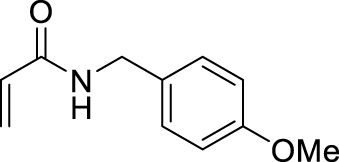
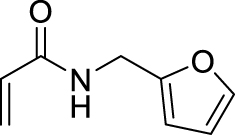
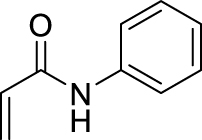
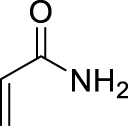
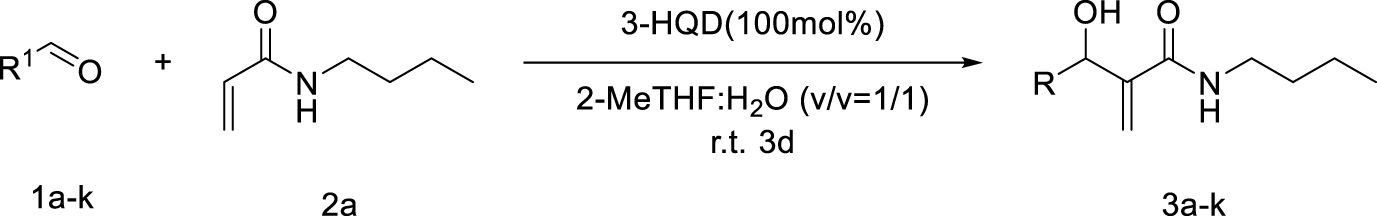1. Introduction
Morita–Baylis–Hillman reaction of acrylamides.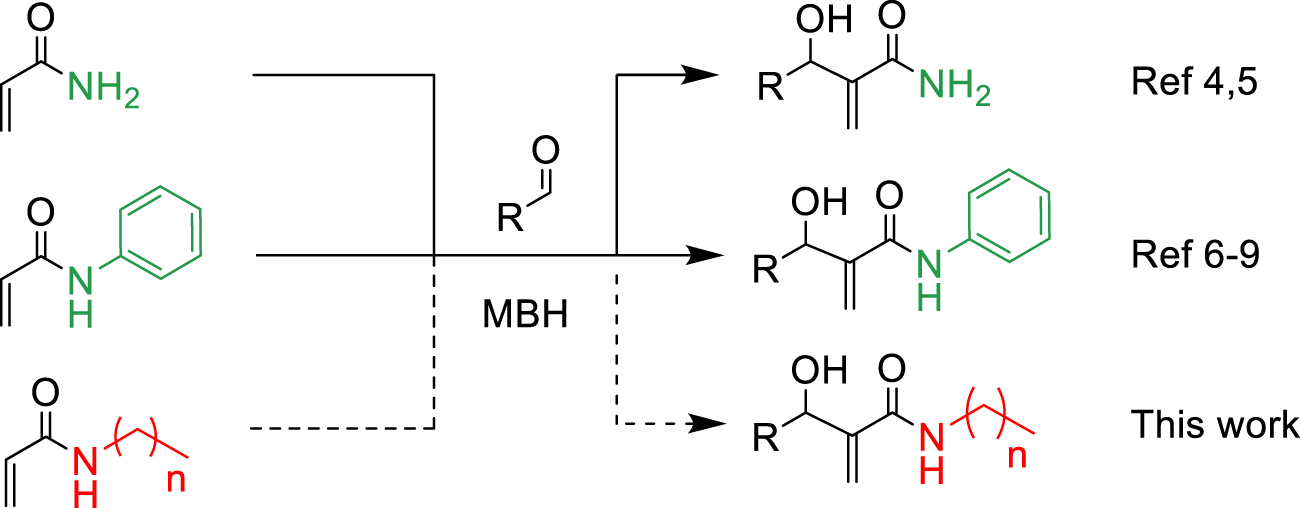
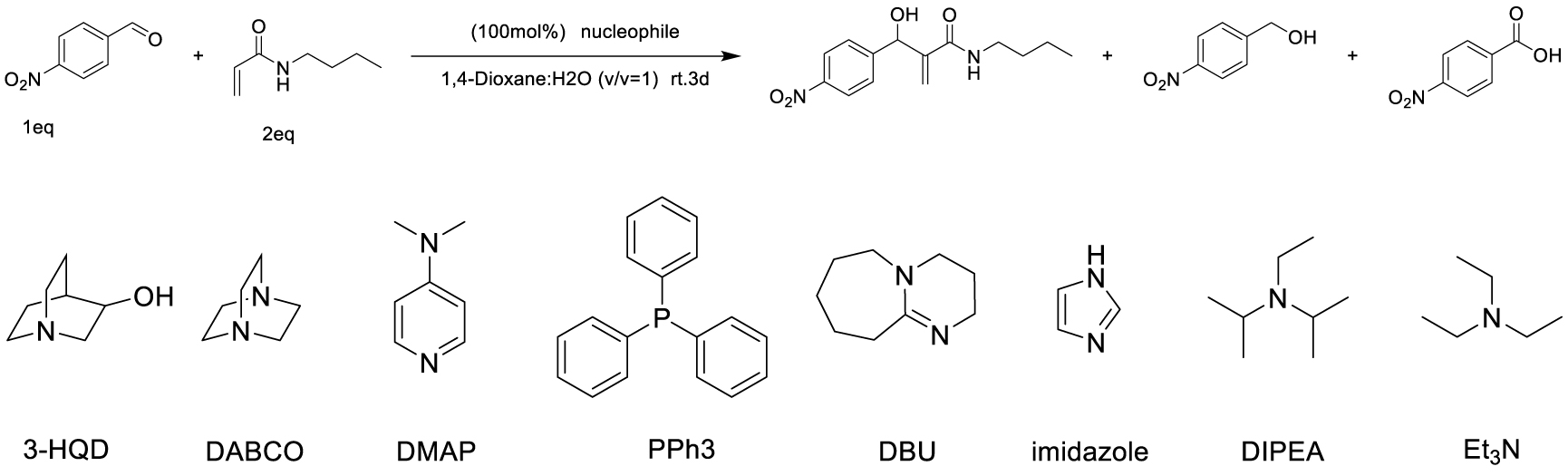
Among carbon–carbon bond forming reactions, the Morita–Baylis–Hillman (MBH) reaction is an elegant one to build complex molecules by coupling aldehydes and activated alkenes. Reported in 1968 by Morita as a phosphine-promoted reaction of acrylic compounds with aldehydes [1], the tertiary amine-promoted equivalent was discovered soon after by Baylis and Hillman in 1972 [2]. Its advantages are notably the high level of functionality in the products, its atom economical character and ability to run under mild conditions using a variety of reported promoters, and its versatility in terms of generality toward starting aldehydes and alkenes [3]. However, with respect to the alkene partner, the use of N-substituted acrylamides has been very limited, being reported either as inert or poorly reactive, due to a weaker electron-withdrawing ability as compared to acrylates or nitriles for example, as well as a competitive acido-basic reaction when a basic tertiary amine is used as promoter, due to the presence of a relatively labile proton on the nitrogen atom of secondary amides. Up to now, only a few examples of MBH reactions using unsubstituted acrylamide have been published. Hu et al. reported in 2002 the reaction of acrylamide with aromatic aldehydes in dioxane water, mentioning that N-isopropylacrylamide and N,N-dimethylacrylamide were both inert under the same conditions [4]. In 2004, Connon’s work dealt also with acrylamide MBH reactions but did not include any substituted ones in the scope [5]. Among N-mono-substituted acrylamides, only N-aryl ones have been studied, with Guo’s work on MBH reactions of 𝛼-substituted N-arylacrylamides mentioning also lower reactivity for acrylamide or N-alkyl acrylamides [6], and Bharadwaj’s studies on reactions of isatin derivatives with N-phenylacrylamide [7] and N-arylacrylamide activated by intramolecular hydrogen bond [8]. Only one example of MBH reaction using a secondary N-alkyl acrylamide was reported by Tang et al., but the focus of this work was more on the comparison between ester or amide chiral auxiliaries in asymmetric MBH reactions than on the structural scope [9].
