1. Introduction
Urinary stones are common all over the world [1]. Thanks to recent technological developments in medical engineering, laser lithotripsy is now the first-line treatment modality in endoscopic surgical urolithiasis treatment [2]. The Holmium:YAG (Ho:YAG) laser is the most popular technology employed in lithotripsy for over 20 years, and is still considered the gold standard [3]. However, the high energy applied during the procedure, the strong absorption of laser light in water, anatomical factors, and inadequate surgical experience can all contribute to injuries to surrounding tissues in the urinary tract and to endoscope damage [4]. Beyond this direct effect, insufficient irrigation during an endourological laser intervention might cause an uncontrolled temperature rise that damages adjacent tissue indirectly and delayed in time [5]. Photonic technologies have recently been used to experimentally identify urinary stones to increase safety while employing the Ho:YAG laser [6]. More recently, Winfree et al. applied an autofluorescent imaging method to facilitate identification of Randall’s plaque [7].
Our research group found that continuous monitoring of the fluorescence spectra of urinary calculi is sufficient to distinguish precisely and in real time between stone, tissue, and endoscope components [8]. Relying on the foundation of this basic research work, our group developed a target identification system that autonomously supports the surgeon during lithotripsy to enable automatic real-time urinary stone detection via autofluorescence. For this review we introduce our new automatic target identification system and the results of the experimental studies we conducted.
The aim of this study was to provide basic scientific evidence for the development of an autofluorescence-based imaging system for real-time urinary stone target identification and subsequent experimental evaluation for performance, reliability and safety in an in vivo animal study.
2. Materials and methods
