1. Introduction
Medullary sponge kidney (MSK) is a common anomaly in recurrent stone formers who present more severe stone disease than other conditions [1, 2, 3]. MSK is a congenital disease with a familial transmission in some cases where GNDF/RET genes mutations were suspected to play a major role to explain abnormal development of the kidneys in affected patients [4, 5, 6]. The occurrence of MSK among stone formers is controversial in the literature, ranging from 2% up to 25% [7, 8, 9, 10, 11, 12, 13, 14]. Indeed, tubular ectasias may be located only in a caliceal group of one kidney or may be extended to all calices of both kidneys, making very diverse the clinical expression of this malformative uropathy. In addition, metabolic disorders that favour urine supersaturation and crystal formation may be diverse, including hypercalciuria, hyperoxaluria, hypocitraturia, hypomagnesuria. As a result, clinical signs of the disease range from the absence of any kind of stones to multiple and recurrent stones with nephrocalcinosis affecting one or both kidneys. It is largely accepted that stones are made of calcium salts, including calcium oxalate and calcium phosphate, but also in some cases uric acid [2, 10, 15]. However, very few studies have investigated more precisely the stone composition and morphology in MSK patients [13, 14, 16, 17]. Examining our stone database, we found that about one thousand calculi analysed in our lab were from patients suffering from MSK. The aim of this work is to provide accurate information regarding composition and morphology of the stones associated with MSK and to compare these data with the ones recorded in common stone disease.
2. Materials and methods
From 1990 to 2020, we received about 77,500 stones for morpho-constitutional analysis based on optical examination of the surface and the section of the stones in order to classify them according to a morphological type completed by a sequential infrared analysis from the core to the surface providing a quantitative crystalline composition as previously described [18, 19, 20]. Among them, 32,532 stones were accompanied with a query sheet providing clinical information on comorbidities and the presence or not of urological or kidney anomalies: 31,494 stones came from patients without any malformative anomaly (control group) while 1036 stones came from MSK patients. In case the query sheet mentioned MSK and other clinical disease such as primary hyperparathyroidism, renal tubular acidosis or other cause of nephrocalcinosis, the stone was excluded from the study. MSK diagnosis was made by trusted urologists or nephrologists aware of the limitations of imaging for such a diagnosis. Most urologists who mentioned MSK have performed endoscopic examination of the kidney(s). For nephrologists, the diagnosis was based on the results of a CT scan with injection of contrast product and furosemide and, for the oldest cases, on the results of an intravenous urography.
Scanning electron microscopy was performed on a selected sample of MSK stones to better describe crystal morphologies and stone organization. For this purpose, surface and section of selected stones were examined using a FEI/Philips XK-40 environmental scanning electron microscope. Such apparatus does not need conductive coating and permits direct observation of the sample.
Statistical comparisons were based on the Chi-square test and Fisher’s exact test when appropriate. Statistical significance was considered for p < 0.05.
Demographic and clinical data
| MSK | Non-MSK | |||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| Number | 618 | 418 | 1036 | 21,315 | 10,179 | 31,494 |
| Mean age | 45.5 ± 12.6 | 45.9 ± 13.7 | 45.5 ± 13.3 | 48.4 ± 14.4 | 48.1 ± 16.4 | 48.3 ± 15.3 |
| BMI (kg/m2) | 25.0 ± 3.7 | 23.8 ± 6.4 | 24.6 ± 5.0 | 25.8 ± 4.4 | 25.8 ± 6.3 | 25.8 ± 5.2 |
| Stone recurrence | 86.7a | 82.2a | 85.0a | 41.31 | 35.7 | 39.5 |
ap < 0.000001 vs non-MSK.
1p < 0.000001 vs females in the same group.
Mode of stone removal
| MSK | Non-MSK | |||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| Spontaneously passed | 328 (53.1)d,5 | 145 (34.7)a | 473 (45.7)d | 8066 (37.8)5 | 2841 (27.9) | 10,907 (34.6) |
| Endoscopy | 167 (27.0)c,4 | 178 (42.6) | 345 (33.3) | 7425 (34.8)5 | 3986 (39.2) | 11,411 (36.2) |
| ESWL | 72 (11.6) | 35 (8.4)a | 107 (10.3)a | 2987 (14.0) | 1321 (13.0) | 4308 (13.7) |
| NLPC | 27 (4.4)a | 31 (7.4)a | 58 (5.6)b | 1611 (7.6)5 | 1228 (12.1) | 2839 (9.0) |
| Surgery | 16 (2.6)a,1 | 24 (5.7) | 40 (3.9)a | 1102 (5.2) | 746 (7.3) | 1848 (5.9) |
| Coelioscopy | 0 | 0 | 0 | 13 ( <0.1) | 11 (0.1) | 24 ( <0.1) |
| Unknown | 8 (1.3) | 5 (1.2) | 13 (1.25) | 111 (0.5) | 46 (0.45) | 157 (0.5) |
| Total | 618 | 418 | 1036 | 21,315 | 10,179 | 31,494 |
ap < 0.01; b p < 0.001; c p < 0.0001; d p < 0.000001 vs non-MSK.
