1 Introduction
Biomass-derived compounds, such as pinanes, hold great potential for the pharmaceutical, bioenergy, fine chemistry, and flavor industries [1–3]. The low abundance of the natural cis- and trans-pinane isomers in essential oils obliges the industry to use synthetic products. Pinane is an alkane that has the potential for an excellent added value as it can be synthesized from very abundant natural products such as pinenes. In addition to being very useful in cosmetology [2], pinane is an attractive building block for the preparation of various molecules used by the pharmaceutical industry such as β-carotene, p-cresol, dihydromyrcene, dihydromyrcenol, and citronellene [4,5]. Yara-Varón et al. [6] have recently used pinane as a bio-based solvent for the extraction of some natural products preventing the need of n-hexane, a crude oil-based solvent.
Recently, there has been an increased demand for bio-based solvents for many applications, including cosmetics, personal care products, pharmaceuticals, and so forth [7]. Also, the growing number of literature over the past few years on the subject attests of the general interest to the development of green and bio-based solvents for that purpose [7–16]. The possible use of pinane as a bio-based solvent is very promising provided that its preparation method qualifies as environmentally friendly while remaining economical. Few green methods of pinane synthesis have recently been reported. Cis-pinane was obtained by Liu et al. [17] through the hydrogenation of α-pinene over ruthenium (Ru) stabilized by amine-functionalized magnetite nanoparticles. The selectivity toward cis-pinane was quantitative (97%). Fe3O4/1,6-hexanediamine/Ru catalyst was repeatedly recovered with the application of an external magnetic field and was able to maintain its catalytic efficiency, which was not significantly altered even after 10 repeated cycles. Polyoxyethylene–polyoxypropylene–polyoxyethylene triblock copolymer (P123)-Ru micellar catalyst was also used by Hou et al. [18] for the hydrogenation of α-pinene in water. The selectivity for cis-pinane reached 98.9%. The isolated catalyst aqueous phase was used seven times with no treatment, whereas its catalytic activity and selectivity remained unaffected.
Under neat conditions, hydrogenation of α- and β-pinene over palladium (Pd) has been investigated by our team in the previous study [19]. Pd catalyst on carbon, alumina, and silica supports yielded to a quantitative conversion of pinene to cis- and trans-pinanes and a higher selectivity for the cis isomer (72–89%). Pd/C and Pd/alumina were successfully recycled 13 and 14 times, respectively, with maintained efficiency, whereas Pd/silica could be used only once to convert pinenes into pinanes. The hydrogenation was exclusively heterogeneous because of the extremely low leaching rate of palladium into pinenes and pinanes. Pd/C remained effective after 13 cycles and was the best catalyst/support of the three tested supports for the environmentally friendly hydrogenation of α- and/or β-pinenes.
In this study, we have pursued our ongoing research for a green and simple synthetic method to obtain pinanes from a solvent-free hydrogenation of pinenes. For that purpose, we have tested and compared the most frequently used supported noble metals as catalysts for the hydrogenation reaction: palladium (Pd) [20], platinum (Pt) [21], ruthenium (Ru) [22,23], and rhodium (Rh) [24]. We have systematically investigated the influence of the catalyst supports (carbon and alumina) as well as the effect of temperature and use of sonication during synthesis. Although heating could increase the efficiency of hydrogenation, past essays have also proven that sonication could lead to improved conversion yields [25,26]. Enhanced selectivity and activity were occasionally reported using ultrasound treatment in a heterogeneous system [27–29]. To the best of our knowledge, this work is the first attempt of simultaneously investigating these parameters for such variety of catalysts and reaction conditions for a solvent-free hydrogenation of pinenes.
Finally, we have assessed the ability of the obtained pinane to be used as an alternative bio-based solvent for the solubilization of three bioactive compounds (β-carotene, vanillin, and rosmarinic acid). Our synthetic product was compared to n-hexane and to cis-rich pinane (cis/trans, 7/3) using an experimental as well as a predictive approach based on the COnductor like Screening MOdel-Realistic Solvation (COSMO-RS) software developed by Klamt [30].
2 Experimental section
Safety warning. high-pressure experiments with compressed H2(g) must be carried out only with appropriate equipment and under rigorous safety precautions!
