1 Introduction
High purity rare earth metals (REMs) have multiple scientific applications in various sectors, which has not only increased the demand but also made their production technically challenging. Thus, recovery of highly pure REMs has become a thrust research area and need for a viable extraction process is always desirable to reduce environmental issues. Attempts have been made to recover REMs from leach liquor/solution generated from ore, concentrate, and slurry of mining. For this purpose, solvent extraction (SX) was found to be an effective tool usually applied to separate a particular metal from mixed metals solution or to get their pure mixed solution and compounds. Literature survey indicates that the researchers have used various solvents viz. cationic, anionic, and solvating for the extraction of REMs from their leach liquor/aqueous solution containing REMs. It was observed that organophosphorus compounds were mostly preferred for separating REMs using SX. The characteristics of variation in the basic nature of REMs and species formation in aqueous phases were applied to achieve their selective oxidation/reduction, partial precipitation, crystallization, solid–liquid ion exchange (ion exchange), and liquid–liquid ion exchange (SX). The extractants D2EHPA, Cyanex 272, Versatic 10, naphthenic acid, TBP, Aliquat 336, HEHEHP (PC88A), Ionquest 80, P-507, SME 418, and so forth were reported for the extraction of REMs from different medium [1–4].
Most of the authors used D2EHPA to study the extraction of REMs from sulfate, nitrate, chloride, and perchlorate aqueous solution. D2EHPA diluted in kerosene was used for the separation of Sm, Eu, and Gd from Ce-depleted nitric–hydrochloric acid solution generated during processing of lanthanide hydrous oxide cake of monazite [5]. SX studies carried out using D2EHPA showed increase in the extraction efficiency for REMs in the order La < Ce < Pr < Nd < Sm < Eu < Gd < Tb < Dy < Ho < Er < Tm < Yb < Lu with increase in the atomic number [6]. The mixer–settler units (MSUs) having several stages were used for the separation of REMs on large scale. Apart from D2EHPA, other acidic organophosphorus extractants viz. HEHEHP, commercially known as PC88A, SME 418, P-507, and Ionquest 80, were also used for the separation of mostly heavy REMs because of its comparatively lower affinity with REMs than D2EHPA. Thus, REMs from loaded PC88A are easily stripped at low acid concentration and require less number of stripping stages as compared with D2EHPA [3,7]. Studies were carried out using PC88A and saponified PC88A to extract Sm from chloride solution and the equilibrium constant was estimated by ionic equilibria. The reactions of Sm involved with PC88A and saponified PC88A are described in Eqs. 1 and 2, respectively [8]. SX studies to separate Y(III) from sulfate, nitrate, and chloride medium using PC88A diluted in kerosene were carried out, which conclude that Y(III) separation from light REMs and heavy REMs worked well with nitric acid and sulfuric acid, respectively [9].
| (1) |
| (2) |
Apart from D2EHPA and PC88A, amine extraction process was also reported for the recovery/separation of REMs from the sulfate leach liquor of monazite [10,11]. La(III) and Ce(III) were extracted from chloride solution using LIX-70 dissolved in kerosene/n-heptane. The extraction efficiency of both metals depends upon the pH and solvent concentration but was independent of the metal/chloride concentration [12]. Cyanex 272 diluted in toluene was reported to extract La(III) from acidic nitrate–acetato media, where La(Ac)2·A·3HA and LaA3 are the species extracted during the SX reaction [13]. Cyanex 272 has been reported to have best extraction ability toward Pr and Nd than La [14]. Aforementioned studies revealed that D2EHPA, PC88A, TBP, Versatic 10, and Aliquat 336 have been widely used to extract REMs via SX technique where hundreds of stages of mixers and settlers were required to achieve the desired separation [15]. Literature survey revealed that most of the work reported were carried out using synthetic solution and few with actual but not systematic. Presented research work is based on systematic SX of REMs from actual leach liquor of monazite mineral.