In the initial study we developed a fiber-based autofluorescence measurement system consisting of commercially available components to analyze spectral information [8]. The excitation laser light was emitted by a 15 mW diode pumped, solid state FDSS532 laser (CryLas, Berlin, Germany) and was connected to an optical core therapeutic fiber with a 365 mm core (PercuFib optical core treatment fiber, LISA laser products, Katlenburg-Lindau, Germany). Fluorescent light re-entering the fiber was separated from the excitation light with a beam splitter and analyzed on a fiber-coupled spectrometer. Fluorescence signals were collected and evaluated in random areas on the stones. The spectra of 82 human kidney stones were analyzed and compared to porcine urinary tract tissue and polytetrafluoroethylene coating of a common ureterorenoscopic working channel in a series of standardized measurements (Figure 1). Artificial stones made of pure inorganic components were also analyzed. Each sample’s chemical composition was consistently analyzed by an infrared spectrometer.
Autofluorescence measurement of urinary stone fragments, teflon element and tissue.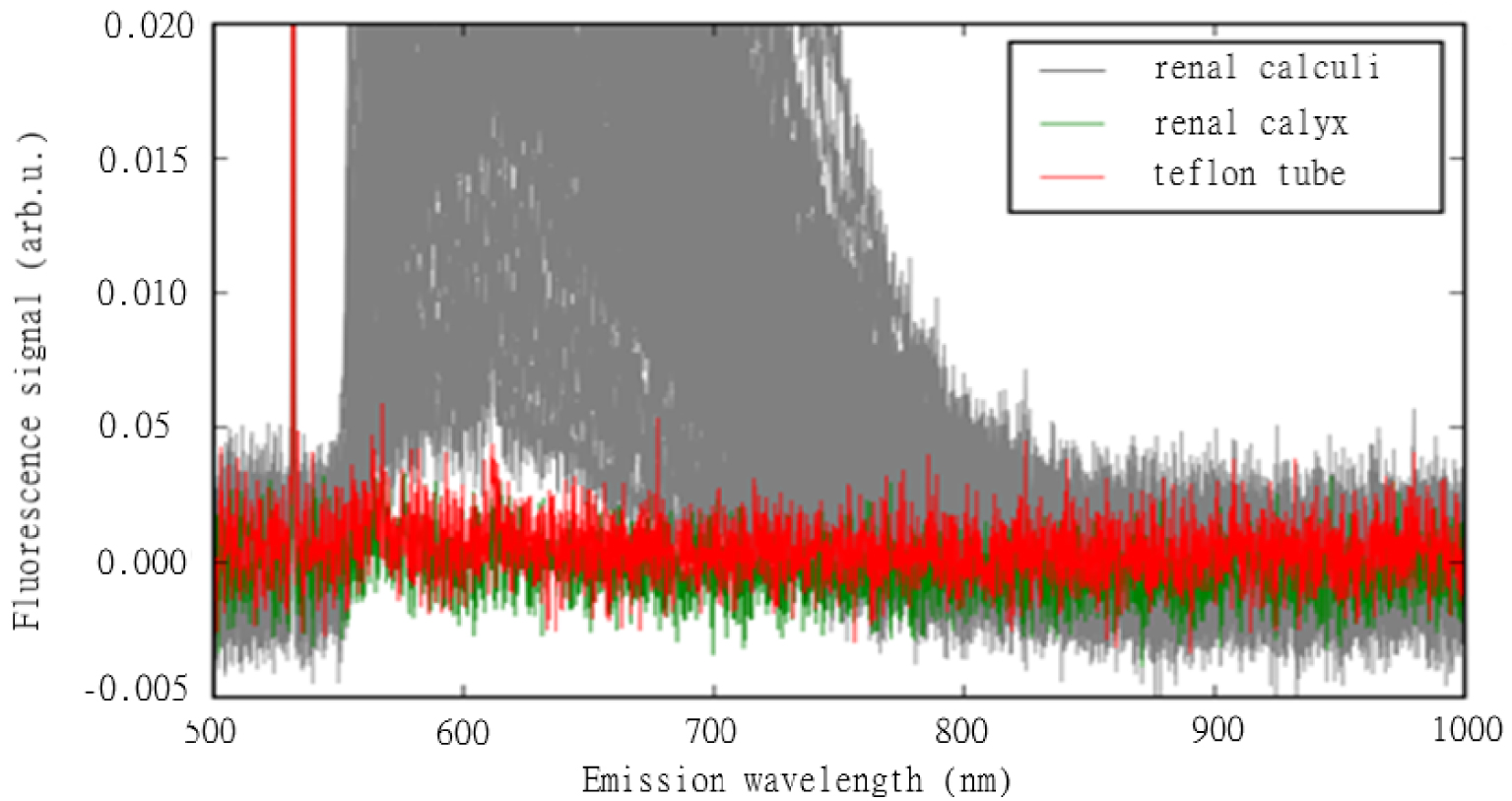
In the second experiment, we developed a real-time compact demonstration system based on the aforementioned laboratory setup to make automatic identification possible [9]. In this system, the Ho:YAG laser was connected to a therapeutic laser fiber, so that the entire system was equipped with all the technical components needed for automatic target identification during stone treatment. Most importantly, this setup actively utilized a feedback loop between the spectral detection unit and Ho:YAG laser. First, the setup was applied in a simulated surgical intervention in a porcine kidney in vitro. The experimental setup is descripted in Figures 1 and 2. Finally, the performance was assessed performing conventional Ho:YAG laser lithotripsy with and without target recognition in an in vivo setting in an animal study on domestic pigs [10]. Laser lithotripsy procedures were performed in the porcine collecting system of the kidney, in the ureter and bladder after retrograde placement of human stone material in the porcine urinary tract under general anesthesia. These experiments were approved by the Ethics Committee of the Albert-Ludwigs University of Freiburg, Germany (IRB: 296/15). To detect and evaluate any tissue damage related to the intervention and laser light’s endoluminal application, the entire urinary tract was surgically removed and examined by a pathologist. The experimental setup can be seen in Figure 2.
