
1 Introduction
Finding of novel potential anticancer drugs, as well as development of efficient methods for their synthesis and preparation of appropriate building blocks, is one of the most challenging problems in medical chemistry. Because of the electrophilic nature of the aziridine ring, derivatives of aziridine-2-carboxylic acid are interesting synthetic substrates for creating various amino acids, alkanolamines and heterocyclic compounds [1]. Some derivatives of aziridine-2-carboxylic acid, namely, imexon, azimexon [2] and leakadine [3] have shown anticancer immunomodulatory activity themselves and were developed as anticancer drug candidates.
Only a small number of activation methods of aziridine-2-carboxylic acid derivatives for ester and amide bound formation are known in the literature. Some examples of N, N′ - dicyclohexylcarbodiimide (DCC) [4–6], 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) [7] and Castro reagent [8] activation have been reported. Other methods include alkylation of metal aziridine-2-carboxylates [9], using anhydride [10], mixed anhydride [11,12] and in case of aziridine 2,2-dicarboxylic diesters, chloroanhydride activation have been shown [13–15]. Some examples of solid phase peptide synthesis have also been demonstrated [16,17]. These methods are limited only to aziridine substrates with free carboxyl or carboxylate salt functionality. Promising method of AlMe3–mediated aziridine-2-carboxylic acid ester aminolysis was reported [18]. However, that method gave only N-trityl–protected aziridine-2-carboxamides.
Here, we report a convenient route for facile synthesis of various aziridine-2-carboxamides using Davidsen bis-Boc activation methodology [19].
2 Materials and methods
The 1H NMR spectra were recorded using a Varian Mercury 200 (200 MHz) and using Varian Mercury plus 400 (400 MHz) spectrometers, TMS or CDCl3 remaining signal (δ 7.26 ppm, solvent CDCl3) was used as an internal standard. The 13C NMR spectra were recorded using Varian Mercury plus 400 spectrometers. CDCl3 remaining signal (δ 77.16 ppm) was used as an internal standard. High-resolution mass spectrometry (HRMS) was measured using a Waters Synapt G2-Si mass spectrometer. Melting points were determined on a Gallenkamp heating stage and are uncorrected. Thin-layer chromatography was carried out using DC Alufolien plates of Kieselgel 60. Column chromatography was carried out on silica gel (Merck), 0.023–0.070 mm, pore diameter ca. 6 nm. All solvents were purified by standard procedures. Starting materials were synthesized according to the known methods [19] for compounds 3 and [20] 2 described in Section 5. General procedures for compounds 4 and 6 are also described in Section 5.
3 Results and discussion
Using aziridine-2-carboxamide (leakadine) (1) [3] as a starting material protected and activated substrate 2 was synthesized first by N-acylation of amide 1 with benzyl chloroformate (Cbz-chloride) and second by reaction of protected amide 2 with 2 equiv of Boc-anhydride under DMAP-catalysed conditions (Scheme 1).

Synthesis of acylimidodicarbonate 3.
Activated aziridine 3 is isolable and stable at room temperature.
Bis-Boc–activated amide 3 in reaction with a series of primary (Table 1, entries 5–10) and secondary (Table 1, entries 1–4) amines as well as anilines (Table 1, entries 11–15) gave amides 4a–o mostly in good yields (Scheme 2). The main withdraw is formation of bis-Boc-amine 5 as a byproduct, therefore purification by chromatography is required. It was observed that Cbz-protected compounds 4 often exhibited rather low stability by storing at room temperature. That might be explained by electron deficiency in aziridine. Therefore, aziridines 4 were deprotected shortly after purification.
Reaction conditions and yields of intermediate amides 4.
| No. | Product 4 | Reaction time (h) | Yield (%) |
| 1 | Image 2 | 2 | 72 |
| 2 | Image 3 | 1.5 | 72 |
| 3 | Image 4 | 2 | 43 |
| 4 | Image 5 | 2 | 91 |
| 5 | Image 6 | 1.5 | 57 |
| 6 | Image 7 | 4 | 85 |
| 7 | Image 8 | 2.5 | 62 |
| 8 | Image 9 | 5 | 66 |
| 9 | Image 10 | 2 | 38 |
| 10 | Image 11 | 3 | 90 |
| 11 | Image 12 | 7 | 55 |
| 12 | Image 13 | 4 | 86 |
| 13 | Image 14 | 3 | 51 |
| 14 | Image 15 | 12 | 62 |
| 15 | Image 16 | 12 | 57 |

Synthesis of amides 4 and 6.
Deprotection of 4 was performed by palladium-catalysed hydrogenation at atmospheric pressure at room temperature in short (1.5–3 h). The results are summarized in Table 2. It is worth to notice that low hydrogen pressure used in the reaction allows selective removal of the Cbz-protecting group providing benzyl amides in good to high yields (entries 2, 7–10).
Reaction conditions and yields of deprotected aziridines 6.
| No. | Product 6 | Reaction time (h) | Yield (%) |
| 1 | Image 17 | 2 | 85 |
| 2 | Image 18 | 1.5 | 84 |
| 3 | Image 19 | 3 | 78 |
| 4 | Image 20 | 2 | 32 |
| 5 | Image 21 | 2 | 51 |
| 6 | Image 22 | 2 | 75 |
| 7 | Image 23 | 1.5 | 90 |
| 8 | Image 24 | 3 | 96 |
| 9 | Image 25 | 1.5 | 100 |
| 10 | Image 26 | 1.5 | 92 |
| 11 | Image 27 | 1 | 83 |
| 12 | Image 28 | 2 | 75 |
| 13 | Image 29 | 2 | 74 |
| 14 | Image 30 | 2 | 81 |
| 15 | Image 31 | 2 | 83 |
4 Conclusions
We have demonstrated a convenient synthesis of substituted amides of aziridine-2-carboxylic acid.
Activated acylimidodicarbonate obtained from aziridine-2-carboxamide reacts with various amines as nucleophiles to form secondary and tertiary aziridine-2-carboxylic acid amides. Hydrogenolythic deprotection of obtained Cbz-protected compounds leads to corresponding NH-aziridines. Reactions undergo at mild conditions and are regioselective. No nucleophilic ring opening of aziridines is observed.