2.1 General experimental conditions
α- and β-pinene were purchased from Aldrich and Alfa Aesar; 10 wt % Pd/C, 10 wt % Pd/Al2O3, 5 wt % Pt/C, 5 wt % Pt/Al2O3, 5 wt % Ru/C, 5 wt % Ru/Al2O3, 5 wt % Rh/C, 5 wt % Rh/Al2O3 were purchased from Aldrich. H2 (purity ≥99.9%) was purchased from Praxair Canada Inc. NMR spectra were recorded using a Bruker Avance III 400 MHz spectrometer. Gas chromatography–mass spectrometry (GC–MS) analysis was recorded using an Agilent 6890 Series GC System coupled with an Agilent 5973 Network Mass Selective Detector and capillary column (Zebron ZB-5MS, 30 m × 0.25 mm × 0.25 um).
Dissolved metal concentrations into the pinane products were determined using the Thermo Scientific iCAP-Q inductively coupled plasma mass spectrometer (ICP/MS; Bremen, Germany) interfaced with the ASX-520 autosampler from CETAC Technologies (Omaha, USA). Before analysis, all synthesis product samples were filtered using 0.45 μm capsule filters (cellulose acetate membrane, VWR International) and then digested using aqua regia (∼150 μL of sample in 0.5 mL of 7:3 v/v HCl/HNO3, trace metal grade, Fisher Scientific). Samples were heated at 65 °C for 4 h using a hot water bath. Before ICP/MS analyses, 12 mL of Milli-Q water (18.2-ΩM grade water provided by an EMD-Millipore water purification system, Darmstadt, Germany) was added to each digest to reduce the sample matrix to a maximum of 4% v/v acid. Recoveries of spiked solutions were on average 98.9 ± 1.6% (n = 3). The limit of detection and limit of quantification calculated from a method blank analysis considering a digestion of 150 μL of sample were, respectively, 0.0002 and 0.0005 μg g−1 for Ru and Rh, and 0.007 and 0.023 μg g−1 for Pt (n = 5).
2.2 Hydrogenation of α- and β-pinene
In a 45 mL autoclave equipped with a glass liner containing a stirring bar, 5 g of pinene (36.7 mmol) and 0.5 g of Pd/C (10 wt %, 0.469 mmol, 0.012 equiv), Pd/Al2O3 (10 wt %, 0.469 mmol, 0.012 equiv), Pt/C (5 wt %, 0.128 mmol, 0.003 equiv), Pt/Al2O3 (5 wt %, 0.128 mmol, 0.003 equiv), Ru/C (5 wt %, 0.247 mmol, 0.006 equiv), Ru/Al2O3 (5 wt %, 0.247 mmol, 0.006 equiv), Rh/C (5 wt %, 0.243 mmol, 0.006 equiv), or Rh/Al2O3 (5 wt %, 0.243 mmol, 0.006 equiv) were placed. The autoclave was purged four times with hydrogen (200 Psi) and then pressurized to 400 Psi during synthesis. The reaction was stopped after nonchange indicated by GC–MS in percentage of the mixture constituent. Hydrogen was vented from the autoclave, and the reaction mixture was filtered through a Celite pad to obtain the hydrogenated pinane products. A Qsonic a ultrasonic processor equipped with a probe was used for sonication (54 kHz, 1 h) essays before hydrogenation at room temperature (RT) or at 100 °C.
2.3 Natural product solubilization
2.3.1 Computational solubilization prediction
Solubility abilities of cis-pinane, trans-pinane, cis-rich pinane (cis/trans, 7/3), and n-hexane to dissolve β-carotene, vanillin, and rosmarinic acid were investigated using COSMO-RS software. The results were expressed in log10(x-solub) (best solubility is set to 0). The chemical structures of the solvents and solutes discussed in this article were mutually transformed by JChemPaint version 3.3 (GitHub Pages, San Francisco, CA) to their simplified molecular input line entry syntax notations, which were subsequently used to calculate the solubility parameters.
2.3.2 Experimental solubilization essay
Solubilization by bead milling was performed using Precellys 24 (Bertin Technology, Ozyme) operating in a 2-mL tube with 1 g of ceramic beads. One hundred milligrams of compound was mixed with 1 mL of a standard solvent (n-hexane, pinane) and submitted in drive tube operating at 6500 rpm for 2*20 s. After extraction, the beads were filtered before solid/liquid separation by centrifugation. The samples were stored at −20 °C until analysis. All experiments were carried out in triplicates. The total content of each compound was measured spectrophotometrically (Biochrom Libra S22 UV/vis Spectrophotometer) in a 1-cm optical path-length quartz cell at the maximum wavelength of each compound in each extract using DMSO as a blank. The Beer-Lambert law was used to determine the carotenoid, vanillin, and rosmarinic acid concentrations in each extract using a calibration curve prepared using β-carotene, vanillin, and rosmarinic acid standard. The straight calibration curve of absorbance versus compound concentration (milligrams per liter) was reliant on the Beer-Lambert law. Finally, the quantity of compound dissolved in each solvent was calculated and expressed as milligrams per liter.