Promoter screening for the Baylis–Hillman reaction of 4-nitrobenzaldehyde with N-butylacrylamidea
| Entry | Promoter | NMR yield (MBH) | NMR yield (alcohol) | Yield (acid) | Conversion (aldehyde) |
|---|---|---|---|---|---|
| 1 | 3-HQD | 52% | 18% | 21% | 97% |
| 2 | DABCO | 34% | 15% | 14% | 70% |
| 3 | DBU | — | 47% | 45% | 95% |
| 4 | DMAP | — | 35% | 30% | 93% |
| 5 | Et3N | — | — | — | 6% |
| 6 | DIPEA | — | — | — | 28% |
| 7 | PPh3 | — | — | — | 45% |
| 8 | Imidazole | — | — | — | 3% |
aReaction conditions: p-nitrobenzaldehyde 0.5 mmol, N-butylacrylamide 1 mmol, promoter, 0.5 mmol, 1,4-dioxane: H2O (1:1) 250 μL (2 M), r.t, 3d.
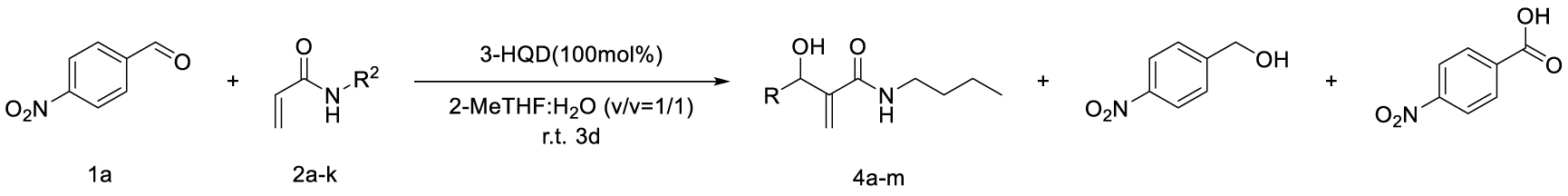
Considering the availability of N-alkyl acrylamides, and the usefulness of amide-containing products in the fields of fine chemicals and polymer chemistry (high stability, strong inter- or intramolecular interactions) [10, 11], this paper addresses the specific case of the use of secondary N-alkyl acrylamides in MBH reactions. Below we report our results on the search for appropriate reaction conditions notably with respect to the promoter and the solvent, as well as on the investigation of the structural scope with different secondary N-alkyl acrylamides and aromatic aldehydes (Scheme 1).
2. Experimental section
2.1. General information
The reagents and solvents were purchased from Sigma-Aldrich, Alfa Aesar and TCI and utilized without any purification unless mentioned specifically. The solvents for the column chromatography were purchased from Carlo Erba company. The thin-layer chromatography (TLC) analysis was conducted on the aluminum-backed plates pre-coated with 0.2 mm silica gel 60. The flash chromatographies were performed using Merck Si 60 silica gel (40–63 μm). 1H and 13C NMR spectra were recorded on a Bruker DRX-300 spectrometer (1H: 300, 400 or 500 MHz; 13C: 75, 100 or 125 MHz). High-resolution mass spectra (HRMS) were recorded on Bruker MicrOTOF-Q II XL spectrometer using ESI as ionization source. NMR spectra are available in the Supporting information.
