1. Introduction
Kidney stone disease (KSD) is a chronic disorder of mineral metabolism with preventable acute renal manifestations and chronic extrarenal complications [1, 2, 3, 4, 5].
The chemical composition and the structural characteristics of kidney stone (KS) vary with age and gender and country [6]. In industrialized countries [7, 8], Arabian Peninsula [9] and Maghreb [10], calcium oxalate (CaOx) accounts for the majority of KS, followed by calcium phosphate (CaP), uric acid (UA) and in a minority by cystine (Cys).
The growing prevalence of stones is likely related to dietary factors (high intake of animal protein, fructose, and salts), low water intake, hot climate, economic status, and urbanization [11, 12, 13, 14, 15, 16, 17]. Moreover, KSD is strongly associated with obesity and type 2 diabetes mellitus [18, 19]. Metabolic syndrome has been identified as an independent risk factor for stone formation [20, 21, 22, 23]. Although rare, inborn disorders need to be appropriately identified and efficiently treated as the risk of stone recurrence and loss of kidney function is high in this background [24]. Stone formation could also be promoted independently by urine flow slowdown secondary to acquired or congenital abnormalities of kidney and urinary tract (CAKUT) or urinary tract infections [25].
Therefore, each patient displays a specific combination of both biological (metabolic) abnormalities and/or clinical factors (urinary tract disease, use of some drugs, etc.) creating an individual micro- and macroenvironment driving firstly crystallization and secondly growth of urinary stones [26, 27, 28, 29, 30, 31, 32]. All the above induce prolithogenic modification in urine composition allowing formation and recurrence of stones [27]. International guidelines and recent consensus recommend a metabolic assessment of kidney stone formers (KSF) to identify the aetiology of stone and a correction of all lithogenic factors, especially in the case of recurrent disease [33, 34].
Given the continuous increase in the prevalence of KSD in adults during the last 30 years, and worrying occurrence in what were previously considered lower risk groups (post-menopausal and pregnant women and the paediatric population) [35, 36, 37, 38, 39, 40], KSD stands as a worldwide public healthcare burden [41, 42]. Therefore, global improvement in aetiological diagnostics and collaborative care among healthcare providers in pediatrics as well as adults is urgently needed [43, 44, 45, 46].
The morpho-constitutional classification (MCC) established by Prof Michel Daudon [47] allows very granular understanding of the whole lithogenesis process within each stone, which can vary during the patient’s life [48, 49]. The fundamental clinical value for clinicians of using MCC is an opportunity to accurately identify all lithogenic factors responsible for stone formation and/or recurrence as well as offering the opportunity to identify easily KSF at a high risk of recurrence [47, 48, 50, 51, 52].
In our laboratory we built such expertise through theoretical learning and practice guided by the experts in this field [53]. In the present work, we report the demographic distribution of stones classified according to MCC in our centre during a five-year period. We discuss available evidence supporting our findings in terms of aetiologies proposed by MCC.
2. Materials and methods
This single-center study carried out by University Hospital Erasme in Brussels, Belgium was evaluated and approved by the local Ethic Committee (No.: P2014/444). All procedures were performed in accordance with the institutional and national ethical standards on human experimentation, and with the Helsinki Declaration of 1975, revised in 2013.
We retrospectively analyzed the results of MCC from all samples submitted to this evaluation in our department of clinical chemistry. The data for analysis were extracted from a local perspective database (GLIMS, CliniSys Group, Gent, Belgium). The KS were provided by hospitals and laboratories located in Brussels as well as from neighbouring provinces (Hainaut, Brabant-Wallon and Namur). Between January 2007 and January 2013, a total of 5480 samples were investigated. Only stones larger than 0.5 mm have been included.
The KS morphology was described under optic stereomicroscope study (MOTIC-ST-39-Series). The stones were assessed for shape, color, organization from surface throughout the section into the nucleus. After detailed morpho-constitutional analysis, each stone was classified into one of seven main morphological types and 22 subtypes. The comprehensive specification of each type and subtype has been extensively described previously and the main characteristics of MCC are summarized for non-familiar readers in Table 1 [48, 50, 54]. The physico-chemical components of stones were identified and semiquantified (in %) by physical methods using FTIR spectroscopy (Bruker-Optics-FTIR-Tensor-27) as described previously [53]. Examples of photomicrographs of various stone types and corresponding FTIR spectra are depicted in Figure 1.