5 Experimental section
5.1 Synthesis of starting materials
5.1.1 Benzyl-2-(aminocarbonyl)aziridine-1-carboxylate (2)
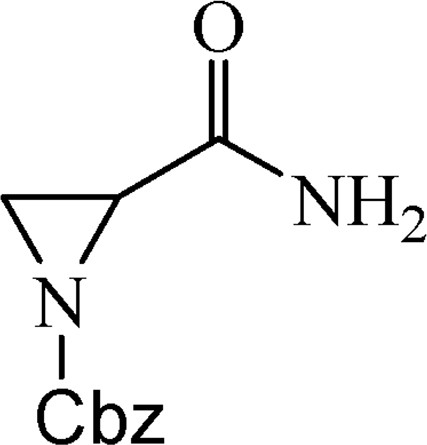
Compound 1 (10.00 g, 0.12 mol) was dissolved in saturated NaHCO3 (125 mL), EtOAc (125 mL) was added and then CbzCl (16.49 mL, 0.12 mol) was added slowly. Reaction mixture (RM) was stirred at room temperature for 3 days. It was extracted with EtOAc (2 × 100 mL) and washed with water. Organic phase was dried over Na2SO4, filtered and evaporated. The product was obtained as a white crystalline solid (22.70 g, 89%), mp 92–93 °C.
1H NMR (CDCl3, 200 MHz), δ, ppm: 2.40 (1H, dd, J = 0.6, J = 3.3 Hz), 2.57 (1H, d, J = 6.6 Hz), 3.04 (1H, dd, J = 3.3, J = 6.6 Hz), 5.15 (2H, m), 5.77 (1H, br s), 6.17 (1H, br s), 7.20–7.45 (5H, m) [20].
LC-MS (ESI, m/z): [M + Na]+ 243.
5.1.2 Benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3)

Benzyl-2-(aminocarbonyl)aziridine-1-carboxylate (2) (3.00 g, 13.62 mmol) was suspended in dry DCM (30 mL), DMAP (170 mg, 1.36 mmol) was added, RM was cooled to 0 °C and Boc2O (5.95 g, 27.24 mmol) solution in dry DCM (10 mL) was added slowly. RM was stirred at room temperature until benzyl-2-(aminocarbonyl)aziridine-1-carboxylate was completely dissolved (∼30 min), filtered through silica and evaporated. Product was purified by column chromatography on silica gel, eluent ether/EtOAc (4:1). Note: evaporation has to be done in vacuum with heating at less than 30 °C; purification has to be done immediately after evaporation. The product was obtained as a colourless oil (4.7 g, 82%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 1.52 (18H, s), 2.53–2.63 (2H, m), 4.05–4.12 (1H, m), 5.14 (2H, m), 7.30–7.40 (5H, m).
13C NMR (D2O, 100 MHz), δ, ppm: 27.6, 28.0, 32.7, 36.1, 68.6, 81.9, 85.5, 128.4, 128.5, 135.4, 149.0, 161.0, 168.4.
HRMS (ESI, m/z): [M + H]+ Calcd C21H29N2O7: 421.1975. Found: 421.1968.
5.2 Amides 4a–o
5.2.1 General procedure
Benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.00 g, 2.38 mmol) was dissolved in dry DCM (5 mL) and corresponding amino derivative (2.38 mmol) was added. RM was stirred at room temperature for 1.5–12 h and then evaporated. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc.
5.2.2 Benzyl-2-[(diethylamino)carbonyl]aziridine-1-carboxylate (4a)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.19 g, 2.83 mmol) and diethylamine (0.26 mL, 2.55 mmol). RM was stirred for 2 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as white crystalline solid (562 mg, 72%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 1.10 (3H, t, J = 7.1 Hz), 1.23 (3H, t, J = 7.1 Hz), 2.37–2.44 (1H, m), 2.70–2.76 (1H, m), 3.20–3.27 (1H, m), 3.38 (2H, q, J = 7.2 Hz), 3.40 (2H, m), 5.13 (2H, m), 7.35 (5H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C15H21N2O3: 277.1552. Found: 277.1541.
5.2.3 Benzyl-2-{[benzyl(methyl)amino]carbonyl}aziridine-1-carboxylate (4b)
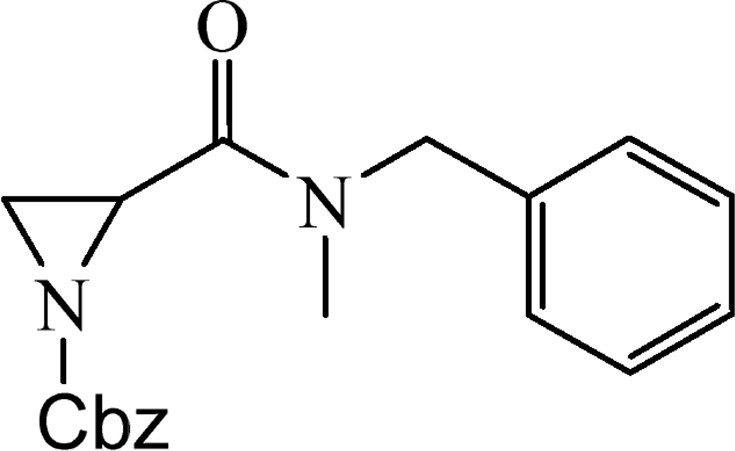
This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.80 g, 1.90 mmol) and N-methyl-N-benzylamine (0.22 mL, 1.71 mmol). RM was stirred for 1.5 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (443 mg, 72%).
1H NMR (CDCl3, 200 MHz), mixture of two rotamers, δ, ppm: 2.35–2.48 (1H, m), 2.73–2.78 (1H, m), 2.94 (1.5H, s), 3.11 (1.5H, s), 3.23–3.29 (0.5H, m), 3.31–3.38 (0.5H, m), 4.57 (1H, s), 4.65 and 4.81 (1H, AB, J = 17.0 Hz), 5.11 (1H, s), 5.11 and 5.17 (1H, AB, J = 12.2 Hz), 7.12–7.40 (10H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C19H21N2O3: 325.1552. Found: 325.1544.