2.2. General procedure for synthesis and treatment
2.2.1. Synthesis of N-alkyl acrylamide
N-alkyl acrylamides were prepared based on the methods in literature [12]. In detail, N-alkyl acryl amine (12 mmol) and anhydrous triethylamine (12 mmol, 1.7 mL) were dissolved in anhydrous DCM (50 mL). Acryloyl chloride (10 mmol, 820 μL) was dripped slowly into the solution under 0 °C. The reaction mixture was left under room temperature overnight. The solution was concentrated and dissolved again in ethyl acetate. The precipitate was removed by filtration and the filtrate was concentrated. The obtained crude product was purified by column chromatography using a mixture of ethyl acetate and pentane (v∕v = 1∕2) as eluent to yield the corresponding N-alkyl acrylamide.
2.2.2. Calculation of NMR yield
The reaction mixture was transferred to a separating funnel with water and EtOAC, followed by the extraction with EtOAc (3 × 30 mL). The organic layer consisted of MBH adduct, remaining 4-nitrobenzaldehyde and 4-nitrobenzyl alcohol. This organic layer was dried (over Na2SO4) and concentrated under vacuum and the obtained crude mixture was analyzed by 1HNMR using 1, 2, 4, 5-tetramethylbenzene (1 equivalent) as internal standard and deuterated chloroform (CDCl3) was used as solvent to check NMR yield. (For NMR yield of MBH adduct, Cannizarro alcohol and the conversion of aldehyde see Tables 1–3).
Solvent screeninga
| Entry | Solvent | NMR yield (MBH) | NMR yield (alcohol) | Yield (acid) | Conversion (aldehyde) |
|---|---|---|---|---|---|
| 1 | Solvent free | 15% | — | — | 29% |
| 2 | H2O | 40% | 24% | 20% | 94% |
| 3 | DMF:H2O (1:1) | 38% | 6% | 8% | 55% |
| 4 | MeCN:H2O (1:1) | 41% | 13% | 16% | 96% |
| 5 | Isopropanol:H2O (1:1) | 43% | 17% | 15% | 95% |
| 6 | EtOH:H2O (1:1) | 50% | 21% | 25% | 98% |
| 7 | 1,4-dioxane:H2O (1:1) | 52% | 18% | 21% | 97% |
| 8 | 2-MeTHF:H2O (1:1) | 62% | 13% | 10% | 92% |
| 9 | 2-MeTHF:H2O (1:9) | 48% | 13% | 11% | 97% |
| 10 | 2-MeTHF:H2O (3:7) | 52% | 12% | 15% | 95% |
| 11 | 2-MeTHF:H2O (7:3) | 43% | 7% | 11% | 70% |
| 12 | 2-MeTHF:H2O (9:1) | 31% | 4% | 6% | 63% |
| 13 | 2-MeTHF | 24% | — | — | 53% |
aReaction conditions: 0.5 mmol p-nitrobenzaldehyde, 1 mmol N-butylacrylamide, 0.5 mmol 3-HQD, 250 μL solvent (2 M), r.t, 3d.
Influence of stoichiometry and concentrationa
| Entry | Equivalent (3-HQD) | Equivalent (N-butylacrylamide) | Concentration | Time | NMR yieldb (MBH) | NMR yieldb (alcohol) | Yieldb (acid) | Conversion (aldehyde) |
|---|---|---|---|---|---|---|---|---|
| Entry 1 | 0.5 | 2 | [2 M] | 3d | 47% | 20% | 17% | 94% |
| 1 | 2 | [2 M] | 3d | 62% | 13% | 10% | 92% | |
| 1.5 | 2 | [2 M] | 3d | 58% | 9% | 11% | 98% | |
| Entry 2 | 1 | 1 | [2 M] | 3d | 41% | 13% | 15% | 88% |
| 1 | 2 | [2 M] | 3d | 62% | 13% | 10% | 92% | |
| 1 | 3 | [2 M] | 3d | 62% | 12% | 10% | 98% | |
| Entry 3 | 1 | 2 | [0.5 M] | 3d | 38% | 24% | 26% | 87% |
| 1 | 2 | [1 M] | 3d | 60% | 15% | 12% | 94% | |
| 1 | 2 | [4 M] | 3d | 58% | 10% | 15% | 98% | |
aReaction conditions: 0.5 mmol p-nitrobenzaldehyde, r.t.
2.2.3. Calculation of isolated yield
The reaction mixture was removed to a separating funnel with water and EtOAc, followed by the extraction with EtOAc (3 × 30 mL). The organic layer consisted of MBH adduct, remaining 4-nitrobenzaldehyde and 4-nitrobenzyl alcohol. This organic layer was dried (over Na2SO4) and concentrated under vacuum and the obtained crude mixture was purified by column chromatography using EtOAc:Pentane = 1:1 as eluent. The aqueous layer was acidified with HCl (1 M), and extracted with EtOAc (3 × 30 mL). This organic layer was dried (over Na2SO4) and concentrated under vacuum to give 4-nitrobenzoic acid. (For isolated yield of acid in all the tables, the isolated yield of MBH adduct and alcohol, and the conversion of aldehyde see Tables 4, 5) [13, 14].
aReaction conditions: 1 mmol p-nitrobenzaldehyde, 2 mmol acrylamide, 1 mmol 3-Hydroxyquinuclidine, 500 μL 2-MeTHF:H2O (v∕v = 1∕1) (2 M), r.t., 3d. cr.t. 4h.
aReaction conditions: 1 mmol aldehyde, 2 mmol N-butylacrylamide, 1 mmol 3-Hydroxyquinuclidine, 500 μL 2-MeTHF:H2O = 1:1 (2 M), r.t, 3d. bNMR yield, 1,2,4,5-tetramethylbenzene (1 mmol) as internal standard.