3 Results and discussion
3.1 Influence of various active metals and supports on pinene hydrogenation
The solvent-free hydrogenation under reduced pressure (Scheme 1) of α- and β-pinene was investigated using commercially available catalysts of various active noble metals (Pd, Pt, Ru, and Rh) and supports (carbon and alumina) (Table 1).
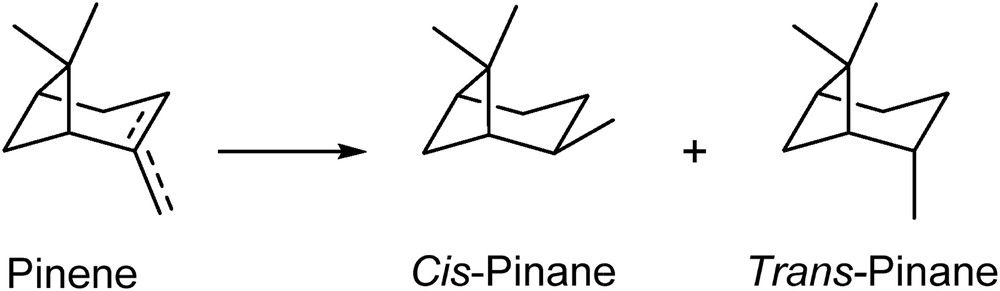
Reagents and conditions: H2 (400 Psi), catalyst, RT.
Conversion and selectivity of solvent-free hydrogenation of α- and β-pinene over different catalysts at 400 Psi and RT.
| Metal | Support | Starting material | Conversiona (%) | Selectivitya (%) | Time (h) |
| Pd [19] | Charcoal | β-Pinene | 100 | 72/28 | 4 |
| α-Pinene | 100 | 68/32 | 4 | ||
| Alumina | β-Pinene | 100 | 78/22 | 4 | |
| α-Pinene | 95 | 76/24 | 4 | ||
| Pt | Charcoal | β-Pinene | 100 | 90/10 | 5 |
| α-Pinene | 96 | 91/9 | 5 | ||
| Alumina | β-Pinene | 96 | 88/12 | 48 | |
| α-Pinene | 95 | 93/7 | 48 | ||
| Ru | Charcoal | β-Pinene | 0 | – | 144 |
| α-Pinene | 0 | – | 144 | ||
| Alumina | β-Pinene | 100 | 100/0 | 144 | |
| α-Pinene | 100 | 98/2 | 144 | ||
| Rh | Charcoal | β-Pinene | 94 | 95/5 | 144 |
| α-Pinene | 92 | 95/5 | 144 | ||
| Alumina | β-Pinene | 96 | 81/19 | 24 | |
| α-Pinene | 96 | 84/16 | 24 |
a Conversion and selectivity (cis/trans-pinane) were determined by GC–MS analysis.
As indicated in Table 1, Pt and Rh on charcoal and alumina had a significant better cis/trans-pinane selectivity as compared with Pd on the same supports. The conversion ratio with these two catalysts ranged from 92% to 100% with a selectivity between 81% and 95% always in favor of cis-pinane. The present solvent-free hydrogenation over Pd and Pt was faster than that over Ru and Rh but was less selective toward cis-pinane (Table 1).
Ru on alumina had a perfect conversion and selectivity rate for cis-pinane because hydrogenation of β-pinene produced exclusively cis-pinane and the hydrogenation of α-pinene produced only 2% of trans-pinane and 98% of cis-pinane (Table 1). However, hydrogenation of α- or β-pinene at these same conditions with Ru on charcoal was unsuccessful. Even after 144 h, only the starting pinenes were recovered.
This first series of tests with these four most used catalysts in hydrogenation motivated us to test the influence of ultrasound or heating on hydrogenation of pinenes and assess their impact in terms of efficiency and selectivity.
3.2 Influence of sonication and temperature on the hydrogenation of pinenes
Except for the hydrogenation of α-pinene after ultrasonic activation (Table 2, entry 3), activation of Pd/C by sonication and/or heating increased the proportion of trans-pinane in the mixture of pinanes (Table 2, entries 4–8). All hydrogenations were carried out for 5 h except for that of α-pinene after activation by sonication, which was completed only after 24 h (Table 2, entry 3).
