1 Introduction
All rare-earth elements (REE) are significant to the world, especially in the magnetic [1], electronic [2], advanced engine [3], medicine engineering [4], and telecommunication technologies [5,6]. REE are divided into three types, i.e. the light REE, medium REE and heavy REE based on the solubility characteristics of REE towards sodium sulphate [7]. The elements highlighted in this paper are praseodymium (Pr), gadolinium (Gd), and dysprosium (Dy) which represent each of the three types of REE. Pr has shown significant contributions in many fields since 2012 with a dramatic increase in demand [8], Gd is often used in medical equipment such as MRI and chiller and Dy is one of the most valuable elements that is recently gaining high demand worldwide [9,10]. These elements are scarce and difficult to be extracted efficiently, so it is critical to find the most effective method to extract these REE from their mineral ores.
The production of REE has been considered a real challenge for years especially in the extraction of individual REE from other REE found in ores [11–13].The process flow starts with mining, beneficiation, separation and extraction and finalises with the product recovery stage [8,14,15]. REE are commonly excavated together with other minerals such as iron, calcium, aluminium, silica and fluorine [14]. These minerals are removed in advance during the separation and extraction phases to obtain a pure product of REE. The separation and extraction of these elements from REE are easier compared to the separation and extraction among REE themselves. Similar physical and chemical bondings between lanthanide groups complicate the chance of obtaining highly pure REE in the final product [8] and most of the current extractants provide poor selectivity of REE. Furthermore, the elements available in ores are inconsistent and mainly influenced by the geological formation effects on the locations where REE are found [16,17].
Solvent extraction is a conventional separation and extraction method that applies various kinds of organic extractants such as D2EHPA, HEHEHP, TBP, Cyanex 272 and DODGAA [18–20]. These conventional extractants are practical in extracting REE with several disadvantages such as the tendency of extractant loss into the aqueous phase due to the acts of multiple contacts [21] and formation of emulsion because of a high viscosity of extractant that declines the extraction efficiency [22]. Many attempts have been made to improve the extractant efficiency including the use of diluents such as benzene, heptane and kerosene [23,24] to reduce the viscosity of the organic extractants. However, these diluents contributed to the release of undesired volatile organic compounds [25,26].
In the past few years, synergist extractants have revolutionised the conventional extractants by reforming the extractants either by merging two or more extractants together [27] or restructuring the organic compound of the extractants [28] to develop extractants with better extraction capability and durability [29–34]. Synergist extractants have the potential to overcome typical issues in solvent extraction such as poor metal extraction, poor selectivity of desired REE and the competition of REE separation from other minerals observed while using conventional extractants. The advantages of using a synergist extractant include the potential in higher selectivity of REE, reduction in the formation of emulsion and reduction in the release of volatile organic compounds [35].
Till date, the issues on conventional extractants, i.e. production of high volatile organic compounds, high tendency to get lost into aqueous solution and poor analysis on synergist effects have not been thoroughly studied. Therefore, this research focuses on the formulation of eight newly designed synergist extractants and their performances were compared with the conventional extractants in the extraction of Pr, Gd and Dy. A screening study was conducted to determine the best synergist extractants followed by the study on the effect of synergist before and after the extraction of REE. The study is expected to develop synergist extractants with higher extraction efficiency thus reducing the use of conventional extractants and to overcome the environmental issue of high volatile organic compounds.
2 Materials and methods
2.1 Materials
The conventional extractants, i.e. Aliquat 336 (A336), 2-thenoyltrifluoroacetone (HTTA) and Adogen 464 (A464) were purchased from Aldrich and bis(2-ethylhexyl) phosphate (D2EHPA) was purchased from Merck. The ionic liquids used were 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, [C2mim][NTf2], and 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, [C4mim][NTf2], and these chemicals were purchased from Sigma Aldrich. The selected REE, praseodymium(III) nitrate hexahydrate (Pr), gadolinium(III) nitrate hexahydrate (Gd) and dysprosium(III) nitrate hydrate (Dy), were purchased from Sigma Aldrich in the form of crystal powder before being dissolved in nitric acid.
2.2 Methods
2.2.1 Preparation of synergist extractants
The design of the synergist extractants is based on the mixing of two solvents at five different ratios. First, the simulated 0.01 M of Pr, Gd and Dy aqueous solutions were prepared by dissolving praseodymium(III) nitrate hexahydrate, gadolinium(III) nitrate hexahydrate and dysprosium(III) nitrate hydrate in 0.1 M of nitric acid (HNO3), respectively. Next, synergist extractants were prepared by combining the conventional extractants and ionic liquids at the ratio of 1:2. Two solvents of A336 and [C4mim][NTf2] were mixed to design the synergist extractant of A336-[C4mim][NTf2] by mixing 0.5 mL of A336 into 1 ml of [C4mim][NTf2]. The mixture was shaken overnight using a mechanical shaker at 200 rpm to ensure homogeneity between the components. The synergist extractants of A336-[C4mim][NTf2] were then analysed using FTIR [36] to verify the presence of compounds in the mixture. Then, using the same ratios and procedure, [C4mim][TNf2] was replaced with [C2mim][NTf2] to form A336-[C2mim][NTf2]. The same method was applied on the other conventional extractants and ionic liquid combinations. Table 1 shows simplified names of the formulated synergist extractants.
List of synergist extractant denoted after the combination of conventional extractants and ionic liquids.
| Conventional extractants | Ionic liquids | |
| [C4mim][NTf2] (1 ml) | [C2mim][NTf2] (1 ml) | |
| HTTA (g) | HTTA-[C4mim][NTf2] | HTTA-[C2mim][NTf2] |
| D2EHPA (ml) | D2EHPA-[C4mim][NTf2] | D2EHPA-[C2mim][NTf2] |
| A336 (ml) | A336-[C4mim][NTf2] | A336-[C2mim][NTf2] |
| A464 (ml) | A464-[C4mim][NTf2] | A464-[C2mim][NTf2] |
2.2.2 Extraction of Pr, Gd and Dy
The extraction of REE was conducted using the respective synergist extractants and independent extractants to determine the extraction efficiency on the selected REE. An equal volume of A336-[C4mim][NTf2] was mixed and shook with the simulated aqueous phase of Pr at 200 rpm for 10 min [37–39]. Then, the mixture was centrifuged to separate the organic extractant from the aqueous phase. The study was conducted at room temperature at approximately 25 °C ± 1 °C and the pH of the aqueous phase was maintained approximately around 1 ± 0.1. A syringe was used to withdraw the aqueous phase sample and the extraction efficiency of A336-[C4mim][NTf2] on Pr was analysed. The result was recorded and the same procedure was repeated using the rest of the synergist extractants and REE.
The REE extraction efficiency, E, in percentage was determined using Eq. 1 where Co is the initial concentration and Ce is the equilibrium concentration of REE (mol/L) [19,40].
| (1) |
2.2.3 Carbon-13 magnetic resonance spectroscopy (13C NMR) and hydrogen-1 nuclear magnetic resonance spectroscopy (1HNMR)
13CNMR and 1HNMR were used to determine the molecular structure and purity of the synergist extractants. Approximately 5–25 mg of samples was required for the analysis and chloroform was used to dissolve the extractants before being analysed using a 500 MHz NMR JEOL model. Chloroform was used to dissolve the samples before the analysis and the detected peaks were identified using δ NMR Software and processed using MestReNova 64x software.
2.2.4 Gas chromatography mass spectrometry (GCMS)
The synergist extractant was analysed by GCMS using an Agilent Technologies 7890A GC system. For this analysis, the capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm) was coupled with Agilent Technologies 5975C Inert MSD. The oven temperature was programmed to 40 °C for 2 min, ramped at 3 °C/min to 100 °C for 1 min and finally ramped at 4 °C/min to 270 °C for 1 min. Each sample was analysed for a total of 66 min. The injection port temperature was 240 °C, whereas the detector was set at 230 °C with a split ratio of 60. The carrier gas used was helium with a flow rate of 1 mL/min. The MS conditions were as follows: ionisation voltage, 70 eV; ion source temperature, 150 °C; electron ionisation mass spectra were acquired over the mass range of 50–550 m/z. The compounds were identified by comparing the mass spectra data with spectra available from the mass spectra libraries [41–43].