2.3. Characterization of compounds
2.3.1. N-butyl-2-(hydroxy(4-nitrophenyl)methyl) acrylamide (3a/4a)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.08 (d, J = 8.7 Hz, 2H), 7.49 (d, J = 8.5 Hz, 2H), 6.92 (t, J = 5.7 Hz, 1H), 5.87 (s, 1H), 5.51 (d, J = 4.6 Hz, 2H), 5.46 (s, 1H), 3.09 (dq, J = 10.5,6.8 Hz, 2H), 1.36–1.19 (m, 2H), 1.18–0.99 (m, 2H), 0.74 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, Chloroform-d) 𝛿 167.21, 148.94, 147.11, 144.18, 126.89, 123.38, 121.95, 73.89, 39.12, 31.15, 19.81, 13.53. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H18N2NaO4 301.1159; found 301.1165.
2.3.2. N-butyl-2-(hydroxy(2-nitrophenyl)methyl) acrylamide (3b)
1H NMR (300 MHz, Chloroform-d) 𝛿 7.79 (ddd, J = 13.2, 8.0, 1.4 Hz, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.36 (ddd, J = 8.5, 7.4, 1.5 Hz, 1H), 6.61 (s, 1H), 6.03 (d, J = 4.8 Hz, 1H), 5.64 (s, 1H), 5.15 (d, J = 5.2 Hz, 1H), 5.12 (d, J = 1.0 Hz, 1H), 3.13 (td, J = 7.1, 5.8 Hz, 2H), 1.44–1.28 (m, 2H), 1.24–1.07 (m, 2H), 0.78 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, Chloroform-d) 𝛿 168.37, 147.93, 144.19, 136.21, 133.35, 128.87, 128.50, 124.50, 120.45, 69.72, 39.30, 31.30, 19.93, 13.69. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H18N2NaO4 301.1159; found 301.1163.
2.3.3. N-butyl-2-(hydroxy(3-nitrophenyl)methyl) acrylamide (3c)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.18 (t, J = 2.0 Hz, 1H), 8.02 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.62 (dt, J = 7.7, 1.4 Hz, 1H), 7.41 (t, J = 7.9 Hz, 1H), 6.93 (t, J = 5.8 Hz, 1H), 5.90–5.82 (m, 1H), 5.51 (d, J = 2.3 Hz, 2H), 5.45 (s, 1H), 3.11 (dddd, J = 20.6, 13.6, 7.1, 5.7 Hz, 2H), 1.36–1.20 (m, 2H), 1.15–0.98 (m, 2H), 0.72 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, Chloroform-d) 𝛿 167.26, 148.22, 144.28, 143.86, 132.35, 129.28, 122.44, 122.00, 121.00, 73.83, 39.14, 31.23, 19.87, 13.59. HRMS (ESI) m/z: Calcd for [M + Na]+C14H18N2NaO4 301.1159; found 301.1161.
2.3.4. N-butyl-2-(hydroxy(pyridin-2-yl)methyl) acrylamide (3d)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.46–8.33 (m, 1H), 7.59 (td, J = 7.7, 1.8 Hz, 1H), 7.39–7.26 (m, 1H), 7.23 (s, 1H), 7.17–7.01 (m, 1H), 5.97 (s, 1H), 5.45 (d, J = 24.6 Hz, 2H), 3.21–2.89 (m, 2H), 1.24 (dtd, J = 8.7, 7.0, 5.5 Hz, 2H), 1.15–0.90 (m, 2H), 0.71 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 166.73, 159.40, 147.80, 143.84, 137.08, 123.38, 122.56, 120.78, 74.14, 38.81, 31.14, 19.73, 13.55. HRMS (ESI) m/z: Calcd for [M + H]+ C13H19N2O2 235.1441; found 235.1440.
2.3.5. N-butyl-2-(hydroxy(5-(hydroxymethyl)furan-2-yl)methyl)acrylamide (3h)
1H NMR (400 MHz, Chloroform-d) 𝛿 6.19 (s, 2H), 5.88 (d, J = 0.7 Hz, 1H), 5.54 (t, J = 0.8 Hz, 1H), 5.46 (d, J = 0.9 Hz, 1H), 4.50 (s, 2H), 3.33–3.15 (m, 2H), 1.53–1.39 (m, 2H), 1.34–1.21 (m, 3H), 0.88 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, Chloroform-d) 𝛿 167.87, 154.15, 153.96, 121.70, 108.56, 107.87, 69.20, 57.14, 39.21, 31.42, 20.11, 13.78. HRMS (ESI) m/z: Calcd for [M + Na]+ C13H19NNaO4 276.1206; found 276.1199.