Hydrogenation of pinenes over Pd/C under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 100 | 68/32 | 5 |
| 2 | β | 100 | 72/28 | 5 | |
| 3 | 400 Psi, SBH, RT | α | 100 | 71/29 | 24 |
| 4 | β | 100 | 64/36 | 5 | |
| 5 | 400 Psi, 100 °C | α | 100 | 64/36 | 5 |
| 6 | β | 100 | 65/35 | 4 | |
| 7 | 400 Psi, SBH, 100 °C | α | 90 | 55/45 | 5 |
| 8 | β | 96 | 65/35 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
Unlike hydrogenation without any activation (by sonication or heating), no isomerization of α-pinene was observed during hydrogenation over Pd/C when sonication was used alone (Table 2, entry 3). Isomerization was observed only after about 4 h of reaction when Pd/C was ultrasonically activated followed by continuous heating of the reaction. Surprisingly, in this condition the proportion of trans-pinane was the highest (Table 2, entry 7).
As compared with Pd/C, Pd/Al2O3 was more efficient for the pinene hydrogenation and the selectivity toward cis-pinane was also better. Activation by sonification had no effect on the catalytic capacity with conversion and selectivity rates similar (Table 3, entries 3 and 4) to hydrogenation performed without activation (Table 3, entries 1 and 2). Furthermore, sonication greatly increased the reaction time, which went from 4 to 24 h (Table 3, entries 3 and 4). Heating after sonification allowed to reduce this reaction time (Table 3, entries 7 and 8). However, heating increased the proportion of trans-pinane from 24% to 43% in the case of the hydrogenation of α-pinene (Table 3, entry 5). Overall, in the case of Pd on either support (charcoal or alumina), activation by sonication or heating did not seem to improve significantly conversion or selectivity in the hydrogenation of neither α- nor β-pinene.
Hydrogenation of pinenes over Pd/Al2O3 under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 95 | 76/24 | 5 |
| 2 | β | 100 | 78/22 | 5 | |
| 3 | 400 Psi, SBH, RT | α | 100 | 77/23 | 24 |
| 4 | β | 100 | 75/25 | 24 | |
| 5 | 400 Psi, 100 °C | α | 100 | 57/43 | 5 |
| 6 | β | 100 | 78/22 | 4 | |
| 7 | 400 Psi, SBH, 100 °C | α | 100 | 68/32 | 5 |
| 8 | β | 100 | 70/30 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
The hydrogenation of α- or β-pinene over Pt/C was more selective than that with Pd on charcoal or alumina. The proportion of trans-pinane was around 9% and 10%, respectively (Table 4, entries 1 and 2), for pinene hydrogenation at RT and 400 Psi as compared to 32% and 28% in the case of Pd (Table 2, entries 1 and 2). The proportion of trans-pinane did not exceed 21% even when the hydrogenation was carried out with heating and/or ultrasonic activation. Ultrasonic activation of Pt/C for the hydrogenation of α-pinene increased slightly not only the selectivity but also increased the reaction time because the hydrogenation was completed only after 48 h (Table 4, entry 3).
Hydrogenation of pinenes over Pt/C under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 96 | 91/9 | 5 |
| 2 | β | 100 | 90/10 | 5 | |
| 3 | 400 Psi, SBH, RT | α | 95 | 94/6 | 48 |
| 4 | β | 94 | 89/11 | 5 | |
| 5 | 400 Psi, 100 °C | α | 94 | 89/11 | 8 |
| 6 | β | 95 | 83/17 | 5 | |
| 7 | 400 Psi, SBH, 100 °C | α | 95 | 89/11 | 5 |
| 8 | β | 95 | 79/21 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
The hydrogenation of α- or β-pinene over Pt/Al2O3 was slower because the hydrogenation was only completed after 48 h (Table 5, entries 1 and 2). Ultrasonic activation had no effect on the efficiency or the selectivity of the reaction (Table 5, entries 3 and 4).
Hydrogenation of pinenes over Pt/Al2O3 under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 95 | 93/7 | 48 |
| 2 | β | 96 | 88/12 | 48 | |
| 3 | 400 Psi, SBH, RT | α | 95 | 92/8 | 48 |
| 4 | β | 95 | 87/13 | 48 | |
| 5 | 400 Psi, 100 °C | α | 96 | 82/18 | 5 |
| 6 | β | 96 | 83/17 | 6 | |
| 7 | 400 Psi, SBH, 100 °C | α | 89 | 83/17 | 5 |
| 8 | β | 98 | 83/17 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
Similarly to Pd, activation by heating allowed to reduce the reaction time but again had the effect to increase the proportion of trans-pinane (Table 5, entries 5–8). It is interesting to note that the hydrogenation of α- or β-pinene over Pt on charcoal or alumina was much more selective than that over Pd even under heating conditions. The proportion of trans-pinane in that case did not exceed 21%, unlike the 30–45% of trans-pinane obtained with Pd over charcoal or alumina. In summary for Pt catalysts, only activation by heating in the case of Pt on alumina seemed to improve synthesis results by reducing reaction time. However, this gain was significantly counterbalanced by the decreased selectivity in disfavor of cis-pinane.