3 Results and discussions
The performance of each extractant is observed through the extraction efficiency of Pr, Gd and Dy representing light, medium and heavy REE. The most potential extractant is then selected and further analysis was conducted to investigate the synergist effects between ionic liquids and conventional extractants.
3.1 The comparison of performance of extractants in the extraction of Pr
Figs. 1a and b shows the extraction efficiency of Pr using independent and synergist extractants in comparison between [C4mim][NTf2] and [C2mim][NTf2]. Clearly, the independent performance of [C4mim][NTf2] in extraction of Pr is unreliable and similar to the independent extraction of [C2mim][NTf2]. So far, none of the published works proved the capability of sole [Cnmim][NTf2] to extract REE in any medium. However, upon pairing with different conventional extractants, [Cnmim][NTf2] shows different extraction efficiencies. These results are similar to those of many studies and it was proven that the task specific ionic liquid (TSIL) consists of imidazolium, [Cnmim]+ as cation paired with anion of (trifluoromethyl)sulfonyl)imide and [NTfn]− to be effective in extracting at least the elements in light REE [29,44,45]. The reason behind the performance differences is probably the difference in the properties of the organic extractant paired with [Cnmim][NTf2] in TSIL. A similar pattern is observed as most of the synergist extractants in this study shows improvement in the extraction of Pr after pairing the ionic liquids with other conventional extractants as shown in Fig. 1.
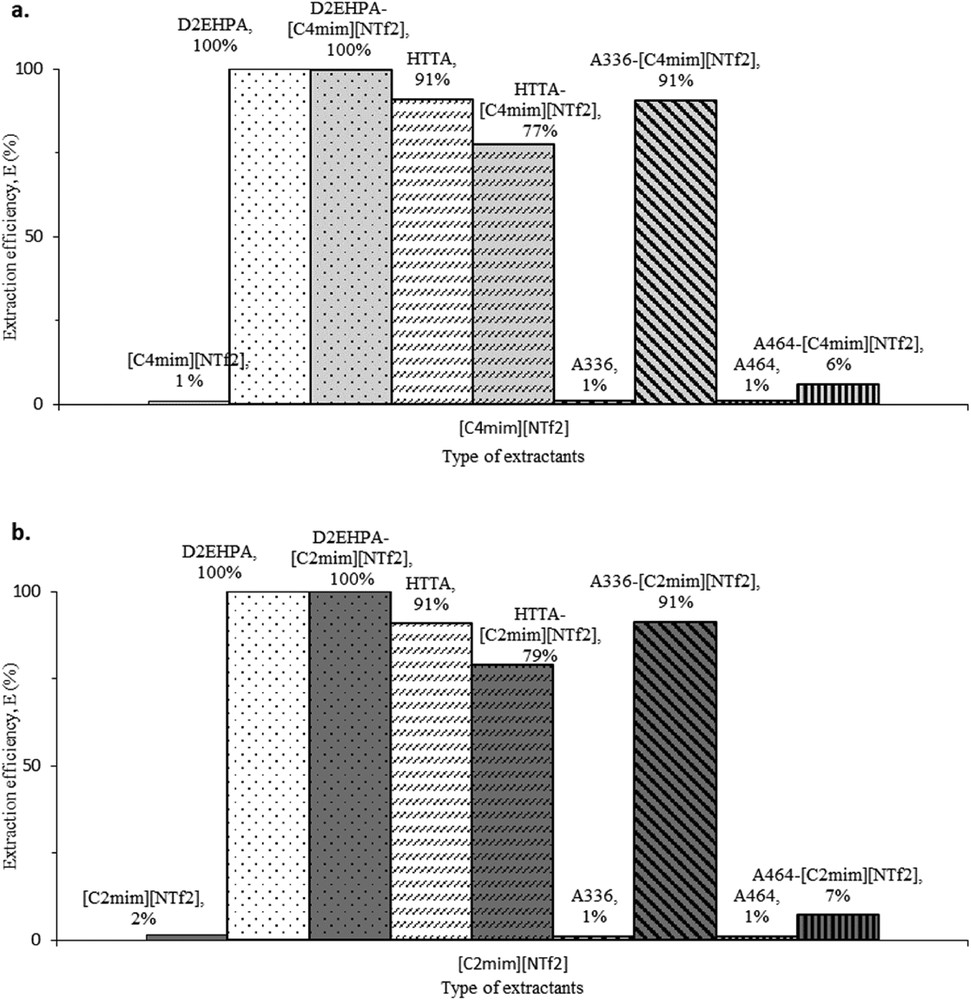
Comparison of the independent extractants versus synergist extractant in the extraction of Pr using (a) [C4mim][NTf2] and (b) [C2mim][NTf2] at room temperature.
Fig. 1 illustrates the outstanding performance of D2EHPA in the extraction of Pr without the support from ionic liquids. This is not a new discovery as D2EHPA is one of the most efficient organophosphorous extractants in REE processing. D2EHPA, which is also known as P204, is widely employed as an organic solvent in REE industry [21,40,46,47]. The selection of D2EHPA as one of the conventional extractants was made after the consideration of its performance under the influence of nitrate media, where the solvent was found to be better than HEHEHP (also known as P507), Cyanex 272 and Cyanex 301 [39,40]. Even after pairing with ionic liquids, the extraction efficiency of Pr is excellent for both D2EHPA-[C4mim][NTf2] and D2EHPA-[C2mim][NTf2]. The extraction efficiency of Pr using synergist extractants composed by D2EHPA was probably not affected much by the presence of ionic liquid.
In the extraction involving D2EHPA-[C4mim][NTf2] and D2EHPA-[C2mim][NTf2], three layers of visible immiscible liquids were formed after the centrifugation process, as shown in Fig. 2. The top layer is inferred as [C4mim][NTf2] or [C2mim][NTf2], followed by the aqueous solution in the middle and D2EHPA as the bottom solution. The orders are based on the density of [C4mim][NTf2], [C2mim][NTf2] and D2EHPA which are 1.524, 1.443 and 0.965 g/mL respectively at 293 K. Moreover, the densities of REE aqueous solutions are in between those of the ionic liquid and D2EHPA. The sample after the extraction was taken from the middle layer and tested positive with Pr using ICPMS. D2EHPA is immiscible with ionic liquids as it is categorised as a non-polar extractant; however, previous studies show that the combination is possible using certain techniques. The technique was studied by Sun et al. (2010); in their study, the inner synergist effects occurred between D2EHPA and A336, where D2EHPA was broken into ammonium salt and it produced a synergist extractant of trialkylethylammonium di-2-ethylhexylphosphate, [A336][P204] [48–50]. Therefore, the excellent extraction efficiency of Pr by both D2EHPA-[C4mim][NTf2] and D2EHPA-[C2mim][NTf2] maybe performed entirely by D2EHPA after considering the poor performance of both independent ionic liquids in Fig. 1.
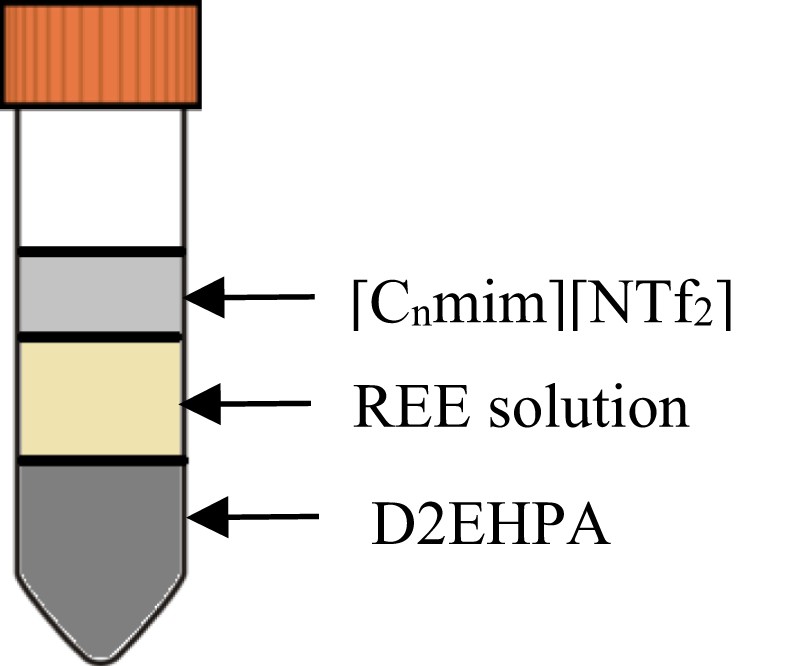
Three layers effect after centrifuging.