2.3.6. N-butyl-2-(hydroxy(5-(𝛼-D-glucopyrano- syloxymethyl)-furan-2-yl)methyl)acrylamide (3i)
1H NMR (300 MHz, Methanol-d4) 𝛿 6.37 (d, J = 3.2 Hz, 1H), 6.22 (d, J = 3.2 Hz, 1H), 5.87 (d, J = 4.0 Hz, 0H), 5.72 (s, 1H), 5.61 (s, 1H), 4.71–4.48 (m, 2H), 3.86–3.75 (m, 1H), 3.75–3.55 (m, 3H), 3.48–3.26 (m, 3H), 3.22 (t, J = 6.9 Hz, 2H), 1.57–1.40 (m, 2H), 1.41–1.20 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, MeOD) 𝛿 169.88, 156.29, 156.22, 152.58, 152.54, 146.01, 145.92, 119.94, 119.69, 111.40, 108.96, 108.89, 99.18, 99.06, 74.99, 73.77, 73.46, 71.76, 71.71, 67.88, 67.75, 62.63, 62.13, 62.05, 49.85, 49.57, 49.28, 49.00, 48.72, 48.43, 48.15, 40.07, 32.43, 30.69, 21.02, 14.09.HRMS (ESI) m/z: Calcd for [M + Na]+C19H29NNaO9 438.1735; found 438.1725.
2.3.7. N-butyl-2-(furan-2-yl(hydroxy)methyl) acrylamide (3j)
1H NMR (300 MHz, Chloroform-d) 𝛿 7.29 (dd, J = 1.8, 0.9 Hz, 1H), 6.26 (d, J = 1.8 Hz, 1H), 6.22 (dd, J = 3.3,0.9 Hz, 1H), 5.83 (s, 1H), 5.46–5.39 (m, 2H), 3.21 (ttd, J = 6.8, 3.5, 1.3 Hz, 2H), 1.49–1.31 (m, 2H), 1.27–1.17 (m, 2H), 0.83 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 167.60, 154.10, 142.34, 131.02, 126.31, 121.62, 110.54, 107.16, 69.78, 39.31, 31.48, 20.10, 13.82. HRMS (ESI) m/z: Calcd for [M + Na]+ C12H18NaO3 246.1101; found 246.1101.
2.3.8. N-butyl-2-(hydroxy(5-(methoxymethyl)furan-2-yl)methyl)acrylamide (3k)
1H NMR (300 MHz, Chloroform-d) 𝛿 6.98 (t, J = 5.8 Hz, 1H), 6.18 (d, J = 9.4 Hz, 2H), 5.88 (s, 1H), 5.44 (d, J = 6.5 Hz, 2H), 4.28 (s, 2H), 3.26 (s, 3H), 3.24–3.13 (m, 2H), 1.47–1.33 (m, 2H), 1.30–1.12 (m, 2H), 0.83 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, Chloroform-d) 𝛿 167.34, 154.54, 151.19, 142.43, 122.15, 110.28, 107.63, 69.29, 66.26, 57.67, 39.17, 31.33, 20.00, 13.69. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H21NNaO4 290.1363; found 290.1362.
2.3.9. 2-(hydroxy(4-nitrophenyl)methyl)-N-octylacrylamide (4b)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.10 (d, J = 8.8 Hz, 2H), 7.51 (d, J = 8.5 Hz, 2H), 6.78 (s, 1H), 5.87 (s, 1H), 5.53 (s, 1H), 5.47 (s, 1H), 3.23–2.96 (m, 2H), 1.39–1.26 (m, 2H), 1.26–0.97 (m, 5H), 0.81 (t, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 167.25, 148.93, 147.21, 144.33, 126.93, 123.46, 121.74, 74.06, 39.50, 31.74, 29.16, 26.78, 22.59, 14.05. HRMS (ESI) m/z: Calcd for [M + H]+ C18H27N2O4 335.1965; found 335.1962.
2.3.10. N-dodecyl-2-(hydroxy(4-nitrophenyl)methyl) acrylamide (4c)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.11 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.7 Hz, 2H), 6.72 (s, 1H), 5.86 (s, 1H), 5.54 (s, 1H), 5.47 (s, 1H), 3.27–2.97 (m, 2H), 1.39–1.27 (m, 2H), 1.21 (s, 4H), 0.90–0.71 (t, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 167.28, 148.90, 147.25, 144.40, 126.94, 123.49, 121.67, 74.10, 39.54, 31.92, 29.64, 29.60, 29.55, 29.35, 29.26, 26.82, 22.70, 14.13. HRMS (ESI) m/z: Calcd for [M + Na]+ C22H34N2NaO4 413.2411; found 413.2408.
2.3.11. N-(sec-butyl)-2-(hydroxy(4-nitrophenyl) methyl)acrylamide (4d)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.10 (d, J = 8.8 Hz, 2H), 7.51 (dd, J = 8.8, 1.9 Hz, 2H), 6.57 (s, 1H), 5.86 (d, J = 11.3 Hz, 1H), 3.84–3.63 (m, 1H), 1.47–1.17 (m, 2H), 0.95 (dd, J = 24.5, 6.6 Hz, 3H), 0.66 (dt, J = 39.4, 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 166.77, 166.59, 148.98, 147.17, 144.50, 126.92, 123.40, 121.85, 121.49, 73.99, 46.76, 29.29, 20.01, 10.16, 10.03. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H18N2NaO4 301.1159; found 301.1156.
2.3.12. 2-(hydroxy(4-nitrophenyl)methyl)-N-isobutylacrylamide (4e)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.16 (d, J = 8.8 Hz, 2H), 7.55 (d, J = 8.2 Hz, 2H), 6.52 (s, 1H), 5.87 (s, 1H), 5.57 (d, J = 5.2 Hz, 1H), 5.51 (d, J = 0.9 Hz, 1H), 4.98 (d, J = 5.7 Hz, 1H), 3.14–2.86 (m, 2H), 1.72–1.58 (m, 1H), 0.76 (dd, J = 6.7,1.5 Hz, 6H). 13C NMR (75 MHz, CDCl3) 𝛿 167.52, 148.81, 147.41, 144.60, 127.02, 123.63, 121.46, 77.58, 77.16, 76.74, 74.42, 46.85, 28.43, 19.98. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H18N2NaO4 301.1159; found 301.1160.