Ru/C was completely inactive for the hydrogenation of α- or β-pinene under our initial reaction conditions (RT, 400 Psi) (Table 6, entries 1 and 2) even after 144 h of reaction. On the contrary, after sonication, the same catalyst was found active only for α-pinene. It was then very selective to transform α-pinene into cis-pinane (99% selectivity at 100% conversion) albeit with a low reaction rate (isomerization at 24 h and full α-pinene conversion at 96 h of reaction, Fig. 1). An improved mass transfer that may have a positive effect on the adsorption/desorption rates at the catalyst surface could explain this efficient and selective hydrogenation.
Hydrogenation of pinenes over Ru/C under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 0 | – | 144 |
| 2 | β | 0 | – | 144 | |
| 3 | 400 Psi, SBH, RT | α | 100 | 99/1 | 96 |
| 4 | β | 0 | – | 96 | |
| 5 | 400 Psi, 100 °C | α | 100 | 90/10 | 5 |
| 6 | β | 95 | 93/7 | 5 | |
| 7 | 400 Psi, SBH, 100 °C | α | 95 | 96/4 | 72 |
| 8 | β | 95 | 95/5 | 96 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
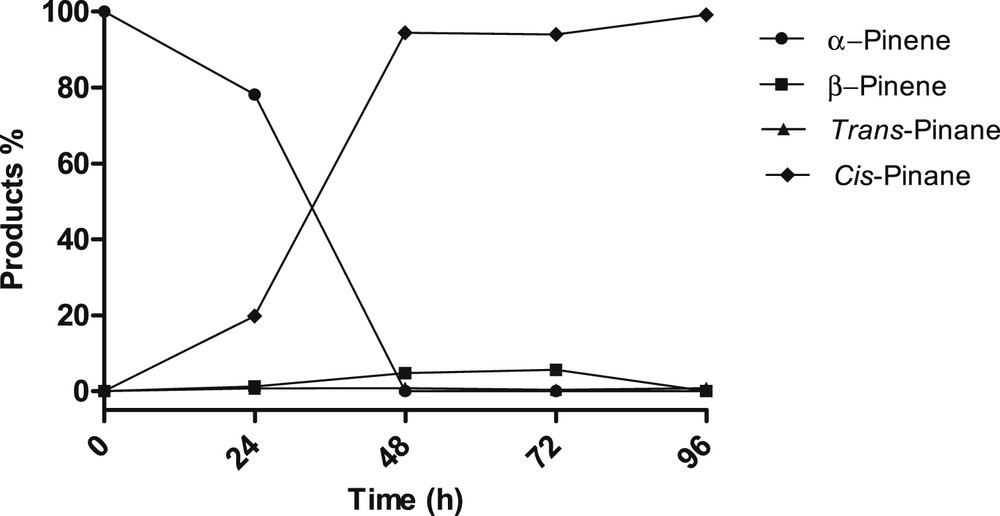
Hydrogenation of α-pinene over 5% Ru/C, with sonication at RT.
Under the same conditions, no traces of pinane or isomerization was detected even after 96 h of reaction with β-pinene (Table 6, entry 4).
Similar to sonication, heating allowed an efficient α-pinene hydrogenation over Ru/C in only 5 h (Table 6, entry 5). Under these conditions, even β-pinene was hydrogenated. On the other hand, the selectivity (90%) was lower as compared with that of the α-pinene hydrogenation after sonication alone (99%). Sonification followed by heating during the hydrogenation increased the selectivity toward cis-pinane but surprisingly increased largely the reaction time (Table 6, entries 7 and 8).
Alumina appeared to be a support with a beneficial chemical effect on the activity of the Ru. As shown in Table 7, Ru/Al2O3 was found very active and led to a highly selective transformation of α- or β-pinene into cis-pinane (99–100% selectivity at 100% conversion) at 144 h of reaction.
Hydrogenation of pinenes over Ru/Al2O3 under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 100 | 98/2 | 144 |
| 2 | β | 100 | 100/0 | 144 | |
| 3 | 400 Psi, SBH, RT | α | 94 | 98/2 | 144 |
| 4 | β | 0 | – | 144 | |
| 5 | 400 Psi, 100 °C | α | 95 | 93/7 | 5 |
| 6 | β | 95 | 89/11 | 5 | |
| 7 | 400 Psi, SBH, 100 °C | α | 95 | 94/6 | 5 |
| 8 | β | 96 | 89/11 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
Sonication did not seem to affect the hydrogenation of α-pinene, but on the other end completely blocked hydrogenation of β-pinene as no traces of pinane were detected even after 144 h of reaction (Table 7, entries 3 and 4). Heating or sonication before heating during the reaction allowed hydrogenations of α- and β-pinene with 95% of conversion and with a selectivity between 89% and 94% toward cis-pinane in 5 h of reaction (Table 7, entries 5–8).