Compared to D2EHPA, HTTA is a chelating kind of extractant that is easily mixed with ionic liquids. Fig. 1 also shows the performance of independent HTTA, which was lower compared to the independent D2EHPA. Generally, a chelating extractant has a poorer extraction efficiency compared to a cation exchanger such as an organophosphorous type of extractant [18,35]. However, independent HTTA gives a higher extraction efficiency of Pr (∼91%) compared to those of synergist extractants of HTTA-[C4mim][NTf2] and HTTA-[C2mim][NTf2], which were 77 and 79% respectively. In this case, the ionic liquids act simply as a diluent to HTTA and the synergist process drops the extraction efficiency. Normally, HTTA is dissolved using toluene, a typical diluent used in the REE industry, and apparently provides better yield of Pr compared to [C4mim][NTf2] and [C2mim][NTf2] [9,27,51,52]. This is believed to occur due to the viscosity effect of toluene, [C4mim][NTf2] and [C2mim][NTf2] which are at 0.59, 59.8 and 38.6 mPa s, respectively [53,54]. HTTA appears to be more efficient once dissolved using a diluent that has a lower viscosity such as toluene. However, toluene is volatile and has stronger tendency to loss into the aqueous phase faster than the ionic liquid. In other opinion, it was pointed that the synergist extractant of HTTA-[C4mim][NTf2] has a lower extraction efficiency of lanthanum (La) compared to that of HTTA-18C6 [31]. La and Pr are in the same group of light REE, so it can be expected that HTTA is potentially incompatible with ionic liquid such as [Cnmim]+ and [NTfn]− in extraction of light REE. However, this finding contradicts the effort to apply ionic liquid as a green alternative solution as indicated by Atanassova et al. (2015), thus abandoning the use of high-VOC chemicals like toluene.
Fig. 1 furthermore analyses the performance of A336, which alone is incapable to extract Pr and this result is reasonable considering that A336 is one kind of anion exchanger. Naturally, the performance of pure A336 is fairly poor towards the extraction of the cation elements of REE. Nevertheless, based on few studies, A336 does extract REE better by pairing it with other organic compounds like Cyanex925 [55], D2EHPA and HEHEHP [34,35,56]. Evidently, the synergist extractants of A336-[C4mim][NTf2] and A336-[C2mim][NTf2] have dramatically increased the extraction efficiency of Pr up to 91% from 1% efficiency of pure A336. A similar behaviour was recorded in several studies for other compounds such as trioctylmethylammonium dioctyl diglycolamate, [A336][DGA] [22], tricaprylylmethylammonium thiosalicylate, [A336][TS] [24,57], trioctylmethylammonium nitrate, [A336][NO3] [58], trialkylmethylammonium di(2-ethylhexyl)orthophosphinate, [A336][P507] [34] and [A336][P204] [18,34,49,58].
Shi and Wang (2016) described that the changes from ionic to organic features due to the re-arrangement of the molecular structures will definitely affect the physiochemical interactions. This consequently affects the extraction of REE from the aqueous phase. Particularly, A336 shows extraordinary performance in nitrate aqueous solution compared to the sulphate media but it is very poor at extraction in chloride media [35].
The application of A464 is also observed from Fig. 1. Apparently, A464 alone is incompetent similar to the independent performance of A336 as both extractants are from the similar class of anion exchanger, i.e.quaternary amines [18]. Unlike A464, the conventional extractants such HTTA, D2EHPA and A336 are commonly known as potential solvents in the REE [18,50]. However, as far as the authors are aware, there was no work carried out on A464 in the extraction of lanthanides. Regardless, A464 is useful for extraction of other metals such as uranium (U) [59] and gallium (Ga) [60]. Therefore, A464 is inferred to be comparable with A336 in terms of performance after the synergist process with [C2mim][NTf2]. Based on the inference, A464 was selected as one of the potential solvents to extract REE in this study. Contradicting this, the inference is incorrect as the results show that the synergist effects of A464 with both ionic liquids, [C4mim][NTf2] and [C2mim][NTf2], have produced the poorest extraction efficiency of Pr compared to other extractants available in this research. Still, poor positive synergist effects are observed on A464-[C4mim][NTf2] and A464-[C2mim][NTf2] judged by the small increase in the extraction efficiency compared to the pure A464.
3.2 The comparison of the performance of extractants in the extraction of Gd
In this sub-section, the performance of each extractants on the extraction of Gd, one of the medium REE, is evaluated and presented in Fig. 3. Similar to the extraction of Pr, the performances of independent [C4mim][NTf2] and [C2mim][NTf2] in the extraction of Gd are unreliable. This indicates that neither of these two ionic liquids is able to extract REE independently. Perhaps, the presence of fluorinated anion such as NTf2- causes the poor extraction, as it is susceptible to loss into the aqueous phase through the hydrolysis process as stated by Rout and Binnemans (2014). However, based on the review by Hidayah and Abidin (2018), the probability of NTf2- to be more resistant to hydrolysis is higher in nitrate media compared to [PF6]− [22,61]. Therefore, one of the ways to reduce the loss of ionic liquid into the aqueous solution is through the formulation of a synergist extractant, which was carried out in this research.

Comparison of the independent extractants versus synergist extractant in the extraction of Gd using a. [C4mim][NTf2] and b. [C2mim][NTf2] at room temperature.
Fig. 3 shows that the extraction pattern of Gd is similar to that of Pr using pure D2EHPA and the synergised D2EHPA with ionic liquids. D2EHPA alone exhibits the greatest performance in the extraction of Gd and Pr compared to the other extractants. A similar extraction efficiency was obtained when synergist extractants of D2EHPA-[C4mim][NTf2] and D2EHPA-[C2mim][NTf2] were used to extract Gd. On the other hand, HTTA dissolved in toluene provides high extraction efficiency of Gd; however, the extraction efficiency declines from 85% to below 30% after HTTA is paired with [C4mim][NTf2] or [C2mim][NTf2]. Apparently, HTTA-[C4mim][NTf2] and HTTA-[C2mim][NTf2] are less efficient at extraction of Pr and Gd compared to HTTA-toluene. This is possibly due to toluene inertness towards H-bonding [23] compared to more complicated bonds of ionic liquids. However, this inertness also contributed to high tendency of HTTA-toluene loss into the aqueous phase, as stated by Atanassova et al. (2015).
Both A336 and A464 are ineffective independently in extracting Gd, which contradicts the performance of D2EHPA and HTTA. Fairly, A336 and A464 are anion exchangers that do not extract cation metals. Instead, A336 and A464 extract metal ions as anionic complexes and are mostly effective in the presence of strong ionic solvents [35]. As seen in Fig. 3, the extraction shows at least 3% similar positive response in extraction of Gd and Pr using A336-[C4mim][NTf2], A336-[C2mim][NTf2], A464-[C4mim][NTf2] and A464-[C2mim][NTf2] compared to the pure A336 and A464 solvents. Still, as stated by Xie et al. (2014), A336 was found to extract light REE better than heavy REE [18,27], which explains the declination in extraction of Gd over Pr with 80% differences using A336-[C4mim][NTf2] and A336-[C2mim][NTf2]. In the study conducted by Sun and Guo (2011), the probability of [Cnmim][NTf2] to hydrolyse into aqueous phase is higher than A336 but far better than other extractants. Therefore, having them paired would make the synergist extractant less prone to the aqueous phase, which can be studied in detail in the future [55]. However, A464-[C4mim][NTf2] has a higher affinity towards Gd for at least 10% increase in extraction efficiency over Pr compared to A336-[C4mim][NTf2]. This is a great finding when the extraction yield has different affinity towards different REE, which is very useful in the extraction of individual REE from a group of REE. This is beneficial especially for Gd as this metal is difficult to be extracted from the group of REE, i.e. Sm/Eu/Gd/Tb/Dy and Eu/Gd [1].