1p < 0.01; 2 p < 0.001; 3 p < 0.0001; 4 p < 0.00001; 5 p < 0.000001 vs females in the same group.
3. Results
3.1. Patients
Demographic and clinical data are summarized in Table 1. Among 31,494 patients without MSK, 21,315 were males and 10,179 were females (sex ratio M/F = 2.09). Among 1036 patients with MSK, 618 were males and 418 were females (sex ratio = 1.48; p < 0.00001 vs non-MSK patients). Recurrence of clinically symptomatic stone episodes accounted for 85% of cases in MSK vs 39.6% in non-MSK patients (p < 0.000001). Because many MSK patients had multiple stones in one or both kidneys, it is difficult to conclude that a new stone episode is actually a stone recurrence. By contrast, in non-MSK patients, it is easier to assert stone recurrence when imaging reveals the patient is stone-free after a previous stone. Males were more prone than females to recurring stones in non-MSK patients (41.4% vs 35.9%, p < 0.000001). No significant difference was found in MSK patients (M: 86.7%, F: 82.2%, NS).
The mean age of MSK patients was moderately lower than in non-MSK patients (45.5 ± 13.3 vs 48.3 ± 15.3 years, p < 0.000001). BMI was similar in both groups at the upper limit of the normal range (24.6 ± 5.0 in MSK vs 25.8 ± 5.2 kg/m2 in non-MSK subjects, NS).
3.2. Urological procedures
Whilst only 34.6% of stones are spontaneously passed in non-MSK patients, we found that 45.7% of the stones were expelled without any urological procedure in MSK subjects (p < 0.000001), the proportion increasing up to 53.1% in male patients. A urological treatment was required for removal of 54.3% of the stones. As shown in Table 2, percutaneous nephrolithotomy was performed in 5.6% of cases and ESWL was used in 10.3% of cases. The most common treatment for stone removal was endoscopy, including JJ stents, rigid or flexible ureteroscope, and basket to trap stones. Endoscopic procedures were applied in 33.3% of cases. Open surgery accounted for only 3.9% of urological treatment and for less than 1% of cases in recent years.
3.3. Stones
3.3.1. Main component
Calcium oxalate was the main component in 76.2% of stones in MSK males and 51.7% in MSK females (Table 3). Such difference between genders was also found in non-MSK patients. Of note, among crystalline phases of calcium oxalate, weddellite was significantly less frequent in MSK patients (p < 0.0001).
Main components in MSK and non-MSK related stones
| Main component | MSK | Non-MSK | ||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| N | 618 | 418 | 1036 | 21,315 | 10,179 | 31,494 |
| Calcium oxalate | 471 (76.2)3 | 216 (51.7)v | 687 (66.3) | 16,759 (78.6)z | 6079 (59.8) | 22,838 (72.5)c |
| Whewellite | 341 (55.2)2 | 167 (40.0) | 508 (48.9) | 11,402 (53.5)z | 4627 (45.5) | 16,029 (50.9) |
| Weddellite | 130 (21.0)1 | 49 (11.7) | 179 (17.0) | 5357 (25.1)z | 1452 (14.3) | 6809 (21.6)b |
| Calcium phosphate | 118 (19.1)*,3 | 181 (43.3)y | 299 (28.9) | 1737 (8.1)z | 2612 (25.7) | 4349 (13.8)e |
| Carbapatite (CA) | 87 (14.1)*,3 | 166 (39.7)y | 253 (24.4) | 1307 (6.1)z | 2369 (23.3) | 3676 (11.7)e |
| CA without MAP | 77 (12.5)**,1 | 144 (34.4)z | 221 (21.3) | 998 (4.7)z | 1553 (15.3) | 2551 (8.1)e |
| Brushite | 27 (4.4)* | 13 (3.1)v | 40 (3.9) | 380 (1.8) | 150 (1.5) | 530 (1.7)d |
| Presence of brushite | 47 (7.6)*** | 24 (5.7)v | 71 (6.9) | 650 (3.0) | 300 (3.0) | 950 (3.0)e |
| Other calcium phosphates | 4 (0.6) | 2 (0.5) | 6 (0.6) | 50 (0.2)z | 93 (0.9) | 143 (0.5) |
| Struvite (MAP) | 0 | 1 (0.2)v | 1 (0.1) | 143 (0.7)z | 251 (2.5) | 394 (1.3)b |
| Presence of MAP | 9 (1.5)2 | 30 (7.2)v | 39 (3.8) | 563 (2.6)z | 1189 (11.7) | 1752 (5.6) |
| Uric acid and urates | 22 (3.6)** | 14 (3.3)v | 36 (3.5) | 2102 (9.9)z | 770 (7.6) | 2872 (9.1)e |
| Uric acid anhydrous | 21 (3.4)* | 12 (2.9)v | 33 (3.2) | 1767 (8.3)z | 650 (6.4) | 2417 (7.7)e |
| Uric acid dihydrate | 1 (0.2)* | 0 | 1 (0.1) | 299 (1.4)y | 73 (0.7) | 372 (1.2) |
| Ammonium urate | 0 | 1 (0.2) | 1 (0.1) | 29 (0.1)x | 42 (0.4) | 71 (0.2) |
| Cystine | 3 (0.5) | 1 (0.2)v | 4 (0.4) | 252 (1.2)z | 212 (2.1) | 464 (1.5)a |
| Proteins | 4 (0.6) | 4 (1.0) | 8 (0.8) | 160 (0.8)v | 109 (1.1) | 269 (0.9) |
| Others | 0 | 1 (0.2) | 1 (0.1) | 169 (0.8)v | 151 (1.5) | 320 (0.3) |
| Total | 618 (100) | 418 (100) | 1036 (100) | 21,315 (100) | 10,179 (100) | 31,494 (100) |
ap < 0.01; b p < 0.001; c p < 0.0001; d p < 0.00001; e p < 0.000001 vs MSK.