Although D2EHPA has commercial viability because of good separation and extraction ability, its stripping problem has gathered attention to explore other suitable extractants. In comparison to D2EHPA, the PC88A can easily be regenerated using low acid concentration. Therefore, present article is focused on the study of SX behavior and mechanism of some REMs viz. La, Nd, Ce, and Pr from the chloride leach liquor of monazite using PC88A mixed with modifier isodecanol (ID) and diluted in kerosene.
2 Materials and methods
2.1 Preparation of the leach liquor
Pretreatment of monazite mineral (supplied by Korea Institute of Geosciences and Mineral Resources, South Korea) was carried out by baking/roasting it with NaOH at 400 °C for easy removal of phosphate ions, which provides hindrance in REM dissolution. The complete study and discussion was previously carried out by our group [16]. The treated monazite was further leached with 6 M HCl at 80 °C for 2 h maintaining pulp density 30 g/L resulting in maximum dissolution of REMs present in monazite. The leach liquor obtained after each set of experiment was mixed together, stirred properly, filtered, and the same was used for the SX studies to notice the behavior of REMs with PC88A. Leach liquor was diluted twice using deionized water to increase the pH by decreasing the concentration of H+ ions in the solution. The content of Th present in the monazite was about 2.8%, which remained in the leached residue with other radioactive metals and was kept safely for further processing.
2.2 Reagents and apparatus
Twice diluted leach liquor containing 0.87 g/L La, 0.97 g/L Ce, 0.39 g/L Nd, and 0.14 g/L Pr along with other REMs in traces was used for experimental purpose. Organic extractant 2-ethylhexyl phosphonic acid-mono-2-ethylhexyl ester (PC88A) diluted in kerosene and mixed with modifier ID was used as extractant to carry out the SX studies. The modifier ID prevents the formation of third phase and provides good separation during extraction. All reagents used were of analytical reagent grade. The presence of metals in different solutions was analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (VISTA-PMX, CCD Simultaneous; Australia).
2.3 Experimental procedures
SX studies were carried out at room temperature, ±25 °C, by mixing equal volume (50 mL each) of aqueous and organic for 5 min in a beaker provided with magnetic stirrer to get equilibrium condition. The mixture of organic and aqueous phases was allowed to settle and separated using a separating funnel. Various process parameters viz. pH, time, O/A ratio, loading capacity, and so forth were optimized. On the basis of the obtained data the formation of complex, stage requirement, separation factors, and suitable stripping condition were determined. The distribution ratio of REMs was calculated by dividing the concentration of metal in the organic phase by the concentration of metal in the aqueous phase, whereas their extraction percentage was calculated by multiplying the distribution ratio of REMs with 100. Separation factor between adjacent REMs was determined by dividing distribution ratio of one metal by another. The equations or formulas for the distribution ratio and extraction percentage of REMs as well as the separation factor between adjacent REMs are presented via Eqs. 3–5, respectively.
| (3) |
| (4) |
| (5) |
3 Results and discussion
SX studies were carried out for the recovery of REMs from the chloride leach liquor of monazite mineral. Various process parameters viz. equilibrium pH, time, O/A ratio, and so forth were studied and optimized for maximum extraction of REMs.
3.1 Effect of mixing time
To minimize the consumption of energy during SX process and get a minimum retention time to achieve equilibrium condition, the studies were initially carried out varying the contact time of organic with aqueous from 4 to 15 min. It was observed that using 5% PC88A diluted in kerosene with 3% ID at an O/A ratio of 1/1 and equilibrium pH 1.2, about 6.15% La, 35.70% Ce, 84.48% Nd, and 67.71% Pr were extracted from the leach liquor in 5 min. Further increase in mixing time showed negligible effect on the extraction of REMs. This might be because of the saturation of the transfer of metal ions from the aqueous to organic phase for complex formation once the equilibrium state was reached. Thus, 5 min was considered optimum for extracting REMs from the leach liquor. Therefore, further studies were carried out maintaining the mixing time 5 min.