3.3 The comparison of the performance of extractants in the extraction of Dy
Fig. 4 presents the performance of conventional and synergist extractants on the extraction of Dy, which is classified under the heavy REE. The independent [C4mim][NTf2] and [C2mim][NTf2] are also ineffective in the extraction of Dy similar to Pr and Gd. This indicates that these two ionic liquids independently are impractical in the extraction of any REE unless being paired to another organic compound.

Comparison of the independent extractants versus synergist extractant in the extraction of Dy using a. [C4mim][NTf2] and b. [C2mim][NTf2] at room temperature.
However, most of the extractants in Fig. 4 show a slight different extraction pattern towards Dy compared to Pr and Gd as shown in Figs. 1 and 3. Even though independent D2EHPA maintains a nearly complete extraction of Dy similar to Pr and Gd, the pairing effect with ionic liquids leads to a declination in extraction efficiency to 70% and 89% for D2EHPA-[C4mim][NTf2] and D2EHPA-[C2mim][NTf2], respectively. This is probably due to the inner synergistic effects between D2EHPA and ionic liquid that hinders the extraction of Dy thus reducing the efficiency [50]. This is a fascinating finding as different classes of REE show different kinds of affinity towards the synergised D2EHPA with [Cnmim][NTf2]. According to the selectivity coefficient, S, the affinity of D2EHPA is medium > heavy > light REE as in Gd > Dy > Pr, as shown in Table 1. This is unexpected as based on the study of Xie et al. (2014), D2EHPA has the opposite affinity of the class of REE, staring from medium > heavy > light REE as in Dy > Gd > Pr [18]. This implies that [Cnmim][NTf2] has certain influence in the extraction of REE after being combined with D2EHPA, and the findings supported by results in Fig. 4a.
The independent HTTA failed to conduct extraction of Dy. Instead, the synergised HTTA of HTTA-[C4mim][NTf2] and HTTA-[C2mim][NTf2] proved to be good in extracting Dy which is the opposite scenario to Pr and Gd. The synergised HTTA has stronger affinity sequences as follows: Pr > Dy > Gd, which is similar to the independent HTTA. The performance of A336 and A464 independently remains poor for Dy, which makes it incompetent to extract REE. The inclination in the extraction efficiency of Dy is observed after the synergist process especially for A336-[C2mim][NTf2]. Still, as stated by Xie et al. (2014), A336 was found to extract light REE better than heavy REE [18] which explains the declination in extraction as shown in the extraction sequence for Pr and Dy over A336-[C4mim][NTf2]. A336-[C2mim][NTf2] extracts Dy 80% better than A336-[C4mim][NTf2] and this is probably because of the shorter alkyl chains of Cnmim+ [62]. Longer alkyl chain increases the viscosity of the extractant, which affects the efficiency of REE extraction compared to a shorter alkyl chain as stated by Kurteva et al., 2015; Atanassova, 2006 and Atanassova et al., 2015. Apparently, Gd is the least extracted by the extractants which makes the sequence of efficiency as Pr > Dy > Gd.
Extractant A464 may not give high expectation in the extraction of REE but it sure shows positive extraction performance through the synergist extractants of A464-[C4mim][NTf2] and A464-[C2mim][NTf2] with the sequence of efficiency from Gd > Dy > Pr. Even though the sequences of synergist extractants are unpredictable, the unique selection of the extractant can potentially increase the metal selectivity in the final purification process. For example, if A464-[C4mim][NTf2] has stronger affinity towards Gd compared to Pr and Dy, this will eventually increase the selectivity ratio, thereby increasing the purity rate for Gd. However, further study is required to confirm the potential of A464-[C4mim][NTf2] in the selectivity study.
Table 2 summarises the selectivity coefficient of each extractant in the extraction of Pr, Gd and Dy consecutively. The table not only shows the affinity sequence variation between the extractants but also provides the idea on sequence of extraction flow in the extraction of REE using each potential extractant.
Summary of distribution coefficient of each extractant on Pr, Gd and Dy.
| Selectivity sequence | Selectivity coefficient | ||||||
| SPr | SGd | SDy | |||||
| Pr/Gd | Pr/Dy | Gd/Pr | Gd/Dy | Dy/Pr | Dy/Gd | ||
| [C4mim][NTf2] | Dy > Gd | 0 | 0 | 0 | 0.1 | 0 | 7949 |
| [C2mim][NTf2] | Dy > Pr > Gd | 1.3 | 0 | 0.8 | 0 | 92.4 | 117 |
| D2EHPA | Pr > Gd > Dy | 4.5 | 7.1 | 0.2 | 1.6 | 0.1 | 0.6 |
| D2EHPA-[C4mim][NTf2] | Gd > Dy > Pr | 0.7 | 0.8 | 1.4 | 1.1 | 1.2 | 0.9 |
| D2EHPA-[C2mim][NTf2] | Pr > Dy > Gd | 5.7 | 1.4 | 0.2 | 0.3 | 0.7 | 4.0 |
| HTTA | Pr > Gd > Dy | 1.7 | 764 | 0.6 | 442 | 0 | 0 |
| HTTA-[C4mim][NTf2] | Pr > Gd > Dy | 1.4 | 7.1 | 0.7 | 5.1 | 0.1 | 0.2 |
| HTTA-[C2mim][NTf2] | Pr > Gd > Dy | 13.5 | 42.1 | 0.1 | 3.1 | 0 | 0.3 |
| A336 | Dy > Gd | 0 | 0 | 0 | 0.1 | 0 | 9.7 |
| A336-[C4mim][NTf2] | Pr > Dy > Gd | 70.9 | 2.4 | 0 | 0 | 0.4 | 29.2 |
| A336-[C2mim][NTf2] | Pr > Dy > Gd | 142 | 82 | 0 | 0.6 | 0 | 1.8 |
| A464 | Dy > Gd > Pr | 1.0 | 0.8 | 1.1 | 0.8 | 1.3 | 1.3 |
| A464-[C4mim][NTf2] | Gd > Pr | 0.9 | 0 | 1.1 | 0 | 0 | 0 |
| A464-[C2mim][NTf2] | Gd > Dy > Pr | 0.4 | 0.5 | 2.4 | 1.1 | 2.2 | 0.9 |
In summary, on the extraction of selected light, medium and heavy REE metal, A363 gives the highest impact on the extraction after being synergised with the ionic liquids, especially A336-[C2mim][NTf2]. Therefore, A336-[C2mim][NTf2] was further analysed using FTIR, NMR and GCMS.
3.4 Characterisation studies of A336-[C2mim][NTf2] synergist extractants
The influence of a synergist extractant is known to have positive stimulus on the extraction of REE in many studies [23,35,48]; however, previous literature hardly elaborates the synergist interaction between the combined solvents. This study attempts to evaluate the interaction of synergist effects between the conventional extractants and ionic liquids employed in designing the synergist extractants. A336-[C2mim][NTf2] is the highly preferred synergist extractant based on the extraction performance of REE. In correlation, A336-[C2mim][NTf2] is analysed using 13C NMR and 1H NMR to verify the carbon and hydrogen atom structures after the synergist process. FTIR analysis is also conducted on the aqueous phase of Pr, Gd and Dy before and after the extraction process. A similar FTIR study is applied to A336-[C2mim][NTf2] before and after extraction.
3.4.1 FTIR analysis on [C2mim][NTf2], A336 and A336-[C2mim][NTf2]
The purpose of this analysis is to verify the functional group of the independent extractants and synergist extractants and thus identify any significant unknown compound. The ionic liquid of [C2mim][NTf2] in Fig. 5 shows similar characteristic peaks at 3158 cm−1 in the range of 3200–3100 cm−1 (ring C–H stretching) [64] corresponded to the imidazolium cation alkyl groups [65]. Other peaks that are detected in [C2mim][NTf2] spectra are 1050 cm−1 (SO bending), 1431 cm−1 (CC stretching), 1573 cm−1 (CN stretching) and 1250–1100 cm−1 (C–F stretching) [64].

FTIR analysis between A336, A336-[C2mim][NTf2] and [C2mim][NTf2].
