1 Introduction
Reduction of azidoarenes to the aniline derivatives is an industrially important process, since the aniline derivatives are crucial intermediates for the synthesis of dyes, polymers, pharmaceuticals, agrochemicals and biologically active molecules [1–5]. Various synthetic approaches for the reduction of azidoarenes proceed through catalytic hydrogenation [6,7], NaBH4/phase-transfer catalysis [8], triphenylphosphine [9], BF3·OEt2/EtSH [10], metal mediated reductions such as NiCl2–Zn [11], FeCl3–Zn [12], Sm/I2 [13] and tellurium metal [14]. These traditional methods have some disadvantages such as high temperature, high pressure, long reaction time and tedious work-up process. Furthermore, desired transformation is more complicated by using polyfunctional azide substrates in which poor chemoselectivity can be observed. Transfer Hydrogenation (TH), hydrogen abstraction from the reagent by catalyst contribution followed by hydrogen addition to the unsaturated functional system, is a more convenient method for the reduction of diverse functional groups bearing mainly carbonyls and imines [15,16]. This method has several important advantages such as lack of explosive free hydrogen, chemoselectivity, inexpensive and readily available hydrogen sources and also the use of readily accessible, recyclable and reusable catalysts [17–19]. A plenty of homogeneous and heterogeneous catalysts have been already reported in the literature for the TH process. Although heterogeneous catalysts are recyclable and reusable, homogeneous catalysts are usually preferred for TH due to their more activity and selectivity. Recently, considerable attention has been focused on arene ruthenium complexes as TH catalysts due to their unique properties, inherent catalytic ability and versatile building blocks for coordination cages and macrocyles [20]. Arene rings provided sterical hindrance of the ruthenium center, which prevent rapid oxidation, and they are also resistant to the substitution reactions. Besides, there are three available coordination sites located on the opposite side of the arene ligand can be easily replaced by donor ligands to employ more catalytically active Ru(II) complexes [21,22]. Although exponential growth has been observed for TH of carbonyl and imine polar bonds by the aid of arene ruthenium (II) complexes, the usage of these catalysts for azide hydrogenation is rare.
Herein we report for the first time that various azido arenes were catalytically converted to aniline derivatives by the contribution of the [Ru(p-cymene)Cl2]2 [23] dimeric structure and sodiumborohydride in the aqueous medium with shorter reaction times and high yields.
2 Results and discussion
Monomeric piano-stool type arene ruthenium (II) complexes are generally derived by the cleavage of the [Ru(p-cymene)Cl2]2 dimeric structure with –N, –S, –P type strong donor ligands to provide efficient performance and catalytic activity for TH [16]. Recently, Cetinkaya et al. described novel monomeric 3,4-dihydroquinazoline ruthenium(II)complexes for the reduction of acetophenone to the corresponding secondary alcohol with high yield after 1 h [24]. They claimed that only 15% conversion was observed when [Ru(p-cymene)Cl2]2 was used as a catalyst. As it is known that, azido compounds are more reactive, therefore we initially utilized the [Ru(p-cymene)Cl2]2 dimer to check the completion of the reduction process. The careful GC analysis indicated that absolute conversions of azidoarenes to corresponding anilines were achieved within 10 min at room temperature. These results demonstrate that there is no need for an extra reaction step for the generation of a new catalyst to afford reduction. Furthermore, [Ru(p-cymene)Cl2]2 is a promising candidate for the chemoselective TH as previously published monomeric arene ruthenium (II) complexes. On the other hand, a hydrogen source played another crucial role in TH. Apparently, a compound which has lower oxidation potential can act as a versatile hydrogen carrier, because these unique properties enable hydrogen transfer from the donor to the substrate in the presence of the catalyst. Alcohols, formic acid, hydrazine and 1,4-dihydropyridine derivatives have been extensively used as hydrogen carriers [25]. Even they are more accessible, eco-friendly and safe, TH requires harsh reaction conditions such as high temperature and long reaction time. The nature of the hydrogen carriers also influences the chemoselectivity and activity of TH [26,19c]. Sodium borohydride was utilized as a hydrogen carrier, because it donates four moles of hydrogen to the substrate [27]. In this study, the [Ru(p-cymene)Cl2]2 coupled NaBH4 system successfully employed the azide reduction process with absolute conversions and high yields (over 95%) within 10 min (Table 1). Although the TH process was rapid, halosubstituted azido arenes were exposed to selective hydrogenation where halogen substituents preserved their functionality (Table 1). Actually, the [Ru(p-cymene)Cl2]2 homogeneous catalyst is more soluble in organic solvents; however catalytic performance was effectively observed in the aqueous medium without any loss of activity where water is the main component. Azidoarenes were also submitted to the reduction reaction under catalyst free conditions, the results indicated that no aniline derivatives were produced after 5 h of the reaction.