2.3.13. 2-(hydroxy(4-nitrophenyl)methyl)-N-isopropylacrylamide (4f)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.08 (d, J = 8.8 Hz, 2H), 7.48 (d, J = 8.2 Hz, 2H), 6.52 (d, J = 7.9 Hz, 1H), 5.80 (s, 1H), 5.49 (s, 1H), 5.42 (d, J = 0.9 Hz, 1H), 5.32 (d, J = 5.4 Hz, 1H), 3.88 (dt, J = 8.0, 6.6 Hz, 1H), 1.00 (d, J = 6.6 Hz, 3H), 0.93 (d, J = 6.5 Hz, 3H). 13C NMR (75 MHz, CDCl3) 𝛿 166.52, 149.00, 147.23, 144.54, 126.98, 123.46, 121.54, 77.59, 77.16, 76.74, 74.07, 41.53, 22.40, 22.33. HRMS (ESI) m/z: Calcd for [M + Na]+ C13H16N2NaO4 287.1002; found 287.1002.
2.3.14. N-(tert-butyl)-2-(hydroxy(4-nitrophenyl) methyl)acrylamide (4g)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.18 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 8.3 Hz, 2H), 6.00 (s, 1H), 5.72 (s, 1H), 5.54 (s, 1H), 5.43 (d, J = 0.9 Hz, 1H), 1.26 (s, 9H). 13C NMR (75 MHz, CDCl3) 𝛿 167.35, 148.91, 147.40, 145.66, 127.04, 123.59, 120.27, 74.56, 63.99, 51.83, 28.62. 13C NMR (75 MHz, CDCl3) 𝛿 167.39, 148.93, 147.43, 145.73, 127.06, 123.61, 120.13, 77.58, 77.16, 76.74, 74.64, 51.86, 28.65. HRMS (ESI) m/z: Calcd for [M + Na]+ C14H18N2NaO4 301.1159; found 301.1156.
2.3.15. N-cyclohexyl-2-(hydroxy(4-nitrophenyl) methyl)acrylamide (4h)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.10 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.7 Hz, 2H), 6.85 (d, J = 8.2 Hz, 1H), 5.86 (s, 1H), 5.51 (s, 1H), 5.47 (s, 1H), 3.59 (ddd, J = 9.7,6.3,3.3 Hz, 1H), 1.73 (dd, J = 12.6,3.8 Hz, 1H), 1.67–1.41 (m, 4H), 1.29–1.15 (m, 2H), 1.15–1.01 (m, 2H), 0.98–0.86 (m, 1H). 13C NMR (75 MHz, CDCl3) 𝛿 166.39, 148.98, 147.19, 144.72, 126.91, 123.43, 121.80, 73.48, 48.21, 32.45, 25.35, 24.49. HRMS (ESI) m/z: Calcd for [M + H]+ C16H21N2O4 305.1496; found 305.1497.
2.3.16. N-benzyl-2-(hydroxy(4-nitrophenyl)methyl) acrylamide (4i)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.15 (d, J = 8.8 Hz, 2H), 7.54 (d, J = 8.2 Hz, 2H), 7.27–7.22(m, 3H), 7.08–7.00 (m, 2H), 6.76 (s, 1H), 5.91 (s, 1H), 5.60 (s, 1H), 5.56 (s, 1H), 4.47–4.24 (m, 2H). 13C NMR (75 MHz, CDCl3) 𝛿 167.38, 148.58, 147.44, 144.41, 137.48, 128.77, 127.52, 127.04, 123.67, 121.69, 74.40, 43.49. HRMS (ESI) m/z: Calcd for [M + Na]+ C17H16N2NaO4 335.1002; found 335.0997.
2.3.17. 2-(hydroxy(4-nitrophenyl)methyl)-N-(4-methoxybenzyl)acrylamide (4j)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.25–7.95 (m, 1H), 7.51 (dd, J = 9.0,0.7 Hz, 1H), 7.11 (t, J = 7.9 Hz, 1H), 6.78–6.67 (m, 1H), 6.62–6.53 (m, 1H), 5.98 (s, 1H), 5.61–5.48 (m, 2H), 4.41 (d, J = 15.0 Hz, 1H), 4.21 (d, J = 15.0 Hz, 1H), 3.70 (d, J = 0.8 Hz, 2H), 2.72 (d, J = 2.3 Hz, 2H). 13C NMR (75 MHz, CDCl3) 𝛿 167.21, 159.78, 148.68, 147.32, 144.48, 139.27, 129.62, 126.94, 123.60, 122.23, 119.63, 113.46, 112.18, 77.59, 77.16, 76.74, 73.55, 55.14, 43.15, 43.03. HRMS (ESI) m/z: Calcd for [M + Na]+ C18H18N2NaO5 365.1108; found 365.1109.
2.3.18. N-(furan-2-ylmethyl)-2-(hydroxy(4-nitrophenyl)methyl)acrylamide (4k)
1H NMR (300 MHz, Chloroform-d) 𝛿 8.21–8.01 (m, 2H), 7.58–7.45 (m, 2H), 7.28–7.20 (m, 1H), 6.23 (dd, J = 3.2,1.9 Hz, 1H), 6.05 (d, J = 3.2 Hz, 1H), 5.92 (s, 1H), 4.44–4.16 (m, 2H). 13C NMR (75 MHz, CDCl3) 𝛿 167.22, 150.73, 148.67, 147.33, 144.45, 142.27, 127.02, 123.59, 110.47, 107.53, 77.58, 77.16, 76.73, 73.54, 36.40, 36.28. HRMS (ESI) m/z: Calcd for [M + Na]+ C15H14N2NaO5 325.0795; found 325.0790.