The bands of A336 in Fig. 5 at 2921 cm−1 and 2852 cm−1 are assigned to the –CH3 groups whereas the quaternary ammonium group (CH3)N+ signal was observed at the peak of 1466 cm−1 and 1377 cm−1 [60,66]. The intensity of C–H at spectrum 2966 cm−1 of [C2mim][NTf2] has shifted slightly to 2961 cm−1 in A336-[C2mim][NTf2] and the peak at 2852 cm−1 of A336 shifted to 2859 cm−1 after being synergised. The shifts were probably influenced by the presence of two different extractants, i.e. [C2mim][NTf2] and A336; however, a new functional group is not detected in the analysis. This is based on the consideration that A336 is a kind of ionic liquid type of extractant [50,67,68]. Moreover, the synergist extractant of A336-[C2mim][NTf2] consists of both A336 and [C2mim][NTf2] as seen in characteristic bands within the range of 2931 and 2859 cm−1. This analysis proved that there is a physical interaction between A336 and [C2mim][NTf2], thus possibly increasing the hydrophobicity of A336-[C2mim][NTf2] [50].
The hydrophobicity features in an extractant is highly expected to reduce the loss of extractant into the aqueous phase [69]. In addition, the increase in the extraction of REE using A336-[Cnmim][NTf2] over poor performance of A336 in Figs. 1, 3 and 4 is probably due to the function of ionic liquids that act as a diluent to the original extractant. For example, A336 shows negligible extraction on Pr and poor performance on Gd and Dy. However, with the support from [C2mim][NTf2], the extraction of REE especially Dy inclines dramatically as shown in Fig. 4b. On the other hand, the longer alkyl chain possessed by [C4mim][NTf2] contributes to reduction in extraction efficiency of Pr, Gd and Dy in most synergist extractants as shown in Figs. 1, 3 and 4. This finding is parallel to the study by Sun et al. (2013) and Yang, F. et al. (2012) that describe a similar pattern, where shorter alkyl chains give better performance in the extraction of REE after the completion of the synergist process [70,71].
3.4.2 13C NMR analysis on A336-[C2mim][NTf2]
Fig. 6 shows the numbered chemical structures of A336 and [C2mim][NTf2] which are significant references to 13C NMR and 1H NMR chemical shifts listed in Table 3 and Table 4, respectively. In addition to the tables, the NMR analysis in this research is supported by the predicted hydrogen and carbon atoms spectra of A336, [C2mim]+ and [C2mim][NTf2] using MestReNova software. With the support from the predicted shifts, the spectra comparison is more convincing as most of the predicted NMR by MestReNova software is based on measurement of the provided chemical structures as shown in Fig. 6. The predicted spectra by MestReNova software are expected to have predicted errors in between 0.8 and 10 ppm (0.5–7%) for 13C NMR and 0.3–0.8 (3–8%) ppm for 1H NMR especially on new chemical structures [43,72]. Table 3 lists the carbon atom chemical shifts of predicted and actual analyses.
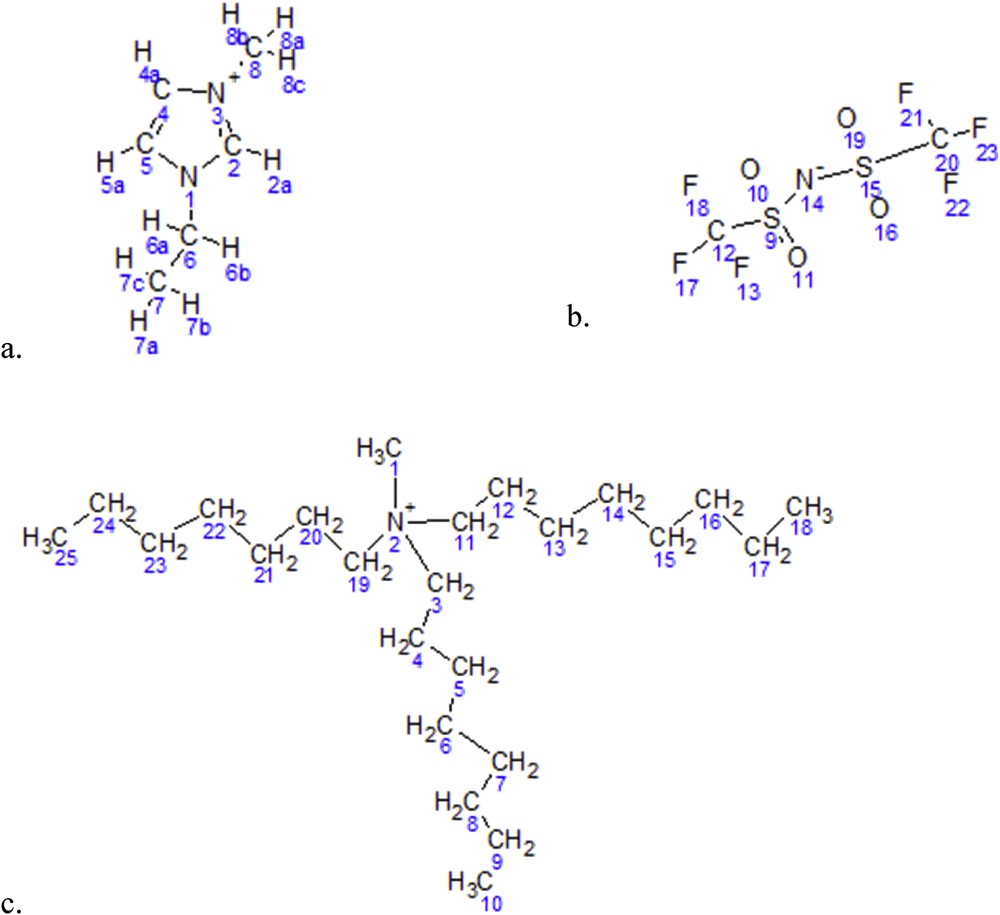
Numbered chemical structures of a. [C2mim]+, b. [NTf2]- and c. A336.
The prediction versus actual analysis of 13CNMR chemical shifts of carbon atoms in [C2mim][NTf2], A336 and A336-[C2mim][NTf2].
| Carbon atoms | [C2mim][NTf2] (ppm) | A336 (ppm) | A336-[C2mim][NTf2] (ppm) | ||
| Prediction | Actual | Prediction | Actual | Availability | |
| 2 CH | 137.6 | 134.9 | 135.0 | ||
| 4 CH | 123.9 | 123.3 | 123.1 | ||
| 5 CH | 120.7 | 121.6 | 121.5 | ||
| 6 CH2 | 44.0 | 44.7 | 44.4 | ||
| 7 CH3 | 15.4 | 14.2 | 14.2 | ||
| 8 CH3 | 36.4 | 35.5 | 35.3 | ||
| 12 CF3 | 118.3 | 118.0 | 117.9 | ||
| 20 CF3 | 118.3 | 118.0 | 117.9 | ||
| 1 CH3 | 48.9 | 47.5 | 47.5 | ||
| 3 CH2 | 61.5 | 60.5 | 61.2 | ||
| 4 CH2 | 22.8 | 21.3 | 21.8 | ||
| 5 CH2 | 23.4 | 25.0 | 25.3 | ||
| 6 CH2 | 29.1 | 27.7 | 28.1 | ||
| 7 CH2 | 29.1 | 27.8 | 28.2 | ||
| 8 CH2 | 31.7 | 30.3 | 30.9 | ||
| 9 CH2 | 22.7 | 21.2 | 21.4 | ||
| 10 CH3 | 14.1 | 12.7 | 13.2 |
The prediction versus actual analysis of 1HNMR chemical shifts of hydrogen atoms in [C2mim][NTf2], A336 and A336-[C2mim][NTf2].