5.2.4 Benzyl-2-(piperidin-1-ylcarbonyl)aziridine-1-carboxylate (4c)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.66 g, 1.57 mmol) and piperidine (0.14 mL, 1.41 mmol). RM was stirred for 2 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (196 mg, 43%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 1.40–1.65 (6H, m), 2.35–2.41 (1H, m), 2.66–2.71 (1H, m), 3.23–3.31 (1H, m), 3.34–3.76 (4H, m), 5.11 (2H, s), 7.32 (5H, s).
HRMS (ESI, m/z): [M + H]+ Calcd C16H21N2O3: 289.1552. Found: 289.1547.
5.2.5 Benzyl-2-(morpholin-4-yl)carbonyl]aziridine-1-carboxylate (4d)
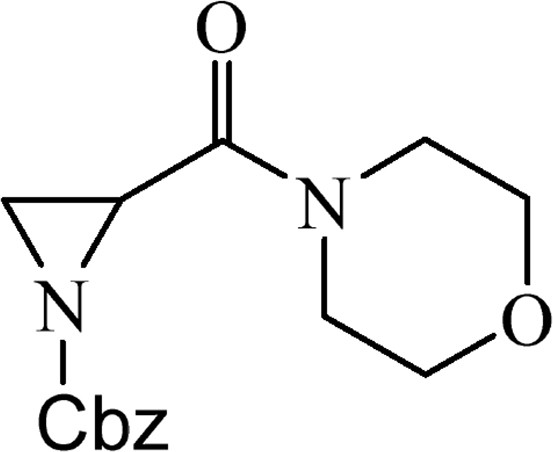
This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.00 g, 2.38 mmol) and morpholine (0.21 mL, 2.38 mmol). RM was stirred for 2 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (630 mg, 91%).
1H NMR 2,45 (1H, d, J = 5.6 Hz), 2.75 (1H, d, J = 3.3 Hz), 3.24 (1H, dd, J = 3.3 Hz, J = 5.6 Hz), 3.44–3.53 (1H, m), 3.54–3.74 (6H, m), 3.77–3.87 (1H, m), 5.12 and 5.14 (AB, 2H, J = 12.2 Hz), 7.30–7.41 (5H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C15H19N2O4: 291.1345. Found: 291.1339.
5.2.6 Benzyl-2-[(isopropylamino)carbonyl]aziridine-1-carboxylate (4e)
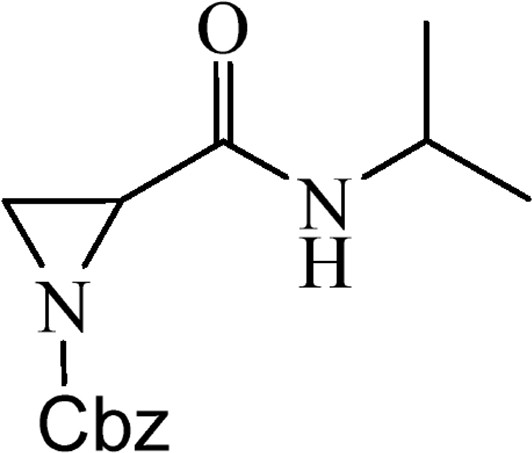
This is obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.91 g, 2.15 mmol) and isopropylamine (0.17 mL, 1.94 mmol). RM was stirred for 1.5 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (311 mg, 57%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 1.10 (3H, d, J = 6.6 Hz), 1.16 (3H, d, J = 6.6 Hz), 2.28–2.32 (1H, m), 2.51–2.56 (1H, m), 3.02 (1H, dd, J = 3.3, J = 6.6 Hz), 3.90–4.12 (1H, m), 5.12 and 5.18 (2H, AB, J = 12.1 Hz), 7.36 (5H, s).
HRMS (ESI, m/z): [M + H]+ Calcd C14H19N2O3: 263.1396. Found: 263.1392.
5.2.7 Benzyl-2-[(pentylamino)carbonyl]aziridine-1-carboxylate (4f)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.04 g, 2.47 mmol) and n-pentylamine (0.26 mL, 2.23 mmol). RM was stirred for 4 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (613 mg, 85%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 0.83–0.93 (3H, m), 1.26–1.35 (4H, m), 1.40–1.54 (2H, m), 2.32 (1H, d, J = 3.3 Hz), 2.56 (1H, d, J = 6.7 Hz), 3.05 (1H, dd, J = 3.3 un 6.7 Hz), 3.22 (2H, m), 5.13 and 5.17 (2H, AB, J = 12.1 Hz), 7.37 (5H, s).
HRMS (ESI, m/z): [M + H]+ Calcd C16H23N2O3: 291.1709. Found: 291.1713.
5.2.8 Benzyl-2-[(benzylamino)carbonyl]aziridine-1-carboxylate (4g)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.70 g, 1.67 mmol) and benzylamine (0.17 mL, 1.51 mmol). RM was stirred for 2.5 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (320 mg, 62%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 2.34–2.38 (1H, m), 2.46 (1H, dt, J = 0.8, J = 6.3 Hz), 3.07 (1H, dq, J = 0.8, J = 3.4 Hz), 4.38 (2H, m), 5.09 and 5.12 (2H, AB, J = 12.2 Hz), 7.16–7.37 (10H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C18H19N2O3: 311.1396. Found: 311.1390.
5.2.9 Benzyl-2-{[(3-methylbenzyl)amino]carbonyl}aziridine-1-carboxylate (4h)
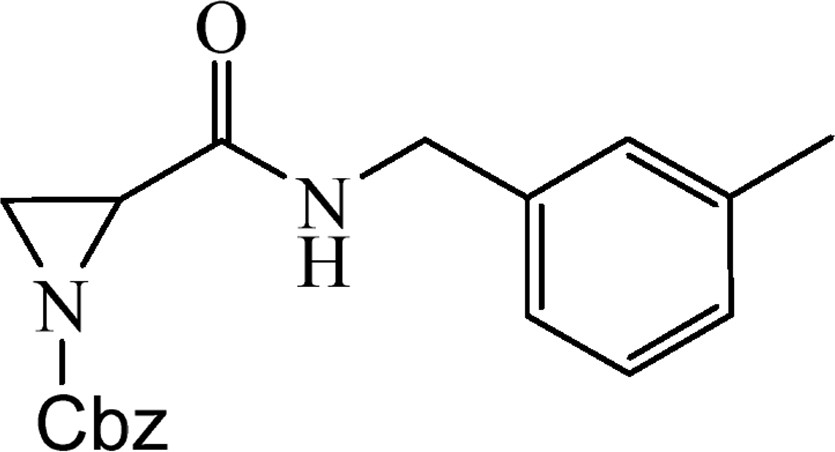
This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.00 g, 2.38 mmol) and 3-methylbenzylamine (0.27 mL, 2.14 mmol). RM was stirred for 5 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as colourless oil (505 mg, 66%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 2.33 (3H, s), 2.36 (1H, d, J = 3.3 Hz), 2.57 (1H, d, J = 6.6 Hz), 3.11 (1H, dd, J = 3.3, J = 6.6 Hz), 4.38 (2H, m), 5.14 (2H, m), 6.97–7.23 (4H, m), 7.36 (5H, s).