System setup including the measurement system and therapeutic laser.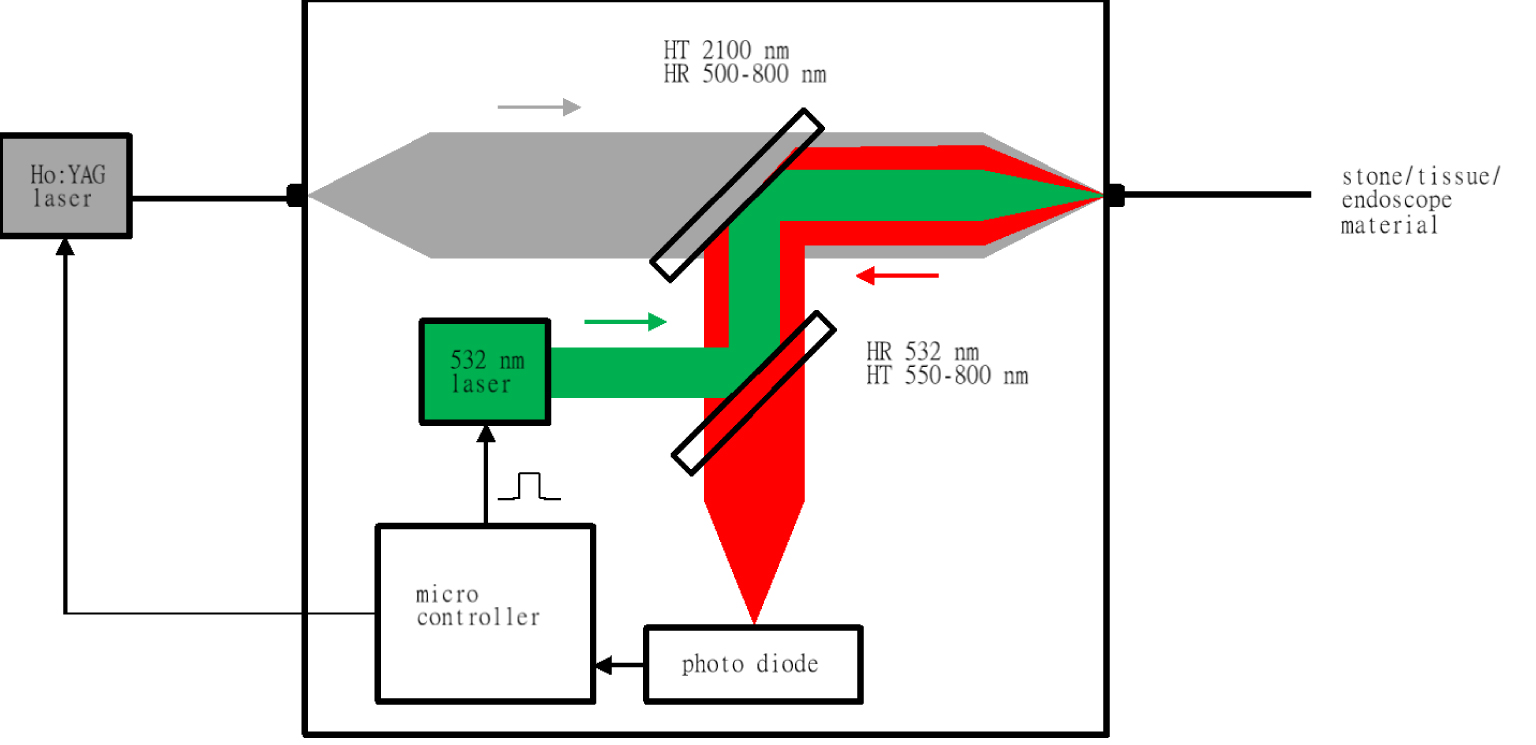
3. Results
In our spectral analyses of various human urinary stones, tissue material from the urinary tract, and surgical instrument (endoscope components) using autofluorescence, we were able to define the spectral characteristics of each of these objects. The shapes of the spectra originating in urinary stones of different composition were similar, which meant that the autofluorescence signals were unable to distinguish between the stones’ chemical compositions. Although the stones’ signal amplitudes varied, all signals were significantly stronger than the measurement values originating from the tissue samples and endoscope components. Note that all the human stones examined in these experiments emitted significantly higher signal values than all the other targeted objects. We found that the weakest stone signal was 3.6 times stronger than the strongest signal from porcine kidney tissue (mean ± SD 0.038 ± 0.043 vs 0.00058 ± 0.00058 arbitrary units). Furthermore, we detected no fluorescence signal from endoscope components.
In our in vitro study, the stones’ mean SD autofluorescence signal amplitudes ranged from 142 ± 29 to 1521 ± 152 ADU (analog-to-digital units), while emissions from tissue and the endoscope coating were practically negligible. All the stone signals were significantly stronger than those from tissue and endoscope components. We discovered that when the minimum threshold is defined as tissue plus six standard deviations, this results in 14 ADU. If the Ho:YAG laser was deactivated under that aforementioned threshold, the emission of laser light on the tissue or endoscope could be prevented. The distance between the laser tip and targeted object is known to play an essential role in ensuring efficient stone disintegration. We observed that the Ho:YAG laser operates optimally when the distance between the fiber tip and target is within a 0.1–0.5 mm range. Note that if a urinary stone emits an average fluorescent signal and is at a distance of more than about 1 mm from the fiber tip, the laser will be blocked. We also experimentally confirmed that automatic target recognition works at threshold values between 50 and 200 ADU. It is important to note, that while the laser was activated during the lithotripsy procedure, only the detection system was controlling its pulse emission—not the surgeon. During in vivo usage of this system, we observed no incorrectly emitted laser energy or tissue damage, or any impact on endoscopes.