| Hydrogen atoms | [C2mim][NTf2] (ppm) | A336 (ppm) | A336-[C2mim][NTf2] (ppm) | |||||||
| Prediction | Actual | Prediction | Actual | Availability | ||||||
| 2 CH | 9.29 | s | 8.39 | s | 8.54 | s | ||||
| 4,5 CH | 8.20, 8.28 | dd | 7.19, 7.24 | dd | 7.24, 7.18 | dd | ||||
| 6 CH2 | 4.41 | q | 4.06 | q | 4.02 | q | ||||
| 7 CH3 | 1.98 | t | 1.037 | t | 1.34 | t | ||||
| 8 CH3 | 2.78 | s | 3.73 | s | 2.08 | s | ||||
| 1 CH3 | 3.32 | s | 2.67 | s | 2.79 | s | ||||
| 5–9 CH2 | 1.20–1.33 | m | 0.7–0.9 | d | 1.0–1.2 | d | ||||
| 4 CH2 | 1.59 | qui | 1.10 | s | 1.48 | s | ||||
| 3 CH2 | 3.58 | t | 2.83 | m | 3.01 | t | ||||
| 10 CH3 | 0.89 | t | 0.27 | t | 0.66 | t |
The spectra between predicted and actual NMR of [C2mim][NTf2] and A336 are dependable with minor and negligible differences. Therefore, the interaction study between the two compounds after the synergist process of A336-[C2mim][NTf2] is simpler. The carbon atom chemical shifts of A336-[C2mim][NTf2] are listed in Table 3 and the comparison of spectra is illustrated in Fig. 7. Similar spectra are visible for both [C2mim][NTf2] and A336 in the synergist extractant of A336-[C2mim][NTf2] based on Table 3. Clearly, there is no new carbon structure formed after the synergist process. Thus, the chemical shifts of A336-[C2mim][NTf2] are quite close to the carbon atom shifts of [C2mim][NTf2] and A336. The arrangement of carbon from Table 3 with Fig. 7c is comparable to Fig. 7a and b with the original structures from Fig. 6. This strongly suggests that there is no chemical interaction between [C2mim][NTf2] and A336 but instead it is maintained as a physical bonding of ionic compounds. Similarly, Kurteva et al. (2015) referred this interaction as intramolecular attraction. This is similar to the synergist of HTTA and [C10mim][NTf2] that has independent proportions of spectra without any interactions to each other [63]. The recent synergist study conducted by Atanassova and Kurteva (2019) on synergist of HTTA and CMPO with an ionic liquid also resulted in negligible interaction between the extractants which is evidently confirmed by the NOESY experiments [73]. To add up, even the –CF3 from [NTf2]- in 13C NMR shows no differences after the synergist process and this further verifies that no interaction occurred during or after the synergist process was performed. The main reason for pointing out –CF3 over other bonds is because of the incapability to detect the presence of C–F and C–S in other characterisation methods such as 1H NMR and GCMS.
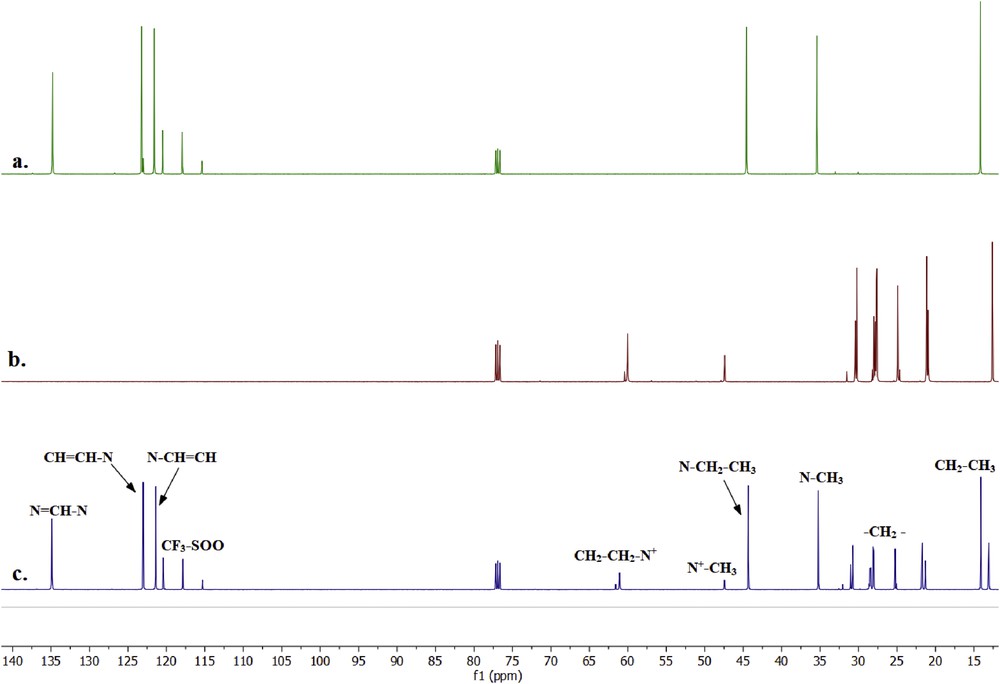
13CNMR spectra of a. [C2mim][NTf2], b. A336 and c. A336-[C2mim][NTf2].
3.4.3 1H NMR analysis on A336-[C2mim][NTf2]
Table 4 shows the list of hydrogen chemical spectra for [C2mim][NTf2], A336 and A336-[C2mim][NTf2] compared to the predicted spectra of [C2mim][NTf2] and A336. Similar spectra are visible from both [C2mim][NTf2] and A336 in the synergist extractant of A336-[C2mim][NTf2]. Similar expected peaks appeared as shown in Figs. 8a, b and c supporting the spectra in Table 4. However, it is notable that the actual peaks of A336 are poorly detected by the actual 1H NMR analysis as it appears in Fig. 8b.
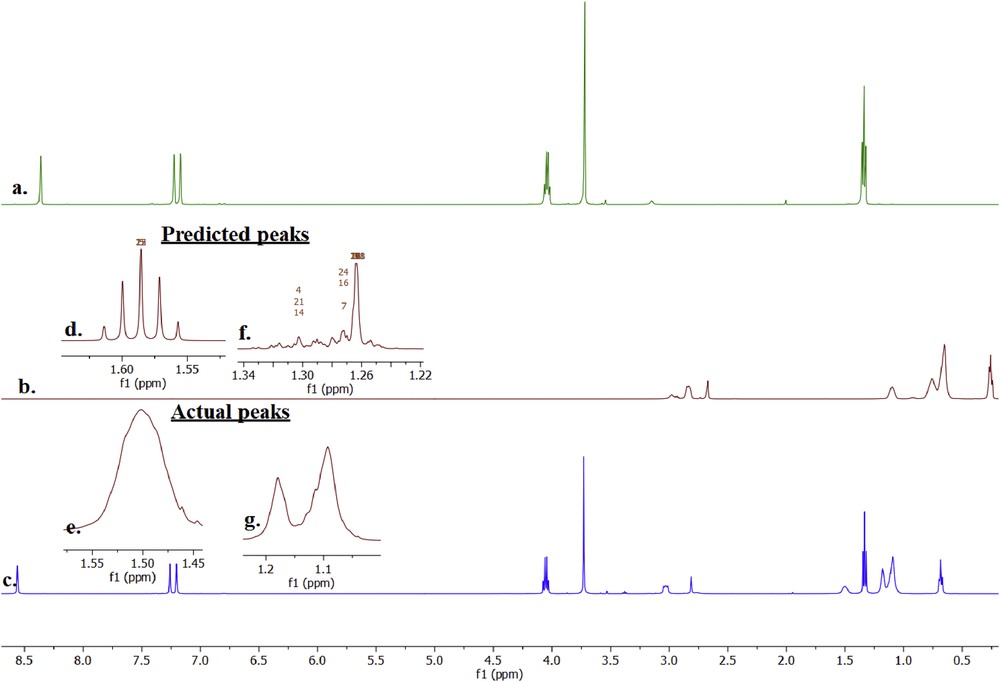
1HNMR spectra of a. [C2mim][NTf2], b. A336 and c. A336-[C2mim][NTf2]; peaks of A336 hydrogen atoms at d. predicted shift of 4 CH2, e. actual shift of 4 CH2, f. predicted shift of 5–9 CH2 and g. actual shift of 5–9 CH2.