1p < 0.001; 2 p < 0.00001; 3 p < 0.000001 vs MSK females.
∗p < 0.0001; ∗∗ p < 0.00001; ∗∗∗ p < 0.000001 vs non-MSK males.
vp < 0.01; w p < 0.001; x p < 10−4; y p < 10−5; z p < 10−6 vs non-MSK females.
The striking difference between MSK and non-MSK stones was the very high proportion of stones mainly composed of calcium phosphates in the case of MSK (28.9% vs 13.8%, p < 0.000001) in both genders. As shown in Table 3, carbapatite and brushite were two times more frequent in MSK patients. Despite stasis induced by tubular ectasias, infection stones seemed infrequent since the presence of struvite was not increased in MSK vs non-MSK patients. In agreement with such observation, the mean content of carbapatite in MSK stones was significantly increased by comparison with non-MSK stones in both genders (17.4% of stone weight in average in MSK vs 9.4% in non-MSK), the difference being more marked in female (26.6% vs 13.7%, p < 0.000001) than in male patients (12.2% vs 7.1%, p < 0.0001).
3.3.2. Stone morphology
Regarding stone morphology, which is an important marker in addition to stone composition for the relation to etiology [21, 22], the first message deduced from morpho-constitutional stone analysis was that pure stone types (Table 4) were significantly less frequent in MSK patients (33.9% vs 44.6% in non-MSK patients, p < 0.000001). As observed in common stone disease, the main type was Ia (13% vs 18.6%, p < 0.00001 MSK vs non-MSK), followed by IVa2 stones (5.2% vs 0.4%, p < 0.000001 MSK vs non-MSK) and by IIa or IIb stones (5.0% vs 9.0%, p < 0.00001 MSK vs non-MSK).
Pure morphological types
| Morphological type | MSK | Non-MSK | ||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| Ia | 94 (15.2)a | 41 (9.8)a | 135 (13.0)c | 4295 (20.2)4 | 1565 (15.4) | 5860 (18.6) |
| Ib | 0 | 1 (0.2) | 1 (0.1) | 162 (0.8) | 72 (0.7) | 234 (0.7) |
| Ic | 0 | 0 | 0 | 23 (0.1) | 10 (0.1) | 33 (0.1) |
| Id | 1 (0.2) | 3 (0.7) | 4 (0.4) | 125 (0.6) | 53 (0.5) | 178 (0.6) |
| Ie | 0 | 0 | 0 | 79 (0.4) | 44 (0.4) | 123 (0.4) |
| IIa or IIb | 43 (7.0)a,1 | 9 (2.2)a | 52 (5.0)c | 2272 (10.7)4 | 573 (5.6) | 2845 (9.0) |
| IIIa | 1 (0.2) | 0 | 1 (0.1)a | 244 (1.1)2 | 65 (0.6) | 309 (1.0) |
| IIIb | 15 (2.4)b | 5 (1.2)a | 20 (1.9)c | 1232 (5.8) | 453 (4.4) | 1685 (5.4) |
| IIIc | 0 | 0 | 0 | 50 (0;2) | 27 (0.3) | 77 (0.2) |
| IIId | 0 | 0 | 0 | 2 (0.01) | 14 (0.1) | 16 (0.05) |
| IVa1 | 11 (1.8) | 16 (3.8) | 27 (2.6) | 177 (0.8)4 | 440 (3.3) | 617 (2.0) |
| IVa2 | 8 (1.3)c,4 | 46 (11.0)d | 54 (5.2)d | 49 (0.2)4 | 74 (0.7) | 123 (0.4) |
| IVb | 5 (0.8)3 | 24 (5.7) | 29 (2.8) | 260 (1.2)4 | 773 (7.6) | 1033 (3.3) |
| IVc | 0 | 0 | 0 | 26 (0.1) | 38 (0.4) | 64 (0.2) |
| IVd | 10 (1.6) | 7 (1.7) | 17 (1.6)a | 180 (0.8) | 76 (0.7) | 256 (0.8) |
| Va or Vb | 2 (0.3) | 0 | 2 (0.2)a | 218 (1.0) | 175 (1.7) | 393 (1.2) |
| VIa, b or c | 3 (0.5) | 0 | 3 (0.3) | 134 (0.6) | 52 (0.5) | 186 (0.6) |
| Others | 1 (0.2) | 5 (1.2) | 6 (0.6) | 95 (0.4) | 32 (0.3) | 127 (0.4) |
| Total | 194 (31.4)d | 157 (37.6)a | 351 (33.9)d | 9623 (45.1) | 4536 (44.6) | 14,159 (45.0) |
ap < 0.01; b p < 0.001; c p < 0.00001; d p < 0.000001 vs non-MSK.