In our in vivo experiment, lithotripsy could be performed successfully with both a standard laser and new target system (Figure 3). No macroscopic damage was detected in the tissue after treatment with this new device. We observed no organ perforation or any other severe lesion in the urinary tract whatsoever in fluoroscopic, macroscopic, and pathological investigations. No relevant thermal damage was detected even in the microscopic and histological work-up.
Presentation of the animal studies: (A) experimental setup of the system; (B) ureteroscopy procedure using the novel laser system (C) percutaneous nephrolithotomy procedure using the novel laser system; (D) endoscopic image from laser lithotripsy—(e) Urinary bladder mucosal (f) Urinary stone, (g) Laser fiber tip.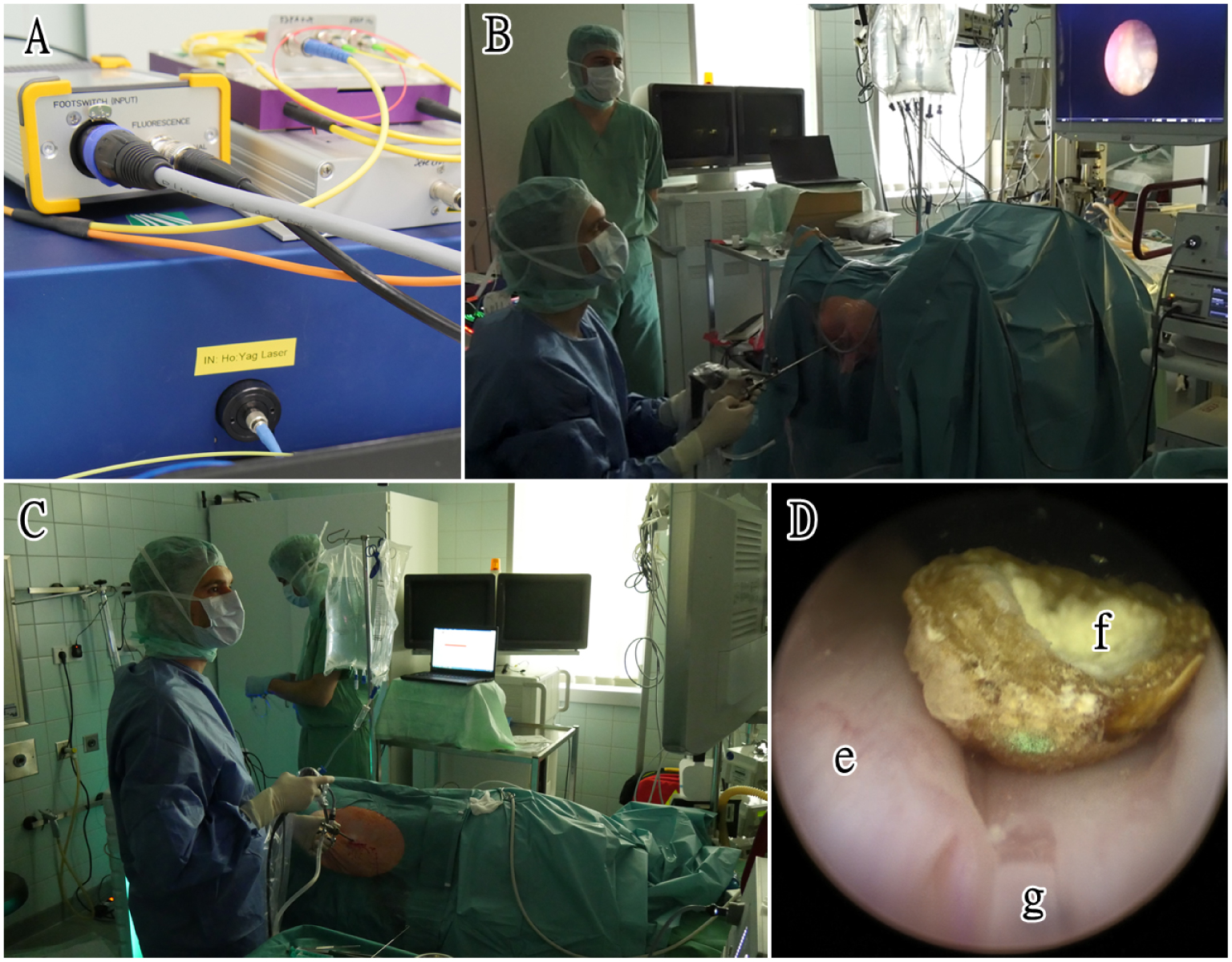
Furthermore, we found that cumulative laser energy was reduced during each procedure while the target recognition system was active. The energy applied lessened significantly with an average reduction of over 30% (27.1% for ureter stone, 52.2% for kidney stone, 17.1% for bladder stone lithotripsy). In addition, according to the personal assessment of the surgeon who conducted the animal studies, the new laser setup functions smoothly and does not hinder workflow.