Overall for Ru, activation by sonication had the positive effect to allow hydrogenation of α-pinene with Ru/C, which was not otherwise possible under normal conditions. Heating in general allowed to reduce the time of reaction in the case of both supports but systematically led to a decrease in selectivity toward cis-pinane without improving conversion rates.
Under our initial hydrogenation conditions, Rh/C was one of the weakest of all catalysts tested in this work in terms of conversion of pinenes into pinanes (Tables 2 and 8). Unlike other catalysts, ultrasonic activation had a negative effect on the conversion rate because it decreased drastically from 92% to 94% (Table 8, entries 1 and 2) down to 67–68% even after 144 h of reaction (Table 8, entries 3 and 4). Heating or sonication followed by heating during the reaction not only increased the conversion rate and trans-pinane proportion but also led to the formation of p-menthene, p-menthane, and p-cymène after 10–15 min of reaction. The selectivity was then comparable to that obtained during essays over Pd or Pt but was much lower than that obtained over Ru/C.
Hydrogenation of pinenes over Rh/C under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 92 | 95/5 | 144 |
| 2 | β | 94 | 95/5 | 144 | |
| 3 | 400 Psi, SBH, RT | α | 67 | 95/5 | 144 |
| 4 | β | 68 | 92/8 | 144 | |
| 5 | 400 Psi, 100 °C | α | 96 | 65/35 | 5 |
| 6 | β | 93 | 82/18 | 5 | |
| 7 | 400 Psi, SBH, 100 °C | α | 96 | 70/30 | 5 |
| 8 | β | 96 | 64/36 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
As compared with Rh/C, Rh/Al2O3 converted more pinene into pinane but was less selective (Table 9, entries 1 and 2). Activation by ultrasound or heating permitted to increase the conversion rate but had no significant effect on selectivity. Unexpectedly heating allowed to decrease the reaction time down to 5 h. However, this time, on contrary of all other catalysts, it had no negative effect on the selectivity (Table 9, entries 4 and 5).
Hydrogenation of pinenes over Rh/Al2O3 under different conditions.
| Entry | Conditions | Pinene | Conversiona (%) | Selectivitya (%) | Time (h) |
| 1 | 400 Psi, RT | α | 96 | 84/16 | 24 |
| 2 | β | 96 | 81/19 | 24 | |
| 3 | 400 Psi, SBH, RT | α | 100 | 85/15 | 24 |
| 4 | β | 100 | 82/18 | 24 | |
| 5 | 400 Psi, 100 °C | α | 100 | 85/15 | 5 |
| 6 | β | 100 | 80/20 | 5 | |
| 7 | 400 Psi, SBH, 100 °C | α | 100 | 81/19 | 5 |
| 8 | β | 100 | 79/21 | 5 |
a Conversion, selectivity (cis/trans-pinane), and isomerization time detection were determined by GC–MS analysis.
3.3 Type of catalysis and recyclability
To confirm the heterogeneous nature of the catalysis, metal leached from the catalysts into the pinane solution was measured by ICP/MS.
As for Pd [19], leaching was excessively low under all conditions tested for both charcoal and alumina supports (Table 10). In the case of Pt and Rh catalysts on either support (alumina or charcoal), metal leaching was always negligible (Table 10). Only Ru as a catalyst led to limited leaching. A maximum of 0.28% was observed for Ru on alumina at RT but was barely detectable after activation by sonication. On charcoal, 0.23% of Ru was released into pinane solution with heating at 100 °C. In both cases, it appeared that sonication did not favor the release of the catalyst from its support. Nevertheless, the amount of catalyst released into solution under all tested conditions remained low suggesting a systematic heterogeneous catalytic hydrogenation of pinenes under our conditions.