The predicted peaks seem to be more reliable and fit the number of hydrogen appeared in the chemical structure of A336. In comparison, the predicted peaks in Fig. 8d and f are compared to the actual peaks as shown in Figs. 8e and g. Figs. 8d and e at the shifts of 1.45–1.55 ppm were compared with the peaks of four CH2 of A336. The predicted peaks were found to be accurate with five peaks indication of four neighbour hydrogen but the actual failed to perceive the particular peaks. Similar to Figs. 8f and g, the peaks that indicate the hydrogen in 5–9 CH2 of actual 1H NMR are vague compared to the predicted peaks from 1H NMR.
Nevertheless, this is a common problem especially if the compound structure has the same bond of long –CH and A336 possess three similar octane structures bonded on –N+. In addition, the actual 1H NMR probably finds it difficult to distinguish the three long CH2, and therefore the close hydrogen neighbours are detected as one. It has been a real challenge to interpret the actual 1H NMR with poor accuracy of peaks; however, the predicted 1H NMR does assist a lot in this study to perceive the possible interaction between A336 and [C2mim][NTf2]. The interaction between [C2mim][NTf2] and A336 in NMR is similar to the act of ionic liquid characteristic where the ions are together in one compound with no actual chemical reaction that forms any new product. The ions are assumed to be [A336]+, Cl−, [C2mim]+ and [NTf2]- that stream in one solvent. This result gives a positive influence on the extraction efficiency thus increasing the hydrophobicity. A similar study of 1H NMR analysis was conducted by Mikola et al. (2006) on A336. This research highlights A336 as a versatile cation source of hydrophobic ionic liquid [74] which explains the reason to the compatibility between [C2mim][NTf2] and A336.
3.4.4 GCMS analysis on A336-[C2mim][NTf2]
The characterisation analysis of synergist extractant of A336-[C2mim][NTf2] is suffice with 1H NMR and 13C NMR study and therefore, GCMS is used only for verification of the components in the selected extractants. One of the complicated parts about GCMS is to determine the right method to analyse the component in compound and it usually takes a significant time and wisdom to develop the right procedure for the method. Therefore, the method used is not designed for the current compound but the closes method applicable. The method was adapted from the study of Holfeltz et al. (2018); they investigated the potential organic extractant for the extraction of REE supported by Limbeck and Puxbaum (1999) and Aljesri (2015) lbib41.
Based on the method, Fig. 9c illustrates the spectra from GCMS to verify the structural identity in A336-[C2mim][NTf2] and compare it with spectra of the actual solvents, i.e. [C2mim][NTf2] and A336 in Figs. 9a and b. According to the analysis, only 1-methylpyrazole from [C2mim][NTf2] and N,N-dioctyl-1-octanamine from A336 are verified. The chemical structures of 1-methylpyrazole and N,N-dioctyl-1-octanamine are both available in [C2mim][NTf2] and A336 from Fig. 6. However, fluorine (F) and sulphur (S) bonds from [NTf2]- are not detected in Fig. 9a or c.
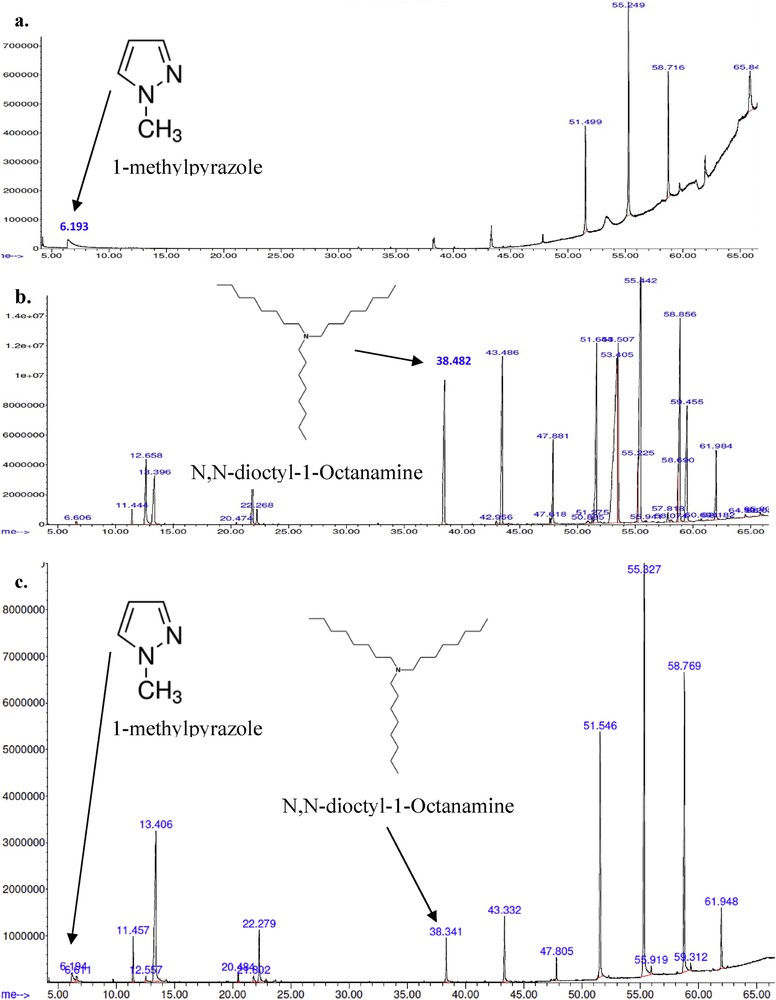
GCMS analysis on a. [C2mim][NTf2] and b. A336 and c. similar peaks detected in A336-[C2mim][NTf2].
Perhaps, the time allocated for the GCMS method is insufficient but the 13H NMR study had verified the bonds availability in A336-[C2mim][NTf2]. Besides, peaks detected are nearly the same among [C2mim][NTf2], A336 and A336-[C2mim][NTf2] in Figs. 9b and c. Because A336-[C2mim][NTf2] is a novel synergist extractant, not much of references are available even in the GCMS library. Thus, the method applied probably not suffice to entirely examine A336-[C2mim][NTf2]. Perhaps, a GCMS method can be studied solely to determine the chemical structure of synergist extractant such as A336-[C2mim][NTf2] in the future.
Based on the description made on all extractants, it can be justified that the synergist extractants are formed with the visibilities of all related independent extractants (either conventional extractants or ionic liquids). In addition, there is no new or unknown compound identified after the synergy process which is safe to state that the synergist extractants are mainly developed from the physical interaction [24,35,49].
3.5 FTIR analysis on the used A336-[C2mim][NTf2]
The final characterisation on A336-[C2mim][NTf2] is conducted on both aqueous phase of REE and the synergist extractant of A336-[C2mim][NTf2] before and after the extraction process of Pr, Gd and Dy. Fig. 10 shows the FTIR analysis on the aqueous phase of Pr, Gd and Dy before and after the extraction which was conducted using A336-[C2mim][NTf2] at the ratio of 1:1. Based on Fig. 10a, the spectra showed no changes that define negligible losses of A336-[C2mim][NTf2] into the aqueous phase after extraction is conducted. However, two minor peaks are detected in the range between 1100 and 1400 cm−1 after the solvent extraction of aqueous REE with A336-[C2mim][NTf2], as shown in Fig. 10b. The most obvious peak is at 1170 cm−1 and it is assumed to be SO, a bond from NTF2- chemical structure as portrayed in Figs. 5d and 6c. The other peak is 1350 cm−1, which is assumed to be CF, also a bond from NTF2-. These two peaks are predicted to be released after the reaction of REE metal ions sorption takes place.