HRMS (ESI, m/z): [M + H]+ Calcd C19H21N2O3: 325.1552. Found: 325.1547.
5.2.10 Benzyl-2-{[(2-methylbenzyl)amino]carbonyl}aziridine-1-carboxylate (4i)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.60 g, 1.43 mmol) and 2-methylbenzylamine (0.16 mL, 1.29 mmol). RM was stirred for 2 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (2:1). The product was obtained as a colourless oil (177 mg, 38%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 2.28 (3H, s), 2.32–2.37 (1H, m), 2.55–2.61 (1H, m), 3.11 (1H, dd, J = 3.4, J = 6.7 Hz), 4.39–4.45 (2H, m), 5.14 (2H, m), 7.14–7.21 (4H, m), 7.34–7.39 (5H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C19H21N2O3: 325.1552. Found: 325.1551.
5.2.11 Benzyl-2-({[2-(trifluoromethyl)benzyl]amino}carbonyl)aziridine-1-carboxylate (4j)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.00 g, 2.38 mmol) and 2-trifluoromethylbenzylamine (0.30 mL, 2.14 mmol). RM was stirred for 3 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (2:1). The product was obtained as a colourless oil (581 mg, 90%).
1H NMR (CDCl3, 200 MHz), δ, ppm: 2.30–2.36 (1H, m), 2.57 (1H, dd, J = 1.5, J = 6.7 Hz), 3.06–3.14 (1H, m), 4.60 (2H, m), 5.13 (2H, m), 7.29–7.54 (8H, m), 7.60–7.69 (1H, m).
HRMS (ESI, m/z): [M + H]+ Calcd C19H18F3N2O3: 379.1270. Found: 379.1266.
5.2.12 Benzyl-2-[(4-chlorophenyl)carbamoyl]aziridine-1-carboxylate (4k)
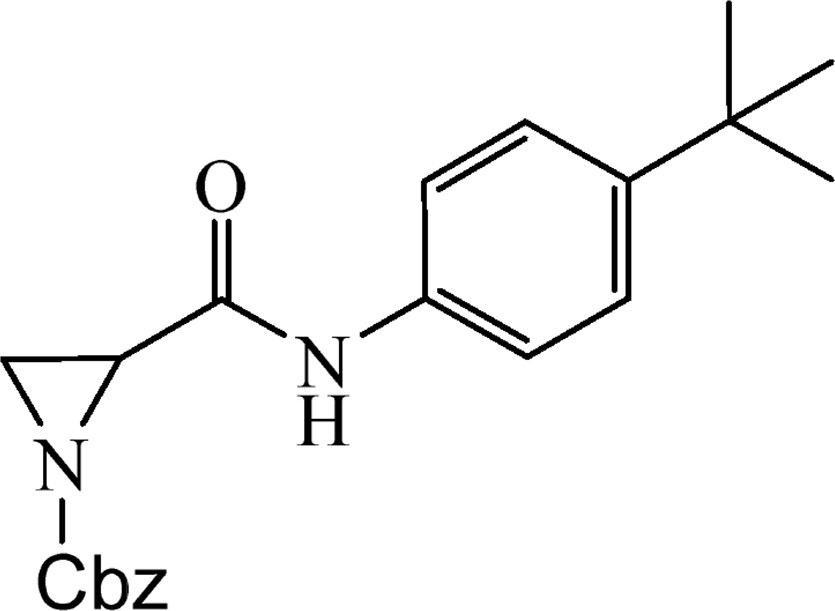
This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.90 g, 2.15 mmol) and 4-tert-butylaniline (0.34 mL, 2.15 mmol). RM was stirred for 7 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (2:1). The product was obtained as a colourless oil (414 mg, 55%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 1.30 (9H, s), 2.43 (1H, d, J = 3.3 Hz), 2.66 (1H, d, J = 6.8 Hz), 3.19 (1H, dd, J = 3.3 Hz, J = 6.8 Hz), 5.17 and 5.20 (2H, AB, J = 12.1 Hz), 7.34 (2H, d, J = 8.6 Hz), 7.37 (5H, s), 7.43 (2H, d, J = 8.6 Hz), 7.89 (1H, br s).
HRMS (ESI, m/z): [M + H]+ Calcd C21H25N2O3: 353.1865. Found: 353.1860.
5.2.13 Benzyl-2-{[4-(trifluoromethyl)phenyl]carbamoyl}aziridine-1-carboxylate (4l)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.08 g, 2.57 mmol) and 4-trifluoromethylaniline (0.32 g, 2.57 mmol). RM was stirred for 4 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (2:1). The product was obtained as a colourless oil (510 mg, 86%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 2.53 (1H, d, J = 3.4 Hz), 2.64 (1H, d, J = 6.4 Hz), 3.26 (1H, dd, J = 3.4, J = 6.4 Hz), 5.19 and 5.21 (2H, AB, J = 12.1 Hz), 7.34–7.40 (5H, m), 7.51 (2H, d, J = 8.6 Hz), 7.61 (2H, d, J = 8.6 Hz), 8.53 (1H, br s).
HRMS (ESI, m/z): [M + H]+ Calcd C18H16F3N2O3: 365.1113. Found: 365.1109.
