oxidation of sulfides to sulfoxides using 30% H2O2 (Scheme 1).

H-ZSM5 catalyzed the oxidation of sulfides to sulfoxides using H2O2.
To optimize the reaction conditions, the oxidation reaction of methyl phenyl sulfide to methyl phenyl sulfoxide was selected as a model reaction. The effect of different amounts of hydrogen peroxide and catalyst was investigated on the model reaction under solvent-free conditions at room temperature (Table 1). The oxidation of methyl phenyl sulfide to methyl phenyl sulfoxide was not completed using H2O2 (4.8 equiv) as an oxidant in the absence of H-ZSM5 as a catalyst even after 24 h (Table 1, entry 1). When 50 mg of H-ZSM5 catalyst and 4.8 equiv of 30% H2O2 were used both sulfoxide and sulfone were obtained with 65% and 35%, respectively, within 24 h (Table 1, entry 2). To control selectivity, the amounts of both catalyst and oxidant were reduced. When the amount of catalyst was reduced to 25 mg, sulfoxide was found as only product in 60 min (Table 1, entry 3). The same result was obtained by decreasing the amount of H2O2 to 2.4 equiv (Table 1, entry 4). After further investigation, 12 mg of H-ZSM5 catalyst and 2.4 equiv of 30% H2O2 under solvent-free conditions at room temperature was found to be ideal for the complete conversion of sulfide to sulfoxide (Table 1, entry 5).
Optimization of the reaction conditions with respect to the effect of the amount of H-ZSM5 and 30% H2O2 on the oxidation reaction of methyl phenyl sulfide.a
| Image 2 | ||||||
| Entry | H-ZSM5 (mg) | H2O2 (equiv) | Time | Conversion (%)b | Yield of sulfoxide (%) | Yield of sulfone (%) |
| 1 | – | 4.8 | 24 h | 80 | 70 | 0 |
| 2 | 50 | 4.8 | 24 h | 100 | 65 | 35 |
| 3 | 25 | 4.8 | 60 min | 100 | 98 | 0 |
| 4 | 25 | 2.4 | 60 min | 100 | 98 | 0 |
| 5 | 12 | 2.4 | 60 min | 100 | 98 | 0c |
a Reaction conditions unless stated otherwise: sulfide (1 mmol), solvent-free, rt.
b Conversion was determined by GC.
c The bold values represent the most effective reaction conditions.
To extend the general application of H-ZSM5 as a catalyst, oxidation reactions of various sulfides were studied. The sulfoxides were obtained in satisfactory yields in relatively short reaction times and the results are shown in Table 2. Interestingly, diphenyl sulfide as a model for the unreactive sulfides was also converted to the corresponding sulfoxide at room temperature (Table 2, entry 10). Also, to investigate the chemoselectivity of the described system, oxidation of 2-(methylthio)ethanol as a model reaction was examined and which alcoholic groups remained intact during the oxidation reactions (Table 2, entry 7).
Oxidation of sulfides to sulfoxides with 30% H2O2 in the presence of H-ZSM5 catalyst under optimized reaction conditions.a
| Image 3 | ||||||
| Entry | Sulfide | Product | Time (min) | Conversion (%) | Yield (%)b | MP (°C) [Reference] |
| 1 | Image 4 | Image 14 | 60 | 100 | 98 | Oil [35] |
| 2 | Image 5 | Image 15 | 73 | 100 | 99 | Oil [35] |
| 3 | Image 6 | Image 16 | 7 | 100 | 96 | Oil [36] |
| 4 | Image 7 | Image 17 | 304 | 100 | 89 | 114–115 [35] |
| 5 | Image 8 | Image 18 | 170 | 100 | 87 | 86–88 [38] |
| 6 | Image 9 | Image 19 | 10 | 100 | 94 | Oil [37] |
| 7 | Image 10 | Image 20 | 1 | 100 | 96 | Oil [36] |
| 8 | Image 11 | Image 21 | 65 | 100 | 82 | Oil [37] |
| 9 | Image 12 | Image 22 | 1 | 100 | 96 | Oil [36] |
| 10 | Image 13 | Image 23 | 50 | 100 | 98 | 59–61 [38] |
a Reaction conditions: sulfide (1 mmol), H2O2 (2.4 equiv), H-ZSM5 (12 mg), solvent-free, rt.
b Isolated yield.
Although the exact role of H-ZSM5 catalyst is not clear, on the basis of the already reported mechanism for the oxidation of sulfides using hydrogen peroxide in the presence of an acid catalyst [11,39–41], in the first step, the H-ZSM5 catalyst might act as protic acid, which activates the OO bond in hydrogen peroxide (Scheme 2).