[Ru(p-cymene)Cl2]2 catalyzed reduction of various R–N3 compounds.a
| Entry | R–N3 | Product | Conversion (%) | Yieldb (%) |
| 1 | Phenyl- | Aniline | 100 | >95 |
| 2 | o-Chlorophenyl- | o-Chloroaniline | 100 | >95 |
| 3 | p-Chlorophenyl- | p-Chloroaniline | 100 | >95 |
| 4 | o-Bromophenyl- | o-Bromoaniline | 100 | >95 |
| 5 | p-Bromophenyl- | p-Bromoaniline | 100 | >95 |
| 6 | p-Iodophenyl- | p-Iodoaniline | 100 | >95 |
| 7 | o-Tolyl- | o-Toluidine | 100 | >95 |
| 8 | m-Tolyl- | m-Toluidine | 100 | >95 |
| 9 | p-Tolyl- | p-Toluidine | 100 | >95 |
| 10 | o-Methoxyphenyl- | o-Methoxyaniline | 100 | >95 |
| 11 | m-Methoxyphenyl- | m-Methoxyaniline | 100 | >95 |
| 12 | p-Methoxyphenyl- | p-Methoxyaniline | 100 | >95 |
| 13 | o-(Trifluoromethyl)phenyl- | o-(Trifluoromethyl)aniline | 100 | >95 |
| 14 | p-(Trifluoromethyl)phenyl- | p-(Trifluoromethyl)aniline | 100 | >95 |
a Reaction conditions, substrate (0.25 mmol), NaBH4 (0.5 mmol) and [Ru(p-cymene)Cl2]2 (10 mg) was used with 1.5 ml of water/methanol (v/v=2/1) at room temperature.
b Isolated yield.
A comparison test of [Ru(p-cymene)Cl2]2 with palladium-based commercially available catalysts was also performed. The 1-azido-2-chlorobenzene served as a substrate for the comparison test. Under the same reaction conditions, 1-azido-2-chlorobenzene was subjected to a transfer hydrogenation reaction to provide 2-chloroaniline in the presence of Pd/C, Pd(OAc)2, PdCl2 and [Ru(p-cymene)Cl2]2 with sodium borohydride. The results with TON/TOF values exhibited that [Ru(p-cymene)Cl2]2 has distinct priority such as high yield and shorter time for aniline formation (Table 2). Furthermore, it is more effective and more stable in the water component of the solvent system. Pd(II) [28] and Pd(0) [29] assisted reduction reactions are not selective, whereas the chlorine group preserved its functionality in the presence of the [Ru(p-cymene)Cl2]2 coupled hydrogen donor system.