Percentage of metal (Pt, Ru, or Rh) leached from the catalyst into the pinanes.
| Metal/support | 400 Psi RT | 400 Psi, SBH, RT | 400 Psi, 100 °C | 400 Psi, SBH, 100 °C |
| Pt/C | 0.0028 | 0.0134 | <0.00002 | 0.0101 |
| Pt/Al2O3 | 0.0019 | 0.0006 | 0.0002 | 0.0016 |
| Ru/C | 0.0007 | 0.0070 | 0.2263 | 0.0004 |
| Ru/Al2O3 | 0.2836 | 0.0001 | n.d. | n.d. |
| Rh/C | 0.0022 | n.d. | n.d. | 0.0004 |
| Rh/Al2O3 | 0.0001 | <0.000001 | 0.0001 | 0.0001 |
To further confirm the heterogeneous nature of the catalysis, we performed essays during which we removed Ru/C or Ru/Al2O3 from the reactive media by filtration after 48 and 72 h of reaction, respectively. As expected, the hydrogenation of the remaining filtrate at the same hydrogen pressure and temperature failed. The remaining pinenes were no further consumed, and no additional pinane was formed, even after 144 h as demonstrated by NMR and GC–MS, which confirmed the lack of Ru catalyst in the filtrate and the heterogeneous catalytic nature of the reaction.
As Ru/C and Ru/Al2O3 were the best catalysts during our essays in general, with high selectivity toward cis-pinane more than 99% and full conversion of pinenes, we decided to conduct recyclability tests using these two catalysts (Table 11).
Recyclability of Ru/C and Ru/Al2O3 at 400 Psi and 100 °C.
| Catalyst | Cycle | Pinene | Conversiona (%) | Selectivitya (%) |
| Ru/C | 1 | β | 95 | 93/7 |
| 2 | β | 95 | 94/6 | |
| 3 | β | 95 | 93/7 | |
| 4 | β | 95 | 93/7 | |
| 5 | β | 95 | 93/7 | |
| 6 | β | 95 | 93/7 | |
| 7 | β | 93 | 94/6 | |
| 8 | β | 65 | 93/7 | |
| Ru/Al2O3 | 1 | β | 100 | 89/11 |
| 2 | β | 77 | 82/18 |
a Conversion and selectivity (cis/trans-pinane) were determined by GC–MS analysis.
The hydrogenation of β-pinene over Ru/C or Ru/Al2O3 at 100 °C under reduced pressure revealed that the support had also an effect on the recyclability of the catalyst. As shown in Table 11, Ru/C could be reused more than seven times with no significant decline in selectivity or activity. On the other hand, the Ru/Al2O3 became much less selective as soon as the second cycle. During these essays, limited Ru leaching was observed, as indicated by the ICP analysis (Fig. 2). For charcoal, it summed up to 1.8% after 7 cycles whereas it was negligible for alumina for both cycles. No correlation was observed between the conversion degree and the amount of Ru leached into solution.
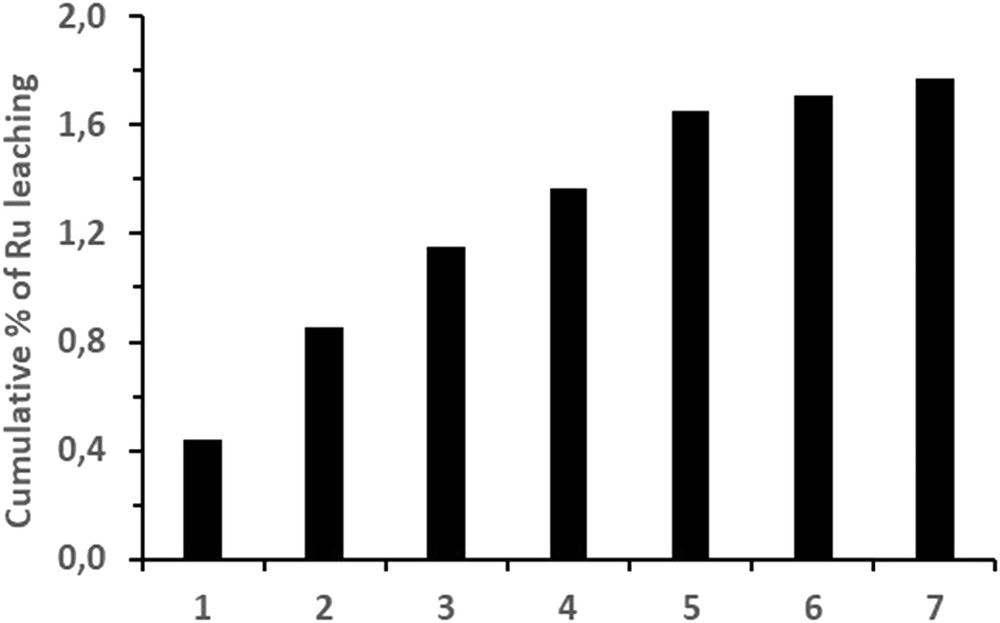
Cumulative percentage (%) of Ru leached from the catalyst over charcoal during recyclability essays. Percentages were calculated from metal concentrations measured into the pinanes obtained from each cycle as compared to the quantity of Ru present at the beginning of the first cycle.