5.2.14 Benzyl-2-[(4-methoxyphenyl)carbamoyl]aziridine-1-carboxylate (4m)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (0.97 g, 2.31 mmol) and 4-methoxyaniline (0.29 g, 2.31 mmol). RM was stirred for 3 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (382 mg, 51%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 2.44 (1H, d, J = 3.4 Hz), 2.66 (1H, d, J = 6.9 Hz), 3.18 (1H, dd, J = 3.4, J = 6.9 Hz), 3.79 (3H, s), 5.15 and 5.20 (2H, AB, J = 12.1 Hz), 6.86 (2H, d, J = 9.0 Hz), 7.38 (5H, s), 7.42 (2H, d, J = 9.0 Hz), 7.84 (1H, br s).
HRMS (ESI, m/z): [M + H]+ Calcd C18H19N2O4: 327.1345. Found: 327.1339.
5.2.15 Benzyl-2-[(3,5-dimethylphenyl)carbamoyl]aziridine-1-carboxylate (4n)

This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.00 g, 2.30 mmol) and 3,5-dimethylaniline (0.29 mL, 2.38 mmol). RM was stirred for 12 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (2:1). The product was obtained as a colourless oil (477 mg, 62%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 2.28 (6H, s), 2.42 (1H, d, J = 3.5 Hz), 2.66 (1H, d, J = 6.9 Hz), 3.17 (1H, dd, J = 3.5 Hz, J = 6.9 Hz), 5.16 and 5.20 (2H, AB, J = 12.1 Hz), 6.77 (1H, s), 7.15 (2H, s), 7.37 (5H, s), 7.86 (1H, br s).
HRMS (ESI, m/z): [M + H]+ Calcd C19H21N2O3: 325.1552. Found: 325.1551.
5.2.16 Benzyl-2-[(2,4-dimethylphenyl)carbamoyl]aziridine-1-carboxylate (4o)
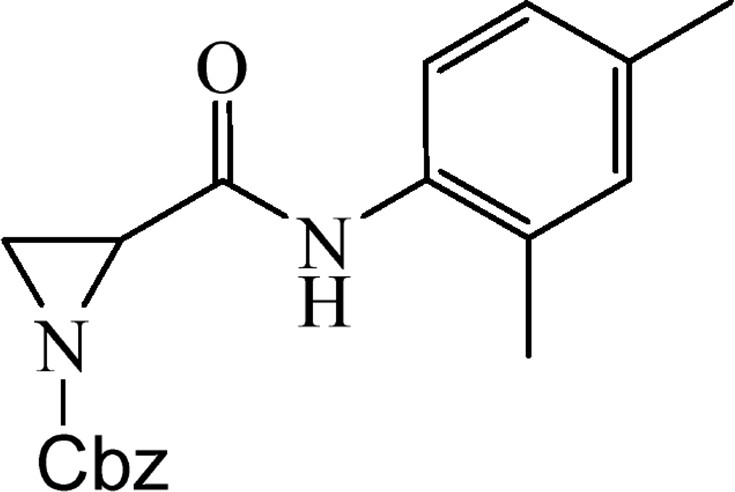
This was obtained from benzyl-2-{[bis(tert-butoxycarbonyl)amino]carbonyl}aziridine-1-carboxylate (3) (1.06 g, 2.52 mmol), 2,4-dimethylaniline (0.29 g, 2.38 mmol) and DMAP (0.03 g, 0.25 mmol). RM was stirred for 12 h. The product was purified by column chromatography on silica gel, eluent petroleum ether/EtOAc (1:1). The product was obtained as a colourless oil (465 mg, 57%).
1H NMR (CDCl3, 400 MHz), δ, ppm: 2.17 (3H, s), 2.28 (3H,s), 2.46 (1H, d, J = 3.4 Hz), 2.69 (1H, d, J = 6.8 Hz), 3.22 (1H, dd, J = 3.4 Hz, J = 6.8 Hz), 5.18 and 5.19 (2H, AB, J = 12.1 Hz), 6.97–7.03 (2H, m), 7.37 (5H, s), 7.68 (1H, d, J = 8.1 Hz), 7.84 (1H, br s).
HRMS (ESI, m/z): [M + H]+ Calcd C19H21N2O3: 325.1552. Found: 325.1546.
5.3 Amides 6a–o
5.3.1 General procedure for elimination of Cbz group
Benzyl-2-substituted aziridine-1-carboxylate 4a–o was dissolved in MeOH and Pd/C (10 mol%) was added. RM was stirred under hydrogen (p = 1 atm) 1.5–3 h. RM was filtered through Celite and evaporated. The products were purified by column chromatography on silica gel, eluent DCM/MeOH (9:1).
5.3.2 N,N-Diethylaziridine-2-carboxamide (6a)

This was obtained from benzyl-2-[(diethylamino)carbonyl]aziridine-1-carboxylate (4a) (0.56 g, 2.03 mmol) and Pd/C (22 mg, 0.20 mmol). RM was stirred for 2 h. The product was obtained as yellow liquid (245 mg, 85%).
1H NMR (D2O, 400 MHz), δ, ppm: 1.12 (3H, t, J = 7.2 Hz), 1.27 (3H, t, J = 7.2 Hz), 1.87 (1H, d, J = 3.4 Hz), 1.94 (1H, d, J = 6.1 Hz), 2.93 (1H, dd, J = 3.4, J = 6.1 Hz), 3.34–3.48 (2H, m), 3.66–3.71 (2H, m).
13C NMR (D2O, 100 MHz), δ, ppm: 12.0, 13.4, 25.3, 27.6, 41.6, 42.2, 170.8.
HRMS (ESI, m/z): [M + Na]+ Calcd C7H14N2ONa: 165.1004. Found: 165.1007.
5.3.3 N-Benzyl-N-methylaziridine-2-carboxamide (6b)
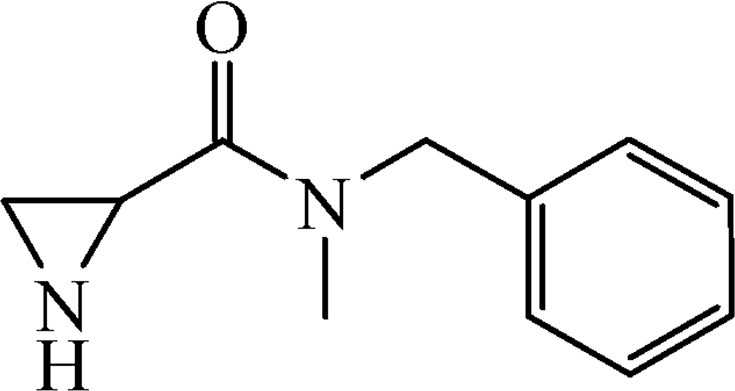
This was obtained from benzyl-2-{[benzyl(methyl)amino]carbonyl}aziridine-1-carboxylate (4b) (440 mg, 1.36 mmol) and Pd/C (15 mg, 0.14 mmol). RM was stirred for 1.5 h. The product was obtained as a colourless oil (yield 217 mg, 84%).