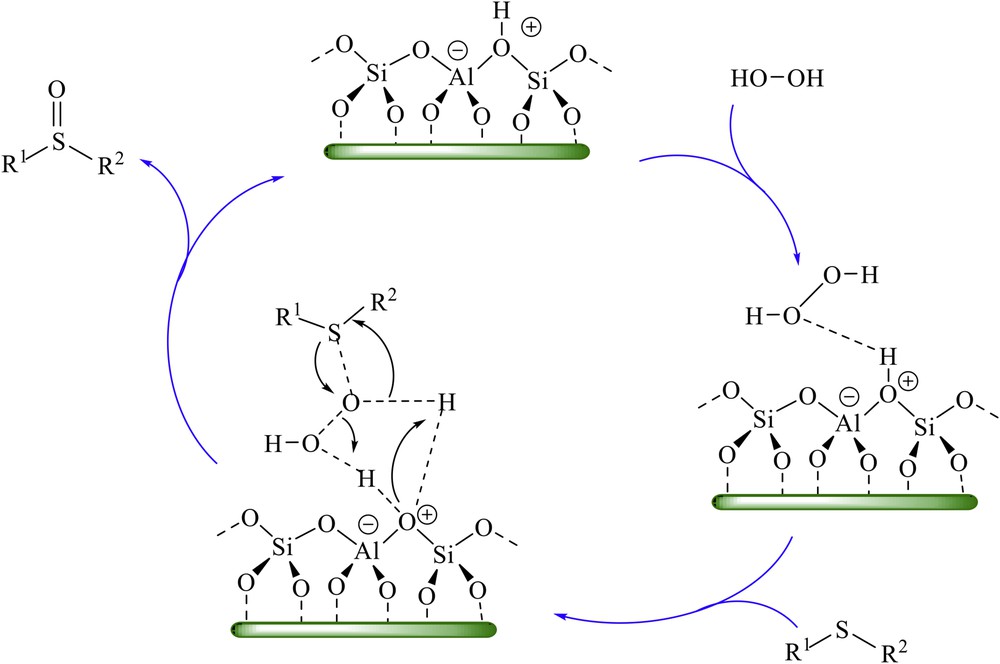
Proposed mechanism for the oxidation of sulfides to sulfoxides using H2O2 catalyzed by H-ZSM5.
To commercial applications of heterogeneous systems, the reusability of the catalyst is an important factor. Therefore, the recovery and recyclability of the H-ZSM5 as a catalyst for the oxidation of methyl phenyl sulfide with H2O2 was evaluated. After the completion of a reaction, the catalyst was recovered by centrifuge technique, washed with EtOAc and distilled water to remove residual product, dried under vacuum at room temperature, and reused for next runs. As shown in Fig. 1, the catalyst can be recovered and reused for 20 runs without any significant loss of its catalytic activity.

Recyclability of H-ZSM5 catalyst for the oxidation of methyl phenyl sulfide with 30% H2O2 at room temperature under solvent-free conditions.
To assess the efficiency of H-ZSM5 as a catalyst, we compared the result of the oxidation of methyl phenyl sulfide to methyl phenyl sulfoxide with previously reported results. As shown in Table 3, the previously reported procedures suffer from one or more limitations such as using a transition metal (Table 3, entries 2–5), the need for volatile and toxic organic solvents (Table 3, entries 2–5), special efforts for the preparation of catalyst (Table 3, entries 2–4), and longer reaction times (Table 3, entries 2–4). The other advantages of H-ZSM5 are the commercial availability and the low cost, nontoxicity, stability, easily separable, and highly reusable.
Comparison of the activity of H-ZSM5 with the various catalysts in the oxidation of methyl phenyl sulfide with 30% H2O2.
| Entry | Catalyst/oxidant | Reaction conditions | Time (min) | Yield (%) | Reference |
| 1 | H-ZSM5 zeolite/H2O2 | Solvent-free, rt | 50 | 98 | This study |
| 2 | Peroxotungstate supported on silica/H2O | CH2Cl2/MeOH, 8 °C | 150 | 91.5 | [42] |
| 3 | VO2F(dmpz)2/H2O2 | CH3CN, 0–5 °C | 300 | 95 | [17] |
| 4 | Ru-PVP/γ-Al2O3/H2O2 | CH3CN, rt | 120 | 98 | [43] |
| 5 | Cr(III)/H2O2 | CH3CN, rt | 72 | 93 | [44] |
4 Conclusion
We have demonstrated that H-ZSM5 can be used as a green, efficient, and reusable nanocatalyst for the oxidation of sulfides to sulfoxides using 30% H2O2 at room temperature. The notable advantages of the present protocol and this heterogeneous catalyst are the use of a commercially available, eco-friendly, inexpensive, and chemically stabile materials, the metal-free and mild reaction conditions, the operational simplicity, practicability, good to high yields of products, and good chemoselectivity. Furthermore, the catalytic system can be easily recycled using simple filtration and reused for 20 times without any significant loss of the activity, which makes it a promising material for practical and large-scale applications.
Acknowledgments
The authors are grateful to the University of Kurdistan Research Councils for partial support of this work.