Use of different commercially available catalysts for the reduction of 1-azido-2-chlorobenzene to 2-chloroaniline.a
| Entry | Catalyst | Amount of catalyst, mg | Time (min) | Yieldc (%) | TON (molproduct/molmetal(s) | TOF (molproduct/molmetal(s)/h |
| 1 | Pd/Cb | 3.4 | 50 | 70 | 109.3 | 131.2 |
| 2 | Pd(OAc)2 | 6.7 | 60 | 72 | 6.0 | 6.0 |
| 3 | PdCl2 | 5.3 | 60 | 35 | 2.9 | 2.9 |
| 4 | [Ru(p-cymene)Cl2]2 | 10.0 | 10 | >95 | 28.8 | 172.7 |
a Reaction conditions, substrate (0.25 mmol), NaBH4 (0.5 mmol) and catalyst (0.03 mmol metal content) was used with 1.5 ml of water/methanol (v/v=2/1) at room temperature.
b 5 wt % Pd.
C isolated yield.
Aniline formation was compared to previously published studies. The [Ru(p-cymene)Cl2]2-assisted aniline formation was successfully achieved with high yield at room temperature. In addition, the reaction was performed in a short time with respect to the previous studies as depicted in Table 3.
Comparison of the designed catalytic system with previously published studies about the reduction of aryl azide.
| Catalyst | Conditions | Temp. °C | Time | Yield, % |
| None [33] | Phenyl azide (1.33 mmol), NaBH4 (0.89 mmol), THF (4 ml), methanol (0.18 ml) | reflux | 1 h | 86 |
| Hexadecyltributylphosphonium bromide [8] | Phenyl azide (1 mol), catalyst (0.1 mol), NaBH4 (1.1 mol), toluene (2 ml) | 20 | 1 h | 92 |
| BF3.OEt2/EtSH [10] | Phenyl azide (0.32 mmol), catalyst (0.8 mmol), CH2Cl2 (5 ml), ethanethiol (1.6 mmol) | 25 | 1.5 h | 95 |
| Tetrathiomolybdate [30] | Phenyl azide (2 mmol), catalyst (1. 1 mmol), acetonitrile/water (v/v=20/1) | 25 | 6 h | 75 |
| Sm/I2 [13] | Phenyl azide (1 mmol), Sm (1.0 mmol), I2 (0.2 mmol), methanol (5 ml), N2 atm | 25 | 6 h | 87 |
| Zn [31] | Benzyl azide (0.03 mol), Zn (0.04 mol), NH4Cl (0.07 mol), ethyl alcohol (80 ml), water (27 ml) | 90 | 10 min | 90 |
| RhCl3.3H2O [28] | Phenyl azide (40 mmol), catalyst (4.3.10−2 mmol), benzene (20 ml), CO (20 atm), water (1.5 ml) | 150 | 4 h | 61 |
| PANF-QAS (A-Hp-Br) [32] | Phenyl azide (4 mmol), catalyst (10 mol %), NaBH4 (0.5 mmol), Na2HPO4/NaH2PO4 (pH=12) | 50 | 4 h | 98 |
| CuSO4 [34] | Phenyl azide (3.4 mmol), catalyst (0.034 mmol), NaBH4 (1 mmol), methanol (10 ml) | 0–5 | 1 h | 80 |
| LiCl [35] | Phenyl azide (1 eq), catalyst (1 eq), NaBH4 (1 eq), THF (15 ml) | 25 | 30 min | 94 |
| [Ru(p-cymene)Cl2]2 (this study) | Phenyl azide (0.25 mmol), catalyst (0.016 mmol), NaBH4 (0.5 mmol), water/methanol (v/v=2/1) | 25 | 10 min | >95 |
According to the catalytic cycle illustrated in Scheme 1, free hydrogen gas was released by the hydrolysis of sodium borohydride with the aid of a ruthenium (II) dimer followed by the oxidative addition of hydrides to the metal center. Coordination of the azide group and subsequent reductive elimination of hydrides remarkably furnished aniline derivatives.