4. Discussion
The increasing clinical need to prevent tissue damage from laser light emitted from laser systems has inspired research on novel photonic technologies. Lange et al. was the first group to demonstrate that autofluorescence is capable of distinguishing between urinary stones and human tissue [6]. Other research groups also proved that tissue and stone differentiation is feasible via target autofluorescence spectra [11, 12]. To transform these findings and technology into therapeutic application, we developed an advanced autofluorescence monitoring system integrated into a Holmium laser system that allows real-time target recognition during laser lithotripsy. This setup proved able to inform the surgeon about the object in front of the laser fiber’s tip in real time, that is, when stone material is within the laser’s therapeutic range. Once a fluorescence intensity threshold level has been set, the feedback mode then functions to autonomously control the Ho:YAG laser. During the procedure, the emission of energy was monitored independently by the feedback system, emitting laser energy only when it “recognized” stone material. The safety and efficiency of the present setup was successfully proven in animal models [10]. Our observations have also been confirmed histologically.
Urinary stones expose characteristic autofluorescent spectra particularly when excited at the wavelength of 520 nm [8]. An essential aim of our project was to define the appropriate wavelength to trigger the targeted object’s excitation, as this would enable us to detect any kind of chemical composite of the stone. Interestingly, stones possessing the same chemical composition reveal different wavelengths upon fluorescent excitation [8]. A stone’s wavelength spectrum is most likely determined by proteins on its surface, and it is most likely these proteins that cause the characteristic autofluorescent properties, rather than the chemical composition. This hypothesis is supported by the evidence that chemically pure compounds (e.g. pure calcium) emit no fluorescence spectra when excited at this wavelength [8].
This new system monitoring intraoperative autofluorescence only allows the laser to emit energy for therapeutic purposes when the stone is within an appropriate distance to the stone surface. The spectra monitoring unit is attached to the therapeutic laser by a glass fiber. The system is equipped with a double control mechanism. The general on-and-off switch is regulated by operating a foot pedal. The monitoring unit also communicates with the treatment laser. It is the surgeon who is in control of turning the entire system on and off. If the foot pedal remains pressed, the system is active. Moreover, the target object must be detected correctly, as only then will energy be emitted. If the foot pedal is not pressed down, it does not matter whether the fiber tip is correctly placed on the target object. In that case, no laser light energy is applied at all. When the system is in the active mode, the surgeon only needs to navigate the endoscope with the treatment fiber in place to the target stone.
Although laser lithotripsy at its current developmental stage might be considered safe and efficient, its most annoying limitation is the uncontrolled and not fully controlled energy release during surgery and its potential damage to surrounding tissues. It is a fact that while direct physical lesions rarely occur, secondary thermal injuries caused by the effect of excessive heat production in the urinary tract can eventually trigger adverse events [5, 13]. Various strategies have been proposed to prevent accidental damage, such as keeping the laser fiber tip coated for better identification and keeping it at a reasonable safety distance from the optical tip of the scope, regularly cutting the fiber tip to prevent retrograde laser emission [14]. The Ho:YAG laser has a therapeutic range of 1–3 mm in liquid. In standard lithotripsy with a Ho:YAG laser system, the emission of high-energy pulses depends solely on the surgeon. When the surgeon presses the foot pedal while the stone is right in front of the laser fiber tip, laser energy is transferred to the stone through the fluid in between. Due to a continuous exchange of irrigation fluid, and to mechanical forces resulting from the laser–target interaction, the stone may move away from laser’s fiber tip. When that happens, laser energy is unavailable for fragmenting the calculus, but it suffices to heat the surrounding liquid. The endoscope can also be damaged if the laser fiber is accidentally retracted too far during treatment [4]. Another potential problem is that by delivering energy in an undesirably large space between the stone and the laser fiber tip, the force of retropulsion becomes excessive and causes the stones’ dislocation. Specifically, if the urinary tract anatomy is unfavorable, situations may arise in which visualization of the intended target becomes difficult. When the threshold values are accurately set, the energy is absorbed almost exclusively by the stone surface, thus minimizing retropulsion.