1H NMR (CD3OD, 400 MHz), mixture of two rotamers, δ, ppm: 1.77–1.92 (2H, m), 2.87–2.95 (1H, m), 2.98 (1.5H, s), 3.13 (1.5H, s), 4.60 and 4.65 (1H, AB, J = 14.8 Hz), 4.76–4.83 (1H, m), 7.22–7.42 (5H, m).
13C NMR (CD3OD, 100 MHz), mixture of two rotamers, δ, ppm: 26.7, 27.0, 28.3, 28.7, 34.6, 35.0, 52.4, 53.6, 127.8, 128.6, 128.8, 128.9, 129.7, 130.0, 138.0, 138.1, 172.3, 172.5.
HRMS (ESI, m/z): [M + H]+ Calcd C11H15N2O: 191.1184. Found: 191.1190.
5.3.4 1-[(Aziridin-2-yl)carbonyl]piperidine (6c)

This was obtained from benzyl-2-(piperidin-1-ylcarbonyl]aziridine-1-carboxylate (4c) (0.19 g, 0.66 mmol) and Pd/C (7 mg, 0.07 mmol). RM was stirred for 3 h. The product was obtained as a yellow oil (80 mg, 78%).
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.53–1.60 (3H, m), 1.68–1.74 (3H, m), 1.78 (1H, dd, J = 1.4, J = 3.4 Hz), 1.83 (1H, d, J = 5.8 Hz), 2.89 (1H, dd, J = 3.4, J = 5.8 Hz), 3.54–3.60 (2H, m), 3.66–3.71 (2H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 24.0, 24.9, 25.2, 26.2, 27.2, 43.5, 45.7, 168.7.
HRMS (ESI, m/z): [M + H]+ Calcd C8H15N2O: 155.1184. Found: 155.1178.
5.3.5 Aziridin-2-yl(morpholin-4-yl)methanone (6d)

This was obtained from benzyl-2-(morpholin-4-yl)carbonyl]aziridine-1-carboxylate (4d) (0.84 g, 2.88 mmol) and Pd/C (31 mg, 0.29 mmol). RM was stirred for 2 h. Yield 145 mg, 32%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.82 (1H, dd, J = 1.4 Hz, J = 3.4 Hz), 1.83–1.89 (1H, m), 2.89 (1H, dd, J = 3.4 Hz, J = 5.6 Hz), 3.61–3.81 (8H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 28.4, 44.1, 46.6, 67.6, 67.8, 82.4, 170.7.
HRMS (ESI, m/z): [M + H]+ Calcd C7H13N2O2
157.0977. Found 157.0981.
5.3.6 N-Isopropylaziridine-2-carboxamide (6e)

This was obtained from benzyl-2-[(isopropylamino)carbonyl]aziridine-1-carboxylate (4e) (0.31 g, 1.19 mmol) and Pd/C (13 mg, 0.12 mmol). RM was stirred for 2 h. The product was obtained as a colourless oil (78 mg, 51%).
1H NMR (D2O, 400 MHz), δ, ppm: 1.12–1.19 (6H, m), 1.84–1.93 (2H, m), 2.53–2.58 (1H, m), 3.90–4.01 (1H, m).
13C NMR (D2O, 100 MHz), δ, ppm: 21.2, 21.3, 27.1, 29.7, 41.9, 171.4.
HRMS (ESI, m/z): [M + Na]+ Calcd C6H12N2ONa: 151.0847. Found: 151.0841.
5.3.7 N-Pentylaziridine-2-carboxamide (6f)

This was obtained from benzyl-2-[(pentylamino)carbonyl]aziridine-1-carboxylate (4f) (0.16 g, 2.10 mmol) and Pd/C (22 mg, 0.21 mmol). RM was stirred for 2 h. The product was obtained as a yellow liquid (246 mg, 75%).
1H NMR (D2O, 400 MHz), δ, ppm: 0.85–0.91 (3H, m), 1.27–1.35 (4H, m), 1.49–1.57 (2H, m), 1.83–1.95 (2H, m), 2.56–2.62 (1H, m), 3.32 (2H, t, J = 6.9 Hz).
13C NMR (D2O, 100 MHz), δ, ppm: 13.2, 21.6, 27.1, 27.9, 28.2, 29.6, 39.5, 172.5.
HRMS (ESI, m/z): [M + H]+ Calcd C8H17N2O: 157.1341. Found 157.1336.
5.3.8 N-Benzylaziridine-2-carboxamide (6g)
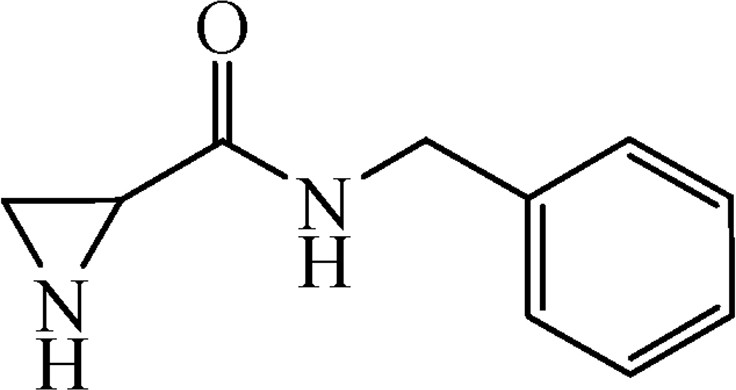
This was obtained from benzyl-2-[(benzylamino)carbonyl]aziridine-1-carboxylate (4g) (0.32 g, 1.02 mmol) and Pd/C (11 mg, 0.10 mmol). RM was stirred for 1.5 h. Yield 161 mg, 90%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.68–1.98 (2H, m), 2.49–2.60 (1H, m), 4.41 (2H, s), 7.23–7.35 (5H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 26.0, 30.6, 44.4, 128.4, 128.6, 129.6, 139.7, 172.6.