1p < 0.001; 2 p < 0.0001; 3 p < 0.00001; 4 p < 0.000001 vs females in the same group.
Mixed morphological types
| Mixed types | MSK | Non-MSK | ||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| I + II∗ | 207 (33.5)b,4 | 72 (17.2) | 279 (26.9) | 5562 (26.1)4 | 1833 (18.0) | 7395 (23.5) |
| II + IV∗ | 65 (10.5) | 50 (12) | 115 (11.1) | 2419 (11.3) | 1280 (12.6) | 3689 (11.7) |
| I + II + IV∗ | 79 (12.8)c | 72 (17.2)c | 151 (14.6)c | 1205 (5.6)3 | 729 (7.2) | 1934 (6.1) |
| I + III∗ | 11 (1.8) | 4 (1.0) | 15 (1.4) | 479 (2.2) | 154 (1.5) | 633 (2.0) |
| I∗+ IVa1 | 6 (1.0)2 | 19 (4.5) | 25 (2.4) | 197 (0.9)4 | 350 (3.4) | 547 (1.7) |
| IVa1 + IVd | 13 (2.1)c | 5 (1.2) | 18 (1.7)b | 84 (0.4) | 50 (0.6) | 134 (0.4) |
| IVd + II∗ | 7 (1.1) | 0 | 7 (0.7) | 134 (0.6) | 44 (0.4) | 178 (0.6) |
| II∗+ IVa1 + IVd | 4 (0.6) | 2 (0.5) | 6 (0.6) | 98 (0.5) | 39 (0.4) | 137 (0.4) |
| I + II + III∗ | 3 (0.5) | 2 (0.5) | 5 (0.5) | 174 (0.8) | 73 (0.7) | 247 (0.8) |
| Ia + II∗+ IVd | 3 (0.5) | 2 (0.5) | 5 (0.5) | 21 (0.1) | 4 ( <0.1) | 25 ( <0.1) |
| Ia + IVa2 + IVd | 3 (0.5) | 0 | 3 (0.3) | 0 | 0 | 0 |
| IVa + VIb | 5 (0.8) | 2 (0.5) | 7 (0.7) | 106 (0.5)4 | 205 (2.0) | 311 (1.0) |
| Others | 21 (3.4)a,1 | 31 (7.4) | 52 (5.0) | 1213 (5.7)4 | 882 (8.7) | 2095 (6.7) |
| Total | 424 (68.6)c | 261 (62.4)a | 685 (66.1)c | 11,692 (54.9) | 5643 (55.4) | 17,335 (55.0) |
∗Subtypes a or b for each type.
ap < 0.01; b p < 0.0001; c p < 0.000001 vs non-MSK.
1p < 0.01; 2 p < 0.001; 3 p < 0.00001; 4 p < 0.000001 vs females in the same group.