The identification for 4l and 4m is based on the data of previous literature [4, 6].
3. Results and discussion
Searching for optimal promoter/solvent couples, the reaction of 4-nitrobenzaldehyde with N-butylacrylamide was performed using several nucleophilic promoters, starting from Hu’s conditions, namely DABCO-1,4-dioxane: H2O (v∕v = 1∕1) which proved efficient for unsubstituted acrylamide [4]. The results are summarized in Table 1. Among all tested promoters, only 3-Hydroxyquinuclidine (3-HQD) and 1,4-diazabicyclo[2.2.2]octane (DABCO) promoted the generation of the corresponding MBH adduct, in 52 and 34% yields respectively, although the conversion of the starting aldehyde was nearly complete. The formation of side products was thus investigated, showing the presence of 4-nitrobenzyl alcohol and 4-nitrobenzoic acid arising from the base-mediated Cannizzaro reaction of the aldehyde. Basavaiah et al. reported that organobases can indeed promote the Cannizzaro reaction [13]. Therefore the process can be evaluated in terms of selectivity toward either the desired MBH or the undesired Cannizzaro pathway, showing significant differences among all tested promoters, either giving only Cannizzaro products like DBU or DMAP, or half and half, like DABCO, or more MBH like 3-HQD.
As for the solvent issue, we focused on the use of protic media, and specifically on aqueous systems, based on their well documented beneficial effect on MBH reactions by stabilization of zwitterionic intermediates [15, 16, 17, 18, 19, 20, 21, 22, 23, 24], The usefulness of water as component of the solvent was already demonstrated for the case of the MBH reaction of unsubstituted acrylamides for which the nitrogen and oxygen atom can be involved in hydrogen bonds [4]. Several solvent systems were thus tested for the 3-HQD promoted reaction of 4-nitrobenzaldehyde with N-butylacrylamide (Table 2). Purely organic solvent conditions, either solvent free or pure 2-MeTHF, gave low yields of MBH adducts, 15% and 24% respectively, though without any formation of Cannizzaro products. With a 40% yield in MBH adducts and concomitant extensive Cannizzaro reaction (44%), pure water is found to accelerate both pathways. Looking for the most appropriate balance between organic and aqueous conditions, mixtures of water with various solvents were used. Aqueous 1,4-dioxane, popular for MBH reactions, was included in the list, however, dioxane being a category 3 CMR (carcinogenic, mutagenic and reprotoxic) substance and regarded as HAP (hazardous airborne pollutant) in US [25], the more efficient and eco-friendly alternative solvent 2-MeTHF [25, 26], available from furfural was also investigated. Interestingly, a better MBH yield was found for the reaction in 1:1 water-2-MeTHF as compared to 1:1 water–dioxane (62% vs 52%). Among all tested systems, 2-MeTHF: H2O (v∕v = 1∕1) appears to be the optimal ratio in terms of yield and selectivity toward the MBH vs the Cannizzaro reactions. Comparing the influence of the ratio of water-2-MeTHF mixtures, it is confirmed that presence of water is indispensable for reasonable aldehyde conversion, even though formation of Cannizzaro products cannot be avoided, with an optimum for the 1:1 (v∕v) ratio. Three days of reaction time was required to get the best results, however extending it further led to only modest improvements. Overall, the known beneficial effect of the presence of water as a solvent component in MBH reactions is confirmed in the present study for the specific case of secondary N-alkyl acrylamides.
The influence of stoichiometry and concentration was also investigated (Table 3). Using less than 1 equivalent of 3-HQD led to significant decrease of the yield, reducing the MBH pathway while favoring the Cannizzaro one, although adding more than one equivalent was of no benefit. Regarding the quantity of N-butylacrylamide, two equivalents were required for pushing the MBH pathway vs the Cannizzaro one and observing acceptable reaction rate, though adding more than two was not useful either. Regarding the concentration of the whole reaction mixture, varying from 0.5 M to 4 M led to significant improvement up to 2 M (38% at 0.5 M, 60% at 1 M, 62% at 2 M, 58% at 4 M).
Having chosen a favorable set of conditions based on the above experiments, the scope of the reaction could be addressed. First, a series of secondary N-alkyl acrylamides were used, with variation in the alkyl chain length and the branching on the first carbon atom of the chain, while keeping 4-nitrobenzaldehyde as the aldehyde (Table 4).
All examples with no branching on the first carbon atom gave rather similar results, with moderate to fair yields in MBH products and quite the same distribution among possible products, either MBH or Cannizzaro. A closer look shows that longer chain gave lower yields in MBH, and lower selectivity for MBH vs Cannizzaro. This can be ascribed to a bulkiness issue or to a solubility issue, but performing the reaction in solvent mixtures with richer organic content did not change significantly the yield. Benzylic amides such as N-benzyl- or N-(4-methoxybenzyl)acrylamides gave good results. Regarding the branched systems, a consistent decrease of the yield in MBH adducts was observed upon bulkiness and branching of the alkyl chain of the starting N-alkylamide. Comparing the butylacrylamides, either n-butyl, sec-butyl or tert-butyl, led to obvious decrease of the MBH product, in 60%, 35% and 16% yield respectively, with concomitant increase of the Cannizzaro products. Consistent with Hu et al.’s remark on the role of the delocalization of the lone pair on the nitrogen atom of the amide group as the main reason for the lower reactivity of alkyl acrylamides as substrates in MBH reactions, [4] we found in the present work that increasing the inductive effect from n-butyl, to sec-butyl and tert-butyl intensifies this loss of reactivity. Increasing the electron-donating ability of the nitrogen atom might increase the enolate form in the amide–enol tautomeric equilibrium, possibly limiting the alkene Michael reactivity required for the MBH pathway, and possibly increasing the basicity of the medium favoring the Cannizzaro reaction. As a matter of comparison, the same conditions were applied to N-phenylacrylamide, which gave a 70% yield with high selectivity toward the MBH pathway, and unsubstituted acrylamide gave a much faster reaction without formation of Cannizzaro products. Overall, the comparison in the series highlights a significant difference among all secondary N-alkyl acrylamides with those for which MBH reaction is preferred, being less bulky and less branched, and those for which the MBH pathway is disfavored as compared to the Cannizzaro reaction, consistent with branching at the first carbon atom of the alkyl chain.