3.2 Effect of equilibrium pH
To reduce the chemical consumption for adjusting equilibrium pH and achieve the optimum condition, SX studies were carried out by varying the pH of solution. Cationic extractant PC88A was used for the extraction of REMs where extraction occurred because of the exchange of hydrogen ion. The equilibrium pH was varied between 1 and 3.5 and mixing time of 5 min. REMs extraction was found to increase on increasing the equilibrium pH as shown in Fig. 1. Maximum extraction of REMs was found to occur between 2.5 and 3.5 pH. Extraction of La, Ce, Nd, and Pr was 99.40%, 99.95%, 99.97%, and 99.05%, respectively, at equilibrium pH 3.5. Further increase in pH does not show any significant increase in extraction of REMs. The reason might be the unavailability of H+ ions in the organic phase for the exchange of metal ions. Fig. 2 shows the plot of log D versus pH for REMs. For La, Ce, Nd, and Pr, straight lines with a slope value of 1.44, 1.31, 1.47, and 1.41, respectively, were obtained. From the aforementioned data, it can be concluded that release of 1.5 mol of H+ takes place for each metal ion complexation forming organometallic bond during the loading of the metal. Thus, the extraction equilibrium of REMs (denoted as M) with PC88A (HX) in kerosene can be expressed as [8,17,18]

Effect of equilibrium pH. (Aqueous: Leach liquor of REMs; Organic: 5% PC88A + 3% ID; O/A ratio: 1:1; Time: 5 min.)

Log D versus pH.
| (6) |
| (7) |
3.3 Effect of O/A ratio
Experiments were carried out to study the effect of an O/A ratio on the extraction of REMs from leach liquor containing 0.87 g/L La, 0.97 g/L Ce, 0.39 g/L Nd, and 0.14 g/L Pr. The O/A ratio was varied from 1:5 to 5:1 to determine the number of stages required for complete extraction of REMs. Increase in the extraction of REMs was observed on increasing the O/A ratio. This was because of the increase in organic available for extraction or increased availability of H+ sites for exchange of metal ions. Maximum extraction of La, Nd, Ce, and Pr was observed at an O/A ratio of 1. About 99.58% La, 99.46% Ce, 99.99% Nd, and 95.32% Pr were extracted at pH 3 and mixing time of 5 min (Fig. 3).

Effect of an O/A ratio. (Aqueous: Leach liquor of REMs; Organic: 5% PC88A + 3% ID; pH: 3; Time: 5 min.)