HRMS (ESI, m/z): [M + H]+ Calcd C10H13N2O: 177.028. Found: 177.1033.
5.3.9 N-(3-Methylbenzyl)aziridine-2-carboxamide (6h)
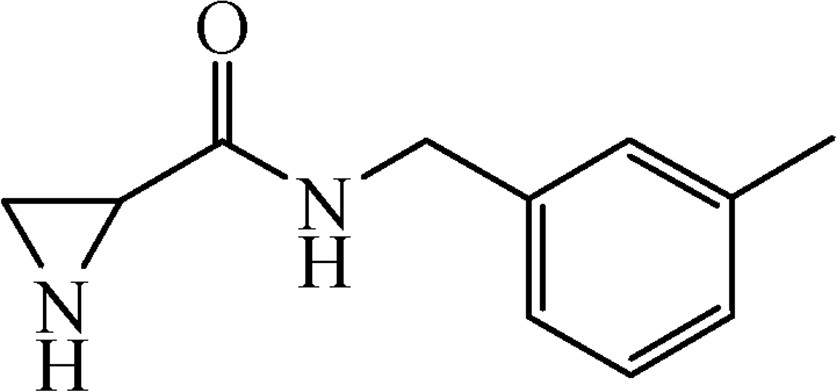
This was obtained from benzyl-2-{[(3-methylbenzyl)amino]carbonyl}aziridine-1-carboxylate (4h) (0.51 g, 1.56 mmol) and Pd/C (17 mg, 0.16 mmol). RM was stirred for 3 h. Yield 284 mg, 96%. Mp 58–59 °C.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.73–1.94 (2H, m), 2.32 (3H, s), 2.51–2.56 (1H, m), 4.37 (2H, s), 7.05–7.12 (3H, m), 7.17–7.23 (1H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 21.4, 26.0, 30.6, 44.4, 125.7, 129.0, 129.3, 129.5, 139.4, 139.5, 172.6.
HRMS (ESI, m/z): [M + H]+ Calcd C11H15N2O: 191.1184. Found: 191.1179.
5.3.10 N-(2-Methylbenzyl)aziridine-2-carboxamide (6i)

This was obtained from benzyl-2-{[(2-methylbenzyl)amino]carbonyl}aziridine-1-carboxylate (4i) (170 mg, 0.52 mmol) and Pd/C (6 mg, 0.05 mmol). RM was stirred for 1.5 h. Yield 129 mg, 100%. Mp 78–79 °C.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.69–1.97 (2H, m), 2.32 (3H, s), 2.51–2.58 (1H, m), 4.42 (2H, s), 7.14–7.19 (3H, m), 7.21–7.25 (1H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 19.0, 26.0, 30.5, 42.7, 127.1, 128.6, 129.3, 131.4, 137.0, 137.4, 172.4.
HRMS (ESI, m/z): [M + H]+ Calcd C11H15N2O: 191.1184. Found: 191.1187.
5.3.11 N-[2-(Trifluoromethyl)benzyl]aziridine-2-carboxylate (6j)
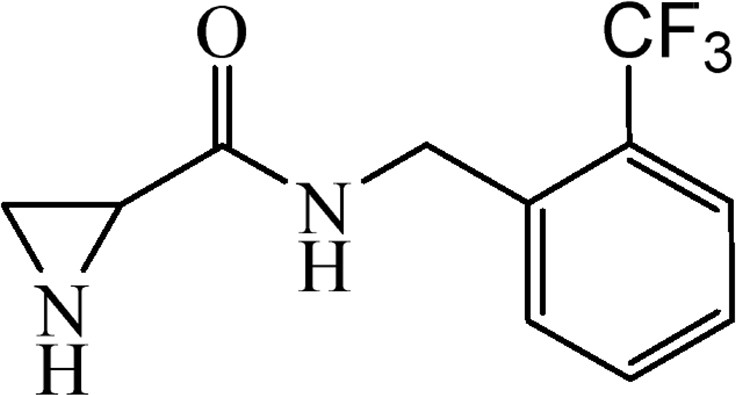
This was obtained from benzyl-2-({[2-(trifluoromethyl)benzyl]amino}carbonyl)aziridine-1-carboxylate (4j) (0.58 g, 1.52 mmol) and Pd/C (16 mg, 0.15 mmol). RM was stirred for 1.5 h. The product obtained was a colourless oil (Yield 341 mg, 92%). .
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.76–1.96 (2H, m), 2.56–2.63 (1H, m), 4.62 (2H, s), 7.43–7.48 (1H, m), 7.51–7.55 (1H, m), 7.58–7.64 (1H, m), 7.68–7.73 (1H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 26.1, 30.5, 41.0, 124.6, 126.0 (q, J = 273 Hz), 127.0 (q, J = 5.7 Hz), 128.8, 130.6, 133.6, 137.8, 173.0.
HRMS (ESI, m/z): [M + Na]+ Calcd C11H11F3N2ONa: 267.0721. Found: 267.0727.
5.3.12 N-(4-tert-butylphenyl)aziridine-2-carboxamide (6k)

This was obtained from 2-[(tert-butylphenyl)carbamoyl]aziridine-1-carboxylate (4k) (0.40 g, 1.14 mmol) and Pd/C (12 mg, 0.11 mmol). RM was stirred for 1 h. Yield 207 mg, 83%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.31 (9H, s), 1.80–2.00 (2H, m), 2.63–2.69 (1H, m), 7.33–7.38 (2H, m), 7.46–7.51 (2H, m)
13C NMR (CD3OD, 100 MHz), δ, ppm: 26.4, 31.1, 31.8, 35.2, 120.9, 126.7, 136.9, 148.5, 170.7.
HRMS (ESI, m/z): [M + H]+ Calcd C13H19N2O: 219.1497. Found: 219.1499.