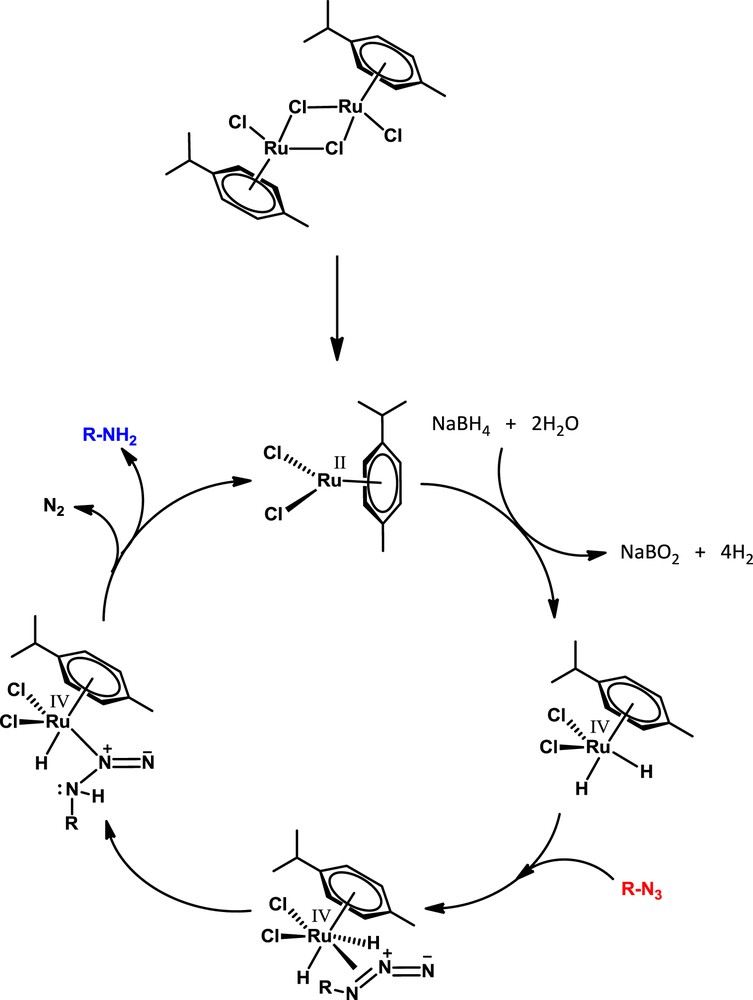
Proposed mechanism for the hydrogenation of aryl azide.
3 Conclusion
A convenient and practical method for the selective reduction of azidoarenes to the corresponding aniline derivatives was described for the first time. The reaction was completed within 10 min at room temperature by the contribution of the cheap, easily accessible and rapid synthesizable [Ru(p-cymene)Cl2]2 homogeneous catalyst. Sodium borohydride was employed as a hydrogen supplier and all aniline derivatives were obtained with absolute conversion and high yield in an ecofriendly solvent system.
4 Experimental
4.1 Materials
[Ru(p-cymene)Cl2]2 was synthesized according to a previously published procedure [23]. Commercially available azidoarenes were obtained from Sigma–Aldrich. NaBH4 was also purchased from Sigma–Aldrich and used as received.
4.2 Characterization methods
The 1H NMR spectra were recorded on a Jeol ECS 400 MHz spectrometer.
4.3 General procedure for the reduction of aryl azides
To an aqueous suspension of [Ru(p-cymene)Cl2]2 (1 ml/10 mg catalyst), azidoarene (0.25 mmol) in 1.5 ml methanol/water (1:2) was added under ambient conditions. Afterwards, NaBH4 (0.5 mmol) was added to the reaction mixture, and the vessel was closed. The reaction continued while the mixture was vigorously stirred and monitored by GC. Most of the reactions were completed over a time period of 10 min. After the reaction, the solvent was evaporated under vacuum and the products were purified by rapid flash column chromatography. The reduced compounds were analysed by 1H NMR with CD3OD or CDCl3 as the solvent, depending on the product separated.
Acknowledgements
This research was supported by Duzce University Research Fund (grant no. 2014.05.03.274).