McCabe–Thiele plot was drawn based on the aforementioned data so as to observe the number of stages required for complete extraction of each REM, during simulation of the process in continuous mode. Single stage for La, Ce, and Nd and two stages for Pr were required for complete extraction in the Mixer Settler Unit (MSU) as presented in Fig. 4(a–d).
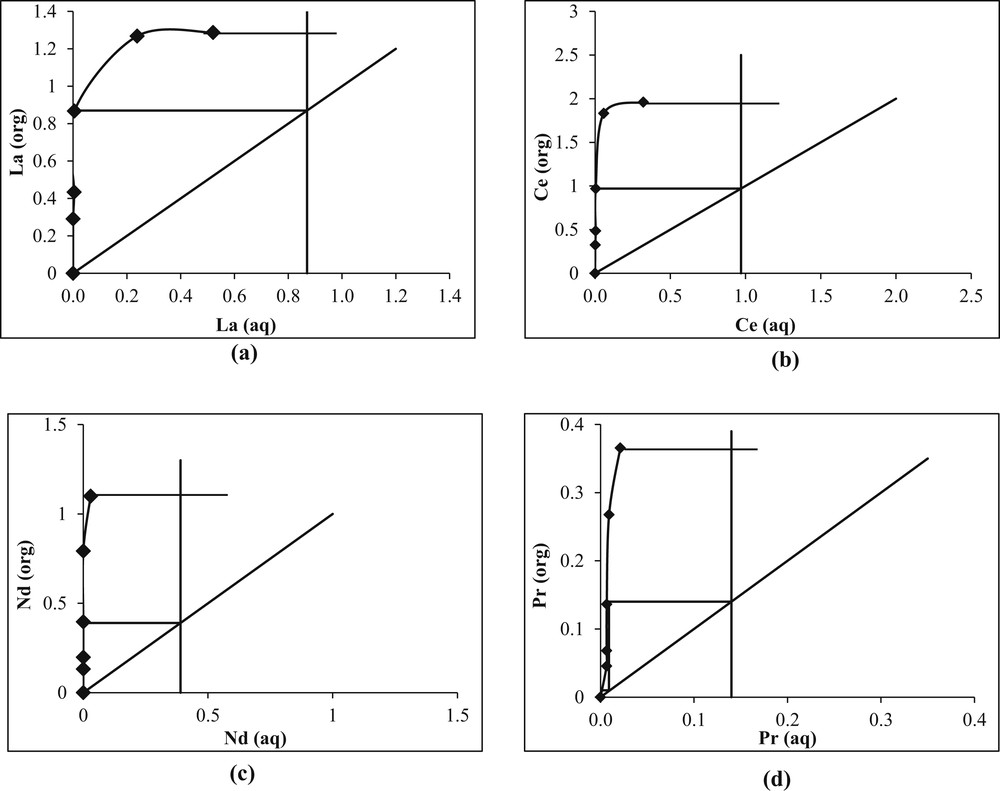
McCabe–Thiele diagram. (Aqueous: Leach liquor of REMs; Organic: 5% PC88A + 3% ID; pH: 3; O/A ratio: 1:1; Time: 5 min.)
3.4 Distribution of various REMs during SX
Separation ability of adjoining REMs can be compared by calculating their separation factor (β). For effective separation between two REMs, the value of β must always be >1. The value of β was calculated for Ce/La, Ce/Nd, Ce/Pr, Nd/La, Pr/La, and Pr/Nd at different pH values. As shown in Table 1, the separation factor was observed to change with change in equilibrium pH. The separation of Ce/La, Nd/La, and Pr/La was 60.85, 72.17, and 98.33, respectively, at equilibrium pH 2, which further decreases with increase in pH. The separation for Ce/Pr was found to be 17.40 at equilibrium pH 3.5, whereas for Ce/Nd and Pr/Nd were 6.45 and 4.20, respectively. The aforementioned results obtained indicate high separation between Ce/La, Nd/La, and Pr/La at equilibrium pH ∼2 and relatively low separation between Ce/Pr, Ce/Nd, and Pr/Nd. These data can be used for selective separation as well as controlling the number of stages required for extraction using MSU.
Distribution of various REMs during separation using SX.
| Distribution | Separation factor of REMs at various equilibrium pH | ||||||
| pH 1 | pH 1.2 | pH 2 | pH 2.2 | pH 2.5 | pH 2.7 | pH 3.5 | |
| Ce/La | 25.66 | 36.10 | 60.85 | 37.23 | 13.55 | 45.96 | 11.01 |
| Ce/Nd | 6.45 | 6.30 | 0.84 | 0.71 | 0.90 | 1.23 | 0.55 |
| Ce/Pr | 1.53 | 2.60 | 0.62 | 0.53 | 0.62 | 2.22 | 17.40 |
| Nd/La | 3.98 | 5.74 | 72.17 | 52.75 | 15.06 | 37.48 | 20.14 |
| Pr/La | 16.73 | 13.86 | 98.33 | 70.58 | 21.90 | 20.71 | 0.63 |
| Pr/Nd | 4.20 | 2.42 | 1.36 | 1.34 | 1.45 | 0.55 | 0.03 |
3.5 Loading capacity of PC88A
Experiments were carried out to determine the loading capacity of PC88A modified with 3% ID and diluted in kerosene. Maximum loading of REMs into the organic phase from the aqueous phase at a constant pH and temperature was carried out by treating fresh leach liquor with the same organic repeatedly. The maximum loading capacity of PC88A was found to be 4.70, 16.37, 3.56, and 1.24 g/L for La, Ce, Nd, and Pr, respectively, in seven cumulative contacts at an O/A ratio of 1:1 in 5 min (Fig. 5).