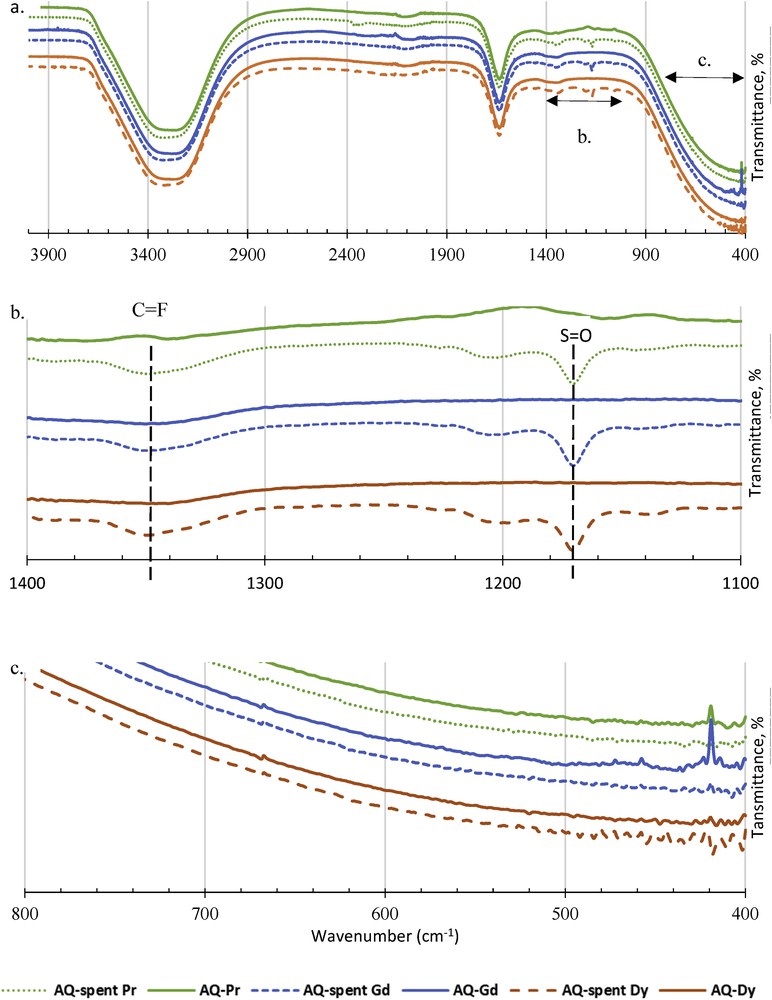
FTIR analysis on the aqueous phase of Pr, Gd and Dy before and after the extraction using A336-[C2mim][NTf2], respectively at unmodified pH, 25 °C and ratio 1:1. a. Full FTIR spectra, b. FTIR spectra at wavelength from 1100 to 1400 cm−1 and c. FTIR spectra at wavelength from 400 to 800 cm−1.
Fig. 11a shows the full scale of FTIR wavelengths of A336-[C2mim][NTf2] before and after the extraction process. No changes in the functional group that need to be highlighted except for the highlighted spectra range are shown in Figs. 11b and c. Fig. 11b shows the changes on the functional group of A336-[C2mim][NTf2] before and after the extraction of Pr, Gd and Dy. The presence of amine group in A336-[C2mim][NTf2] is assumed to be originated from A336. The presence of this amine (N–H) group has been verified in GCMS based on Figs. 9b and c.
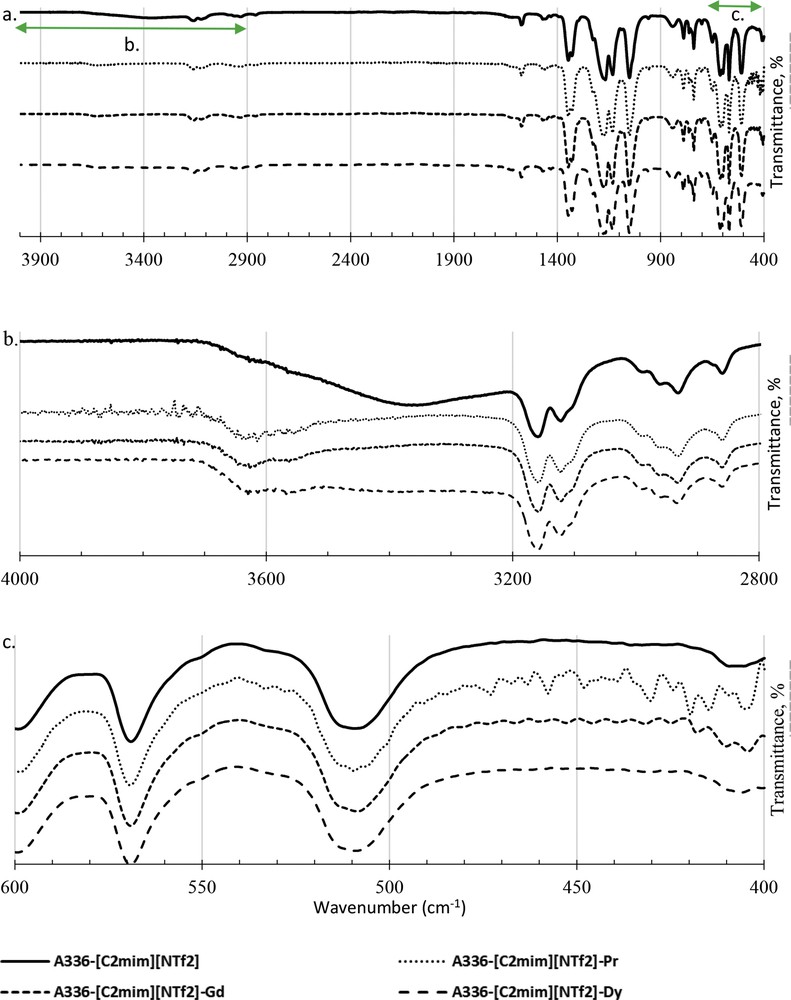
FTIR analysis on A336-[C2mim][NTf2] before and after the extraction on aqueous phase of Pr, Gd and Dy, respectively at unmodified pH, 25 °C and ratio 1:1. a. Full FTIR spectra, b. FTIR spectra at wavelength from 4000 to 2800 cm−1 and c. FTIR spectra at wavelength from 600 to 400 cm−1.
Apparently, after the use of A336-[C2mim][NTf2], N–H disappeared and is replaced with noises that are assumed to be the REE metal ions. The loss of N–H from FTIR spectra is shown in Fig. 11b and similar patterns were applied in A336-[C2mim][NTf2]-Pr, A336-[C2mim][NTf2]-Gd and A336-[C2mim][NTf2]-Dy. It can be assumed that A336 gives a significant contribution in capturing REE metal ions into A336-[C2mim][NTf2].
FTIR may not be reliable to determine metal elements but in comparison, FTIR shows different transmittance percentage among Pr, Gd and Dy extracted based on the slight noises observed, not only in Fig. 10c but also in Fig. 11b and c [75]. Apparently, the noises varied according to the kind of REE. The noises from Pr are larger than those of Gd and Dy, which could be reflected to the size of REE in the ionic phase and their stability in water [1]. Based on the characterisation analysis conducted on A336-[C2mim][NTf2], the interaction between A336 and [C2mim][NTf2] is assumed to be physically bonded at the ionic state and the compound successfully maintained its ionic liquid characteristic. In addition, the synergist extractant of A336-[C2mim][NTf2] is responsive accordingly to REE metal ions during extraction, leaving SO and C–F in the aqueous phase and diminishes the presence of N–H bonds from A336-[C2mim][NTf2].
4 Conclusion
In general, it can be concluded that in the extraction of Pr, Gd and Dy, the type of extractant could increase or decrease the extraction efficiency of REE. The potential of D2EHPA is undeniable but may not incorporate well with certain types of ionic liquids whereas HTTA shows the opposite performance on Pr and Gd after the synergist process. A464 implicates a positive influence towards the extraction after the synergist effect, even though it has the poorest performance as compared to other extractants. A363 is found to be the most compatible extractant after being synergised with ionic liquids as A336-[Cnmim][NTf2] and A336-[C2mim][NTf2] were found to be the most outstanding synergist extractants that enhanced the extraction efficiency. However, only A336-[C2mim][NTf2] is selected as it is more efficient than A336-[C4mim][NTf2] especially in the extraction of Pr and Dy. The characterisation studies of A336-[C2mim][NTf2] show a synergist compatibility between A336 and [C2mim][NTf2]. In addition, the spent A336-[C2mim][NTf2] indicates that the N–H bond from A336 and the SO bond from [C2mim][NTf2] are affected during the extraction reaction of Pr, Gd and Dy.
Acknowledgements
The authors would like to acknowledge Ministry of Education, Malaysia for awarding the FRGS research grant vote FRGS/1/2015/TK02/UMP/02/3 (RDU150115) and Universiti Malaysia Pahang for Doctoral Scholarship Scheme (DRS) and Postgraduate Research Grant Scheme (PGRS170324) for financial support.