Main component of the stone nucleus
| Component | MSK | Non-MSK | ||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| Ca oxalates | 144 (29.1) | 74 (23.0) | 218 (26.7) | 6981 (42.3) | 2330 (30.7) | 9311 (38.7) |
| COM | 110 (22.2)d | 63 (19.6) | 173 (21.2)e | 5124 (31.0)2 | 1801 (23.7) | 6925 (28.8) |
| COD | 34 (6.9)b | 11 (3.4)a | 45 (5.5)d | 1857 (11.3)2 | 529 (7.0) | 2386 (9.9) |
| Ca phosphates | 326 (65.9)f | 235 (73.2)f | 561 (68.8)f | 7606 (46.1)2 | 4165 (55.0) | 11,772 (48.9) |
| Carbapatite | 304 (61.4)f | 223 (68.5)f | 527 (64.6)f | 7231 (43.8)2 | 3946 (52.1) | 11,177 (46.4) |
| Brushite | 10 (2.0)c | 2 (0.6) | 12 (1.5)b | 113 (0.7) | 35 (0.5) | 148 (0.6) |
| Other CaP | 12 (2.4) | 10 (3.1) | 22 (2.7) | 262 (1.6) | 184 (2.4) | 447 (1.9) |
| Struvite | 1 (0.2) | 0 | 1 (0.1) | 78 (0.5)2 | 187 (2.5) | 265 (1.1) |
| Uric acids | 15 (3.0)d | 9 (2.8)b | 24 (2.9)e | 1308 (7.9) | 516 (6.8) | 1824 (7.6) |
| Urates | 1 (0.2) | 2 (0.6) | 3 (0.4) | 51 (0.3) | 57 (0.7) | 108 (0.4) |
| Cystine | 3 (0.6) | 1 (0.3) | 4 (0.5) | 173 (1.0) | 129 (1.7) | 302 (1.3) |
| Proteins | 4 (0.8) | 2 (0.6) | 6 (0.7) | 189 (1.1) | 104 (1.4) | 293 (1.2) |
| Others | 1 (0.2) | 1 (0.3) | 2 (0.2) | 114 (0.7) | 90 (1.2) | 204 (0.8) |
| RP | 153 (30.9)1 | 54 (16.8) | 207 (25.4) | 4459 (27.0)2 | 1447 (19.1) | 5906 (24.5) |
| Total | 495 | 321 | 816 | 16,501 | 7578 | 24,079 |
Other CaP = whitlockite, octacalcium phosphate pentahydrate, amorphous carbonated calcium phosphate.
RP = Randall’s plaque.
ap < 0.05; b p < 0.01; c p < 0.001; d p < 0.0001; e p < 0.00001; f p < 0.000001 vs non-MSK.
1p < 0.00001; 2 p < 0.000001 vs females in the same group.
Interestingly, IVa2 morphology, which is a marker for distal acidification defect [23] was thirty times more frequent in MSK patients. IVa2 morphology was observed as pure or mixed type on the surface or within the stones with a high frequency in MSK patients: 12.1% vs 0.4% in non-MSK (p < 0.000001). The occurrence of type IVa2 was significantly higher in female than in male patients (20.6% vs 6.0%, p < 0.000001).
Examples of stone morphology in MSK patients are given in Figures 1 and 2 for examination under stereo microscope and in Figure 3 for SEM examination. One of the main features of the stones produced by these patients was the high diversity of stone composition and morphology among calculi produced by the same patient. There was no similar finding in non-MSK patients (Table 5).