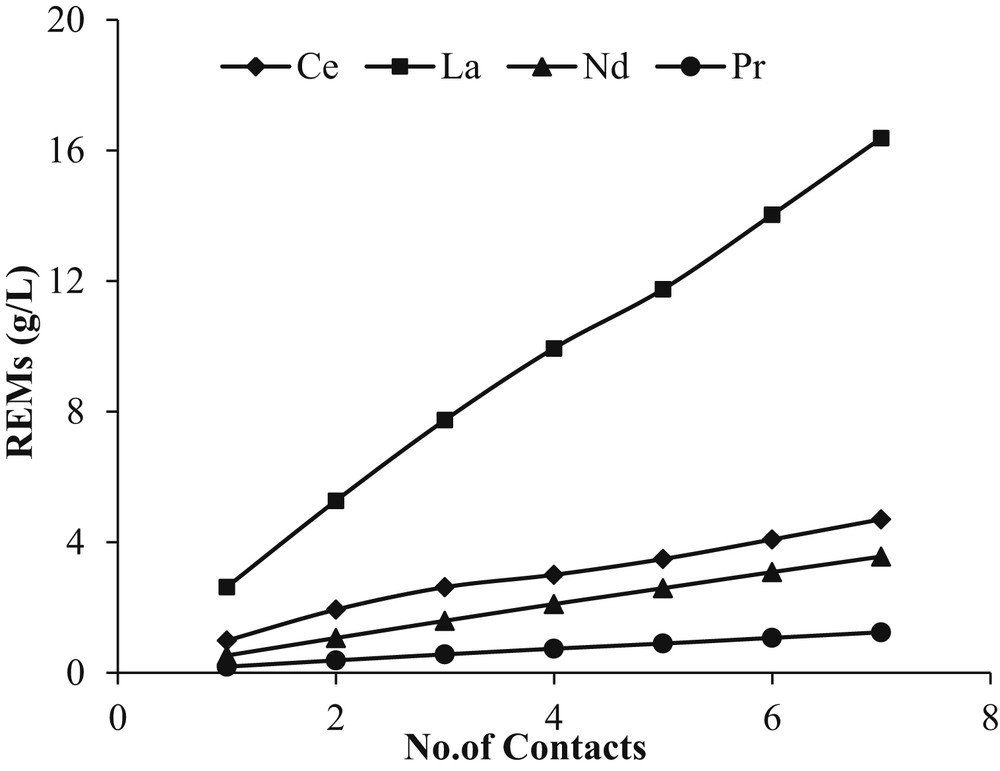
Loading capacity of PC88A. (Aqueous: Leach liquor of REMs; Organic: 5% PC88A + 3% ID; Equilibrium pH: 3.5; O/A ratio: 1:1; Time: 5 min.)
3.6 SX of REMs in continuous mode
Studies were also made to determine number of stages required for complete REMs extraction in continuous mode. At different equilibrium pH, freshly prepared organic was mixed with the same leach liquor in cross-current mode (Fig. 6). On increasing the number of stages, the extraction of REMs was also found to be increased. About 99.77% La, 99.79% Ce, 99.46% Nd, and 99.11% Pr got extracted in seven stages. For all stages, satisfactory mass balance was obtained.