The greatest contribution of our system to the lithotripsy procedure is that it detects stone and tissue automatically, and that it is completely surgeon-independent, requiring no action by or input from the surgeon during lithotripsy. Thanks to this detection function that the feedback mechanism provides, the surgeon can focus solely on navigating and the lithotripsy procedure without having to worry about damaging the tissue.
In addition to reducing the risk of tissue injuries and contributing to a safer, more efficient surgery, our system has further advantages in terms of its clinical performance. We have demonstrated that the total amount of energy required for sufficient and successful fragmentation dropped by about 30%. By preventing endoscope damage, utilizing our system might also deliver a positive economic effect. Another potential benefit of this new technology is that it will be easier to operate by less experienced surgeons or urology residents in training still in a steep learning curve. Thanks to the automatic, surgeon-independent therapy monitoring function, the stress and anxiety associated with a lithotripsy intervention could be minimized. This innovative technology can also be adapted to other laser types as a hardware add-on. Our new target system holds promise for substantial improvements in laser lithotripsy in the future.
Our system has some limitations. While using this system, temperature changes in the operation area are not measured. The long-term thermal effect of temperature changes on human urinary tract tissue is not completely understood [5]. In addition, it is not yet clear how effective this system is when applying different stone disintegration techniques. Its main limitation is that we have not yet gained any practical experience from procedures in humans. However, since this technical approach has proven safe and robust in the preclinical setting and animal model, our group recently started a clinical pilot study with the system being applied for human laser lithotripsy.
5. Conclusion
In this review we summarized our research and development on a novel laser system providing a real-time treatment-monitoring unit using autofluorescence to distinguish urinary stone targets during laser lithotripsy from urothelial tissue and endoscope components. We found this technology to work reliably and be safe in experimental preclinical settings and animal trials. We are currently preparing for its first in-human use.
Author disclosure statement
A. Miernik receives research funds of the German Federal Ministry of Education and Research, Berlin (D). He receives support for his travel activities from the European Society of Urology, Arnhem (NL), and the German Society of Urology, Düsseldorf (D). Furthermore, A. Miernik is consulted for: KLS Martin, Tuttlingen (D), Avateramedical, Jena (D), LISA LaserProducts GmbH, Katlenburg-Lindau (D), Schoelly fiberoptics GmbH, Denzlingen (D), Dornier MedTech Laser GmbH (D), Medi-Tate Ltd. (IL, USA) and B. Braun New ventures GmbH, Freiburg (D). A. Miernik is speaker for the companies Richard Wolf GmbH (D) and Boston Scientific (USA). Additionally, he performed expert activities for the Ludwig Boltzmann Gesellschaft, Wien (A). A. Miernik is involved in numerous patents and inventions in the field of medical technology. L. Kraft provided consulting services for Dornier MedTech Laser GmbH, Weßzling (D). C. Gratzke is advisor for Astellas Pharma GmbH, Munich (D), Ipsen Pharma GmbH, Munich (D), Steba Biotech S.A., Luxembourg (LUX), Bayer Pharma, Leverkusen (D), Olympus Winter & Ibe GmbH, Hamburg (D), Medi-Tate Ltd., Or Akiva (IL), MSD, Haar (D), Astra-Zeneca, Cambridge (UK) and Roche, Basel (CH). C. Gratzke receives speaker fees from Amgen, California (USA), Astellas Pharma GmbH, Munich (D), Ipsen Pharma GmbH, Munich (D), Janssen-Cilag GmbH, Neuss (D), Bayer Pharma, Leverkusen (D), Takeda Pharmaceuticals, Tokio (JPN) and medac GmbH, Wedel (D). D. Schlager, R. S. Ibarrola, A. Schulte, M. Yilmaz declare to have no conflicts of interest.
The manuscript does not contain clinical studies or patient data. This article does not contain any studies with human subjects performed by any of the authors.
Authors’ contribution
DS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. AM and DS conceived the study concept and design. DS, MY, RSI and LK interpreted the data and wrote the manuscript. AM and CG supervised the manuscript. All authors discussed the results and commented on the manuscript.




 CC-BY 4.0
CC-BY 4.0