3.4 Solubilization assays
The cis-pinane obtained from α/β-pinenes was tested as a bio-based alternative solvent for the solubilization of various natural products such as carotenoids, vanillin, and rosmarinic acid. As predicted by COSMO-RS, cis/trans-pinane mixture and cis-pinane showed similar probability of solubility as n-hexane for all analyzed compounds (Table 12). According to the rule “like dissolves like” pinanes and n-hexane showed high probability of solubility for β-carotene. Regarding the vanillin, the two solvents showed less probability of solubility, and for rosmarinic acid, it is even weaker.
COSMO-RS relative solubility (log10(x-solub)) of β-carotenoids, vanillin, and rosmarinic acid in pinane and n-hexane.
| Compounds | Pinane (log10(x-solub)) | n-Hexane (log10(x-solub)) |
| β-Carotene | −0.1116 | −0.0395 |
| Vanillin | −5.14 | −5.24 |
| Rosmarinic acid | −12.27 | −12.43 |
Experimentally, the solubility of each compound in cis-rich pinane (cis/trans, 7/3) and cis-pinane as solvents at RT was investigated by bead milling. The highest carotenoid solubility was observed in cis-pinane (Table 13). β-Carotene was 42 times more soluble in cis-pinane than in n-hexane and 1.6 times more soluble in cis-pinane than in cis-rich pinane. It is clear that β-carotene is much better soluble in cis-pinane than in n-hexane.
β-Carotene, vanillin, and rosmarinic acid solubility in cis-pinane, cis-rich pinane, and n-hexane.
| Solvent | β-Carotene solubility (mg mL−1) | Vanillin solubility (mg mL−1) | Rosmarinic acid solubility (mg mL−1) |
| Cis-pinane | 42.7 ± 2.5 | 2.5 ± 0.03 | 0.185 ± 0.003 |
| Cis-rich pinanea | 26.5 ± 2.3 | 1.23 ± 0.1 | 0.185 ± 0.002 |
| n-Hexane | 1.00 ± 0.03 | 1.10 ± 0.12 | 0.05 ± 0.03 |
a (cis/trans, 7/3).
As shown in Table 13, vanillin was also two times more soluble in cis-pinane than in cis-rich pinane. The presence of the trans-pinane isomer appeared to reduce β-carotene and vanillin solubility, but had no effect on rosmarinic acid solubility because both solvents solubilized the same amount (Table 13).
4 Conclusions
In this study, a series of commercially available catalysts were studied for the hydrogenation of pinenes to systematically evaluate the influence of the active metals, supports, and reaction conditions on catalytic activity and selectivity. Of the four catalysts, Ru was the most selective and Pd was the least selective. In general, the supports, either charcoal or alumina, had a significant influence on the catalytic properties of the catalyst under neat conditions. The effect was very dependent on the metal considered. For most of the essays realized, activation by sonication had little effect on the hydrogenation of the pinenes except when using Ru as a catalyst. In the case of Ru/C, exposure to ultrasound allowed a complete and selective solvent-free hydrogenation of pinenes in favor of cis-pinane. In the absence of ultrasound, the same catalyst was inactive. On the contrary, sonication of Ru/Al2O3 completely deactivated the catalyst that was able to efficiently and very selectively hydrogenate in favor of cis-pinane without ultrasounds. In all essays, activation using heating allowed to reduce the time of reaction very significantly. Each time, however, it has been in the disfavor of selectivity toward cis-pinane. The extremely low leaching rate of Ru and all other metals determined by ICP/MS confirmed the heterogeneous nature of this catalytic solvent-free hydrogenation. In terms of recyclability, Ru/C was recycled seven times with no decline in selectivity and activity, whereas Ru/Al2O3 was selective for only one single cycle. As compared with a cis-rich pinane, cis-pinane solubilized more β-carotene and vanillin. Consequently, cis-pinane could be an interesting bio-based solvent to replace fossil-based solvents for the extraction of several natural products widely used in food and pharmaceutical industry.
Acknowledgments
Authors would like to acknowledge the contribution of the New Brunswick Innovation Foundation (NBIF), the Canadian Foundation for Innovation (CFI), and the Natural Sciences and Engineering Council (NSERC) of Canada (Grant 04560 to M.T.). The authors thanked Dr. A. Nait Ajjou for sharing his autoclave and Dr. Simon Lamarre for sharing his GC–MS.