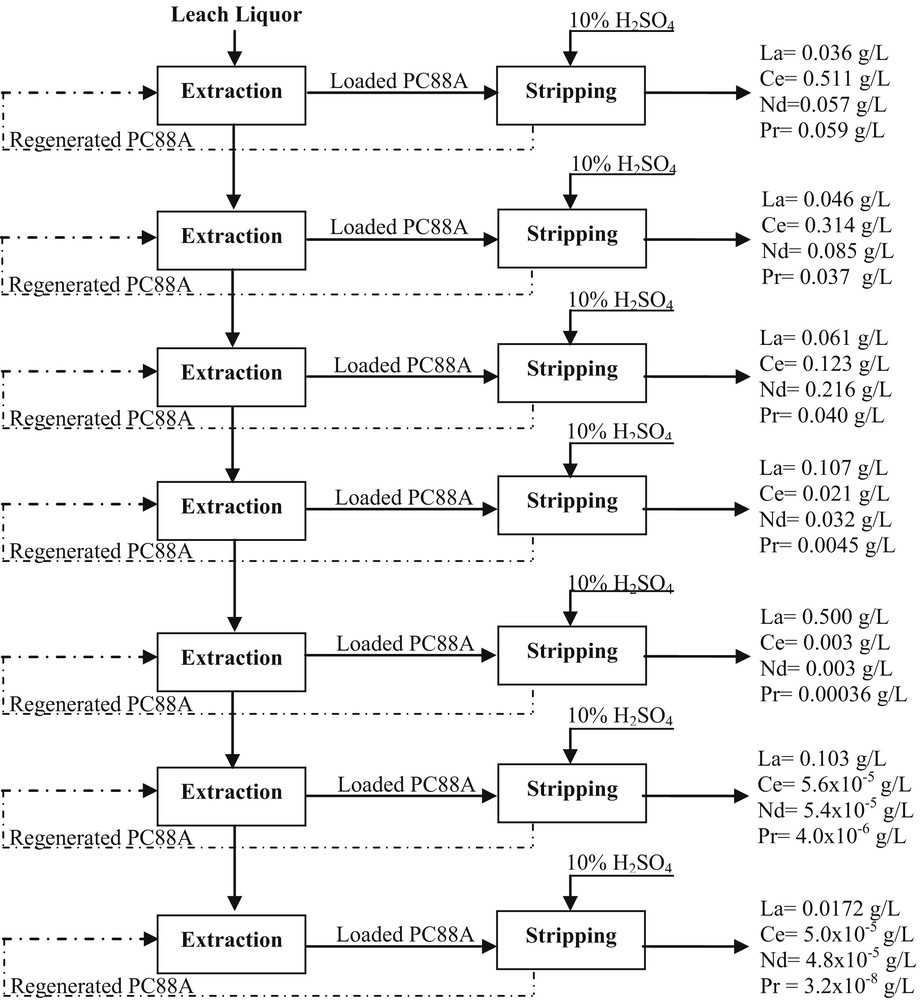
SX of REMs in cross-current mode.
3.7 Stripping of loaded PC88A
In view of environmental and ecological concern, experiments were carried out to study the stripping of REMs from the loaded organic using H2SO4 because of its less hazardous characteristics. It was observed that 10% H2SO4 sufficiently stripped REMs present in the loaded organic. In a mixing time of 10 min at an O/A ratio of 1:1, ∼80.07% La, 88.85% Ce, 83.48% Nd, and 65.42% Pr were stripped from the loaded organic in four contacts. On increasing the number of contacts under the given experimental conditions, complete back extraction of La, Ce, Nd, and Pr was achieved.
4 Conclusions
On the basis of the studies made for the extraction of REMs from the actual leach liquor following conclusions can be drawn:
About 99.40% La, 99.95% Ce, 99.97% Nd, and 99.05% Pr was effectively extracted at equilibrium pH ∼3.5 using 5% PC88A diluted in kerosene and modified with 3% ID in 5 min maintaining an O/A ratio of 1:1. McCabe–Thiele plot suggests requirement of single stage for complete extraction of La, Ce, and Nd whereas in case of Pr two stages will be required using MSU. High separation factors were obtained for Ce/La (60.85), Nd/La (72.17), and Pr/La (98.33) at equilibrium pH 2. Seven stages were required to extract 99.77% La, 99.79% Ce, 99.46% Nd, and 99.11% Pr in continuous mode using MSU. Almost 80.07% La, 88.85% Ce, 83.48% Nd, and 65.42% Pr got stripped from loaded PC88A using 10% H2SO4 in 10 min at an O/A ratio of 1:1 in four contacts. The stripped solution containing mixed REMs can further be treated to get rare earth oxide salts using precipitation/evaporation/crystallization.
Acknowledgments
Authors are thankful to the Director, CSIR-NML for giving permission to publish this article, based on the research work carried out at CSIR-National Metallurgical Laboratory (CSIR-NML), Jamshedpur, India, for the extraction of REMs. One of the authors, Archana Kumari is thankful to CSIR, New Delhi, India for providing grant under CSIR-Senior Research Fellowship scheme (Grant: 31/10(60)/2015-EMR-I) to carry out this research work.