The scope was then developed with respect to various aromatic aldehydes, while keeping N-butylacrylamide as the activated alkene partner (Table 5).
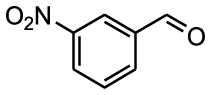
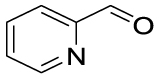
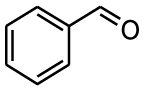
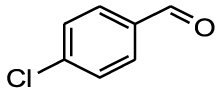
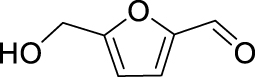
Nitro benzaldehydes (2-, 3- and 4-nitrobenzal- dehyde) are readily transformed to their MBH adducts with 55–60% yields. Logically, the meta-substituted one is less reactive and lower conversion is found over the same reaction time. The renowned highly electrophilic 2-pyridinecarboxaldehyde gave logically the best yield in the study (79%), whereas unactivated benzaldehydes (benzaldehyde, 4-chlorobenzaldehyde or 2-methoxy-benzaldehyde) are virtually unreactive in the reaction. Cannizzaro products are found only for the four highly activated aldehydes. As for furanic aldehydes, 5-(hydroxymethyl)-2-furaldehyde (HMF) gave a 56% yield of MBH adducts without any generation of Cannizzaro products, albeit with a slightly lower conversion rate.
The MBH promoter thus appears to be too weakly basic for enabling the Cannizzaro transformation of HMF, as compared to the ready conversion of HMF to bishydroxymethylfuran and the furancarboxyaldehyde arising under NaOH catalysis or in ionic liquids [27, 28]. Meantime, the results on the four activated aldehydes (entries 1a–d) are in agreement with Bassaviah’s results on tetramethylguanidine-mediated Cannizzaro reaction of pyridine-4-carboxaldehyde, also a very reactive aldehyde, observed while expecting MBH coupling with a vinyl sulfone [13].
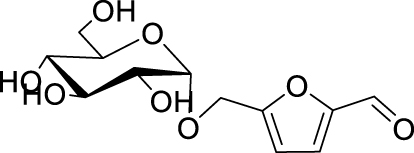
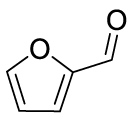
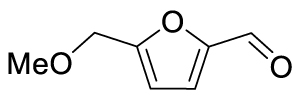
The yields of the MBH adduct from furfural and 5-(α-D-glucosyloxymethyl)furfural (GMF) were both slightly lower than HMF, with 46% and 43% respectively. In order to assay the influence of the hydroxyl group on the reactivity of 5-HMF in this reaction, 5-methoxymethylfurfural was used, and this led to a 42% isolated yield, similar to that of GMF or furfural. The presence of OH is therefore an activating factor making HMF nearly as reactive as 4-nitrobenzaldehyde, anyway much more reactive than benzaldehyde, with respect to the MBH reaction. This observation can be useful for extending the uses of biobased aldehydes as platform molecules in the design of fine chemicals [29, 30, 31, 32].
4. Conclusion
Secondary N-alkyl acrylamides can be used in Morita–Baylis–Hillman reactions despite their notorious lower electron withdrawing ability which caused these to be discarded from earlier studies. Competition between the desired MBH and the undesired Cannizzaro pathways must be addressed, in particular for highly activated aldehydes. A balance between the beneficial effect of the use of aqueous media on both reactions must be found. The promoter/solvent couple 3-HQD/2-MeTHF:H2O (v∕v = 1∕1) was found efficient and provided more eco-friendly conditions than dioxane-containing systems. Overall, the reaction can be applied to a wide range of secondary N-alkyl acrylamides and aromatic aldehydes giving novel MBH adducts in moderate to good yields.
The branching at the first carbon atom of the alkyl chain appears essential, as any further inductive enrichment of the nitrogen amide is deleterious to the desired MBH reactivity. With respect to aldehydes, classical higher reactivity is found for nitrobenzaldehyde and 2-pyridinecarboxaldehyde while unactivated benzaldehydes are virtually unreactive. Interestingly, biobased aldehydes such as furfural and HMF are possible substrates for this reaction, with HMF appearing nearly as activated as 4-nitrobenzaldehyde, but not to the level to undergo Cannizzaro reactions in these conditions. This extends further the possible uses of these platform molecules in the design of fine chemicals.
Conflicts of interest
There is no conflict of interest to declare.
Acknowledgments
The authors are grateful to the Centre National de la Recherche Scientifique (CNRS) and acknowledge the China Scholarship Council for scholarships to XY and the ANR for a post-doctoral fellowship to CV (Bioplatform project, ANR-17-CE07-0053-02).
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.117 or from the author.




 CC-BY 4.0
CC-BY 4.0