Heterogeneity of stone morphology and composition in MSK patients. Each photograph shows multiple stones spontaneously passed by one patient. Figures A, B and F show calculi mainly composed of calcium oxalate monohydrate mixed with calcium oxalate dihydrate and containing only small proportions of carbapatite. Figures C–E illustrate calcium stones where calcium oxalate monohydrate and calcium oxalate dihydrate are associated with high proportions of carbapatite which is the main component of calculi in Figure E. Bars = 2 mm.
In more than 12% of cases (thirty times more frequent than in non-MSK patients), IVa2 type (white arrows on Figures 1C and E) is observed among the stones, suggesting distal acidification defect in the patients.
Examples of inner structure of stones formed in MSK patients. (A) Stone section showing that two initial stones have secondly merged to form only one stone. The partition line between the two stones is indicated by the red arrow. Of note, one of the stones was initially in straight contact with another stone as suggested by the presence of a joining face (white arrow). Bar = 1 mm. (B) Stone section made of numerous concentric cream to pale yellow-brown layers mainly composed of carbapatite. Bar = 1 mm. (C) Stone sections mainly made of calcium oxalate monohydrate (brown crystalline layers with radial organization) surrounding a whitish core made of carbapatite. Bar = 1 mm. Of note, in Figures D and E, which is a magnification of Figure C, the stone core is made of multiple type IVa2 spheres of carbapatite first formed within collecting ducts of the kidney. Bars 1D = 1 mm; 1E = 0.5 mm. (F) Another example of MSK stones mainly composed of carbapatite whose inner structure corresponds to IVa2 morphology which is highly suggestive of distal acidification defects. Bar = 1 mm.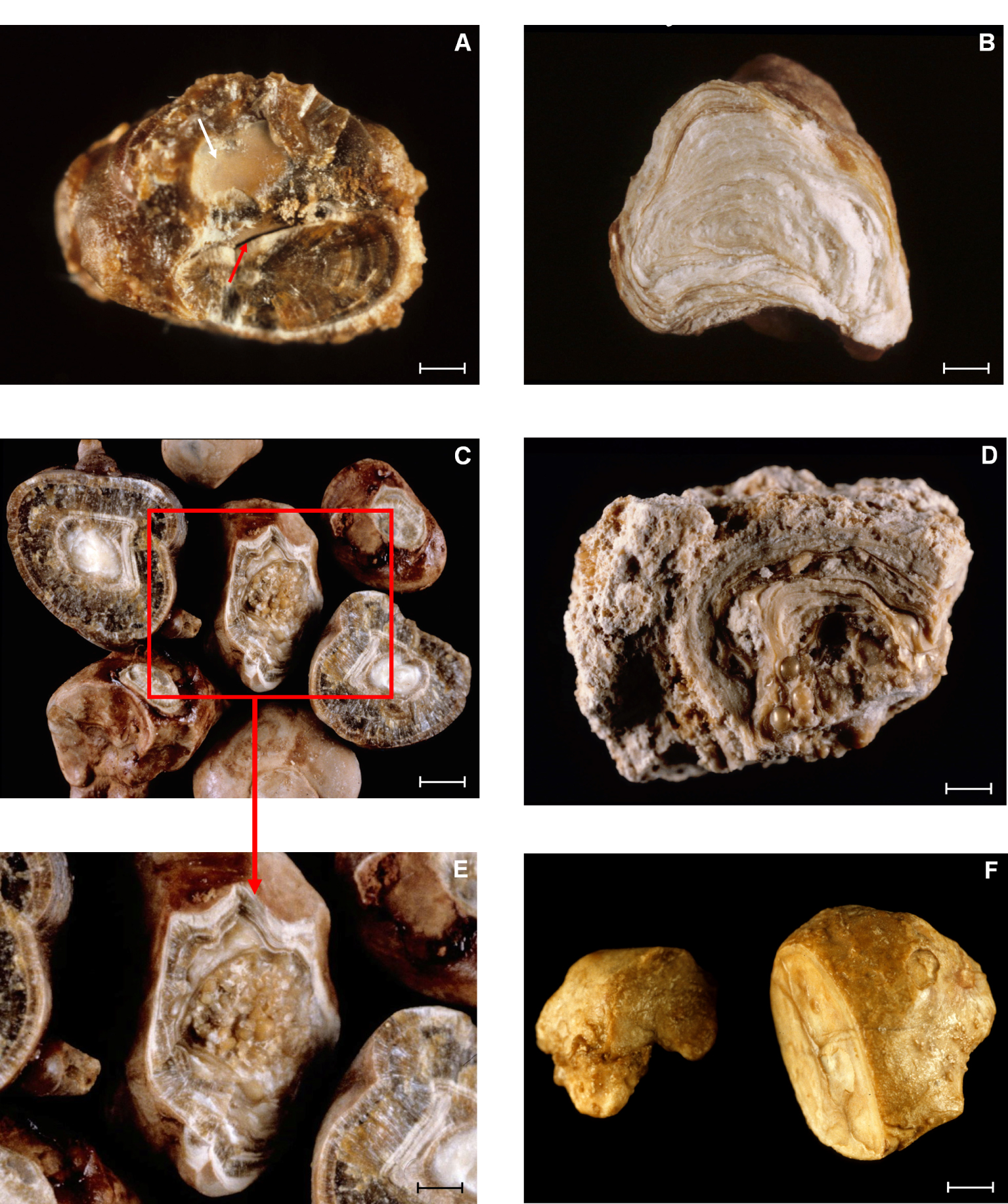
Scanning electron microscopy photographs of MSK stones. (A) Type IVa2 surface, embossed with cracks. Bar = 20 μm. (B) Inner loose layer with carbapatite spherules. Bar = 5 μm. (C) Section of type Ia stone with concentric layers and radial crystallization. Bar = 50 μm. (D) Whewellite crystals poorly organized in deep layers of a stone. Bar = 50 μm. (E) Periphery of a MSK stone with weddellite crystals locally covered by small aggregates of whewellite crystals (black arrows). Bar = 100 μm. (F) Typical crystals of brushite at the surface of a type IVd stone. Bar = 20 μm.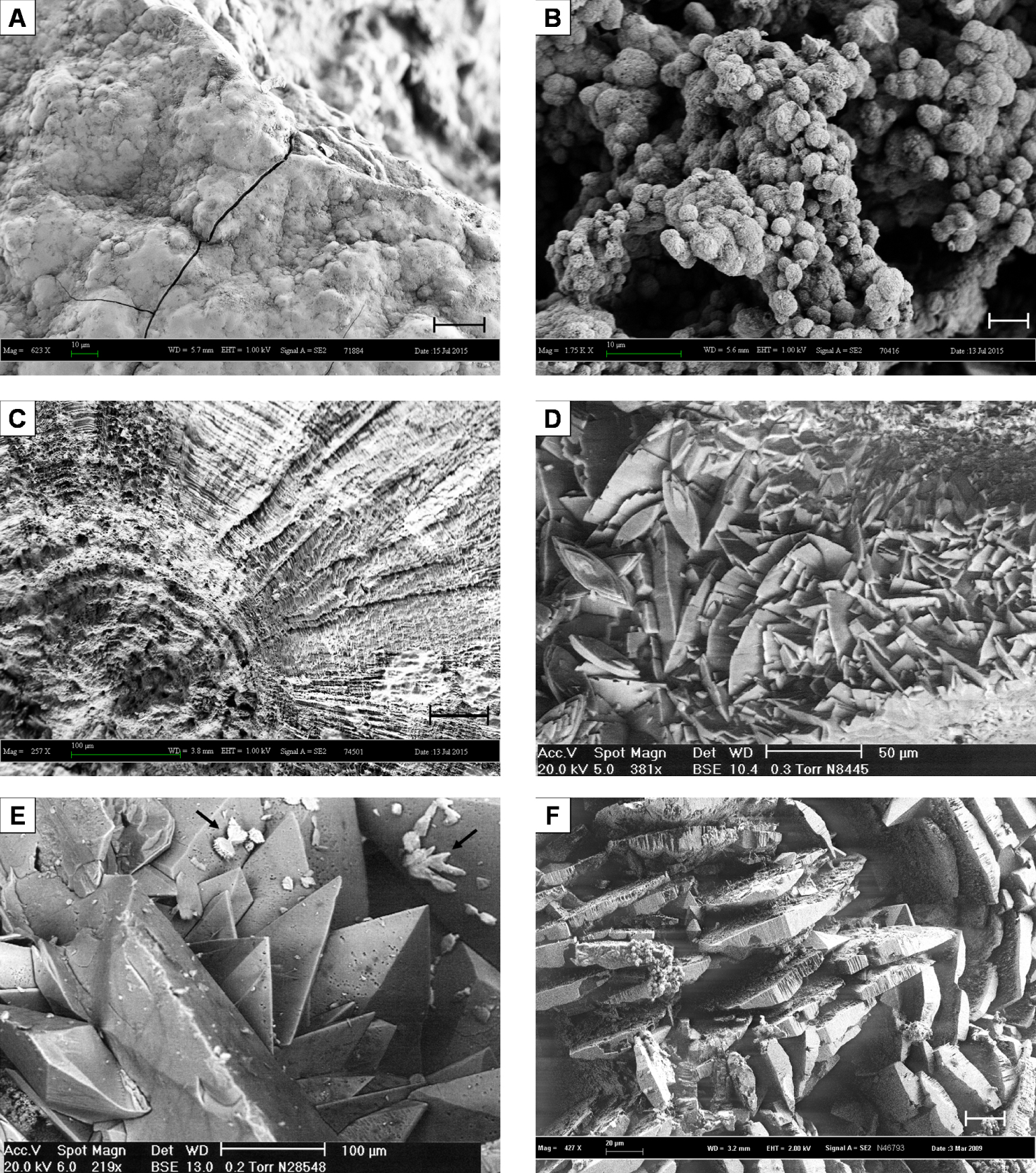