5.3.13 N-[4-(Trifluoromethyl)phenyl]aziridine-2-carboxamide (6l)
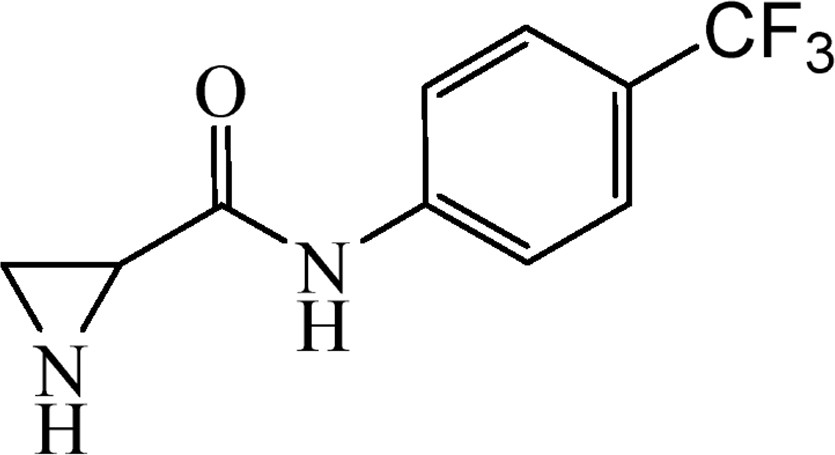
This was obtained from benzyl-2-{[4-(trifluoromethyl)phenyl]carbamoyl}aziridine-1-carboxylate (4l) (0.50 g, 1.37 mmol) and Pd/C (24 mg, 0.22 mmol). RM was stirred for 2 h. Yield 238 mg, 75%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.83–2.02 (2H, m), 2.67–2.73 (1H, m), 7.61 (2H, d, J = 8.4 Hz), 7.80 (2H, d, J = 8.4 Hz).
13C NMR (CD3OD, 100 MHz), δ, ppm: 26.7, 31.2, 120.7, 125.7 (J = 271 Hz), 126.8 (J = 32.9 Hz), 127.1 (J = 3.8 Hz), 143.1; 171.3.
HRMS (ESI, m/z): [M + H]+ Calcd C10H10N2OF3: 231.0745. Found 231.0747.
5.3.14 N-(4-Methoxyphenyl)aziridine-2-carboxamide (6m)

This was obtained from benzyl 2-[(4-methoxyphenyl)carbamoyl]aziridine-1-carboxylate (4m) (0.37 g, 1.13 mmol) and Pd/C (12 mg, 0.11 mmol). RM was stirred for 2 h. Yield 161 mg, 74%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.74–2.05 (2H, m), 2.60–2.69 (1H, m), 3.77 (3H, s), 6.85–6.90 (2H, m), 7.44–7.49 (2H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 26.3, 31.0, 55.9, 115.0, 115.9, 122.9, 132.6, 158.1.
HRMS (ESI, m/z): [M + H]+ Calcd C10H13N2O2: 193.0977. Found 193.0975.
5.3.15 N-(3,5-Dimethylphenyl)aziridine-2-carboxamide (6n)

This was obtained from benzyl 2-[(3,5-dimethylphenyl)carbamoyl]aziridine-1-carboxylate (4n) (0.46 g, 1.42 mmol) and Pd/C (23 mg, 0.14 mmol). RM was stirred for 2 h. Yield 81%, 218 mg.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.83–1.89 (1H, m), 1.91–1.97 (1H, m), 2.27 (6H, d, J = 0.8 Hz), 2,65 (1H, dd, J = 3.2 Hz, J = 5.7 Hz), 6.75–6.78(1H, m), 7.18–7.20 (2H, m).
13C NMR (CD3OD, 100 MHz), δ, ppm: 21.5, 26.4, 31.1, 118.9, 127.0, 139.3, 139.7, 170.7.
HRMS (ESI, m/z): [M + H]+ Calcd C11H15N2O: 191.1184, Found 191.1180.
5.3.16 N-(2,4-Dimethylphenyl)aziridine-2-carboxamide (6o)
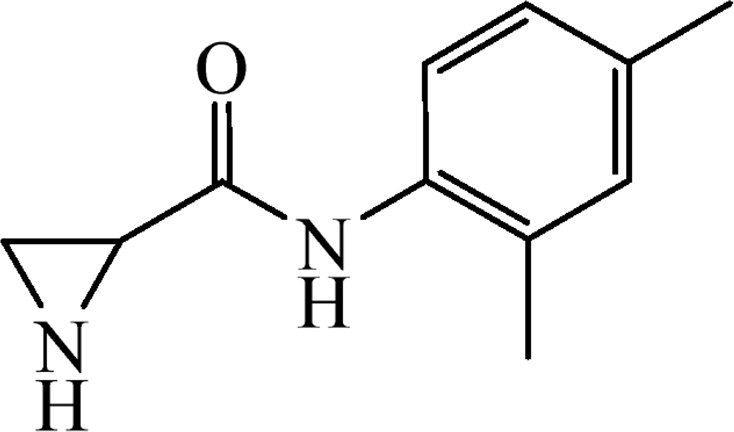
This was obtained from benzyl 2-[(2,4-dimethylphenyl)carbamoyl]aziridine-1-carboxylate (4o) (0.45 g, 1.39 mmol) and Pd/C (15 mg, 0.14 mmol). RM was stirred for 2 h. Yield 218 mg, 83%.
1H NMR (CD3OD, 400 MHz), δ, ppm: 1.79–2.03 (2H, m), 2.21 (3H, s), 2.29 (3H, s), 2.73 (1H, dd, J = 3.1 Hz, J = 5.7 Hz), 6.97–7.02 (1H, m), 7.04–7.07 (1H, m), 7.21–7.30 (1H, m).
13C NMR (CD3OD, 100 MHz), 17.9, 21.0, 26.4, 30.8, 126.4, 127.9, 132.2, 133.9, 137.3, 171.7.
HRMS (ESI, m/z): [M + H]+ Calcd C11H15N2O: 191.1184. Found 191.1176.
Acknowledgements
This work was supported by ERDF project no. 1.1.1.2/VIAA/1/16/242.
Appendix A Supplementary data
The following is the supplementary data to this article:
Characterization data for aziridines 2–4 and 6 including 1H and 13C NMR spectra.