As shown in Table 5, mixed types were more frequent in MSK patients. The main associations were type Ia or Ib mixed with type IIa or IIb (26.9%), ternary associations of type Ia or Ib mixed with type IIa or IIb mixed with type IVa or IVb (14.6%) and associations of type IIa or IIb mixed with type IVa or IVb (11.1%). These associations accounted for more than 52% of all stones in MSK patients.
3.3.3. Stone nucleus
As a result of the high occurrence of stone fragmentation by extracorporeal lithotripsy or in situ fragmentation by laser during ureteroscopic procedures, stone nucleus was available in only 816 stones from MSK patients and 24,079 stones from non-MSK patients. Main components found in stone nucleus are gathered together in Table 6.
In MSK patients, 68.8% of all stones (vs 48.9% in non-MSK patients) were initiated from a calcium phosphate nucleus (p < 0.000001). However, the occurrence of RP was similar in both groups (Table 6). Calcium oxalate nuclei accounted for 21.2% of stones from MSK patients and weddellite was involved only in 5.5% of cases. The main components of RP in MSK are given in Table 7.
Components of Randall’s plaques in MSK patients
| Components found in RP | MSK |
|---|---|
| Carbapatite | 205 (99.0) |
| ACCP | 18 (8.7) |
| Monosodium hydrogen urate | 6 (2.9) |
| Brushite | 2 (1.0) |
4. Discussion
To the best of our knowledge, this series is the largest database regarding stone characteristics from MSK patients. The first information deduced from this cohort is the relatively low male to female sex ratio (1.48/1) in comparison to the control group (2.09/1, p < 0.000001). Thus, women seem more prone than men to present canalicular ectasias and stones in this context, confirming the previous reports of Yagisawa et al. [10] and Fabris et al. [2]. The second point is the high stone recurrence rate in MSK patients (85% vs 39.5% in non-MSK, p < 0.000001) as underlined by several authors [2, 24, 25]. Of note, stones in MSK patients have a high propensity to expel spontaneously as shown in Table 2 (45.7% vs 34.6% in non-MSK patients, p < 0.000001). Spontaneous stone passage is more frequent in men than in women (53.1% vs 34.7%, p < 0.000001). The high rate of stone activity in MSK patients may be explained by stasis in tubular ectasias, in addition to metabolic risk factors found as frequently as in other stone patients, mainly hypercalciuria and hypocitraturia, and less frequently hyperoxaluria and hyperuricosuria [2, 10, 26].
Stone composition shows that calcium oxalate is the main component of 76.2% of stones in males and 51.7% in females. Among crystalline phases, weddellite, which can be considered as a calcium-dependent species [27, 28, 29, 30] appears to be less frequent in MSK patients while hypercalciuria is often reported as a frequent finding among metabolic factors explaining stone formation [1, 2, 8, 24, 26]. However, in agreement with our data, other studies report a less frequent occurrence of hypercalciuria in MSK patients when compared to other stone formers [10].
Stone composition and morphology are both highly suggestive of high occurrence of an acidification defect in MSK patients. Arguments for this are the high proportion of calcium phosphate containing stones (28.9% vs 13.9% in non-MSK patients, p < 0.000001), including carbapatite and brushite crystalline species and the very high proportion of type IVa2 as morphologic characteristics of surface and/or inner structure of the calculi (12.1% vs 0.3% in non-MSK, p < 0.000001). This finding is especially true for stones from female patients: 20.6% vs 6.0% in males, p < 0.000001). Hypocitraturia, which is considered as a marker for renal acidification defect (in the absence of urinary tract infection) was often described as a major metabolic disorder in MSK patients [2, 10, 26] and several works confirm an acidification defect in MSK patients [31, 32] even if other studies failed to demonstrate any abnormal response to dynamic acidification tests [33]. In our experience, proven severe distal acidification defect responsible for stone formation is associated with a very high proportion of calcium phosphate-rich stones exhibiting the type IVa2 morphology [23, 34]. Thus, the relatively high proportion (12.1%) of MSK patients producing IVa2 stones suggests a high occurrence of distal acidification defects in this context but affecting less than 50% of MSK patients. Of note, the occurrence of IVa2 morphology was three times more frequent in females than in males without any evident explanation. Perhaps MSK women in our series presented with more extended and severe form of MSK, but we have no radiographic data to confirm such hypothesis.
Another interesting point regarding stone morphology is the relatively high occurrence of mixed stones in MSK patients (66.1% vs 55% in non-MSK subjects, p < 0.000001), the difference being more marked in males (68.6% in MSK vs 54.9% in non-MSK patients). About 16.5% of stones exhibit three different morphological types, which is two times more than in non-MSK patients (7.4%).
Regarding stone nucleation, we underlined in previous reports that about 50% of calcium stones were initiated from a calcium phosphate nucleus even when the stone was nearly pure in calcium oxalate [35]. A significant part of these stone nuclei were made of a Randall’s plaque (RP), a papillary calcification mainly composed of carbapatite [36, 37]. In MSK patients, the frequency of RP as the nucleus of calcium stones was similar to that observed in non-MSK patients. However, we observed an increased proportion of stones initiated from a calcium phosphate (68.8% vs 48.9% in non-MSK, p < 0.000001). A high occurrence of calcium phosphate plugs in tubular ectasias may explain this phenomenon.
As evidenced by stone analysis, not all MSK patients suffer renal acidification defect. On the other hand, metabolic evaluation of patients in various series found a variety of metabolic disorders as reported in non-MSK, suggesting that some patients, independently of their tubular ectasias, may present risk factors of stone formation related to other causes such as metabolic syndrome or diabetes mellitus. Thus, it is not surprising to find uric acid stones in MSK patients. Of note, 83.3% of MSK patients producing uric acid stones in our database were overweight or obese. However, we can note that uric acid calculi account for only 3.5% of the series, i.e., three times less than in common stone formers. We found also a small proportion (0.4%) of cystine stones.
A limitation of our study is that MSK diagnosis was made by different ways, either endoscopic examination for urologists or intravenous urography or CT scan in other cases. We cannot rule out false negative cases (undiagnosed MSK) but the number of false positive cases was probably limited by the fact that most of stones analysed were sent by trusted urologists or nephrologists aware of the limitations of MSK diagnosis. Of note, repeated misdiagnosis of MSK would have blunted the differences between MSK and non-MSK-related kidney stones.
5. Conclusion
Kidney stones developed in MSK patients present morphological and compositional characteristics significantly different from that observed in non-MSK stones. Both stasis and metabolic disorders may contribute to stone formation. The key point of our results is the high proportion of calcium phosphates, as main components of the stones and even more as the nucleus of a large number of MSK calculi. These data are in agreement with a more frequent acidification defect than in other clinical contexts of stone disease as suggested by the literature and also in our study by the high frequency of stones exhibiting IVa2 morphology.




 CC-BY 4.0
CC-BY 4.0