Main characteristics of surfaces and sections of kidney stones, corresponding to physical components (crystalline phase) and type of Daudon’s morpho-constitutional classification. The value of this method consists in the particularly granular view of associated aetiologies pointing directly to specific diagnostic management and indirectly allowing the improvement of the therapeutic strategy
| Surface | Section | MCC type/subtype | FITR component | Aetiology |
|---|---|---|---|---|
| Mammillary with frequent umbilication and Randall’s plaque indicative of papillary origin. Color: brown | Compact concentric layers with a radiating organization. Color: brown | Ia | Whewellite | Intermittent and moderate hyperoxaluria (a high consumption of oxalate-rich foods or of hydroxyproline-rich foods, low intake of calcium) and /or low water intake resulting in low diuresis |
| Mammillary and rough without umbilication. Color: brown to dark brown | Compact unorganized sometimes, the presence of gaps. Color: brown, dark-brown | Ib | Whewellite | Stasis, low diuresis, crystalline, conversion form weddellite to whewellite |
| Budding. Color: light cream to pale yellow-brown (whitish in children) | Finely granular and poorly organized. Color: light cream to pale yellow-brown | Ic | Whewellite | Primary hyperoxaluria (most commonly type 1 related to AGXT mutation) |
| Smooth. Color: homogeneous, beige or pale brown | Compact with thin concentric layers. Color: beige or pale brown | Id | Whewellite | Malformative uropathy, stasis, presence of multiples stones |
| Locally budding, mammillary, or rough. Color: often heterogeneous, pale yellow-brown to brown | Locally unorganized and loose structure, locally more compact radiating | Ie | Whewellite | Enteric hyperoxaluria related to inflammatory bowel diseases, especially after ileal resection for Crohn’s disease, in children with cystic fibrosis with a severe pancreatic deficit, after bariatric surgery such as jejuno-ileal bypass or Roux-en-Y gastrojejunal bypass |
| Spiculated with aggregated right angles and sharp edged bipyramidal crystals. Color: pale yellow-brown | Loose of radial crystallization. Color: pale yellow-brown | IIa | Weddellite | Hypercalciuria no matter what its cause with high urinary calcium/citrate molar ratio |
| Spiculated and showing aggregated bipyramidal crystals with blunt angles and ridges. Color: pale yellow-brown | Compact, unorganized crystallization. Color: pale yellow-brown | IIb | Weddellite | Both idiopathic hypercalciuria and moderate hyperoxaluria associated with stasis and low diuresis |
| Rough. Color: grey-beige to dark yellow-brown | Unorganized core with a diffuse concentric compact structure at the periphery. Color: grey-beige to dark yellow-brown | IIc | Weddellite | Hypercalciuria and an obstructive anatomic abnormality with multiple stones resulting from stasis conditions. |
| Homogeneous smooth. Color: homogeneous, typically orange, sometimes cream, ochre or yellowish | Homogeneous compact, concentric structure with a radiating organization. Color: typically, orange | IIIa | Uric acid anhydrous | low urine pH, stasis, prostate hypertrophy, metabolic syndrome, ammoniagenesis defect |
| Heterogeneous embossed, rough and porous surface. Heterogeneous color from beige to brown-orange | Poorly organized section with frequent porous areas. Color: orange | IIIb | Uric acid dihydrate ± uric acid anhydrous | Insulin resistance, metabolic syndrome, type 2 diabetes, low urinary pH, ammoniagenesis defect |
| Homogeneous or slightly heterogeneous rough and locally porous. Color: homogeneous, cream to greyish | Unorganized porous. Color: whitish to greyish | IIIc | Urate salts, including ammonium hydrogen urate | Hyperuricosuria, no trial or alkaline urine pH, urinary tract infection by urea splitting microorganisms |
| Heterogeneous embossed, rough and porous, heterogeneous. Color: greyish to dark brown | Alternated layers, thick and brownish or thin and greyish, locally porous. Color: Sometimes, locally purplish | IIId | ammonium hydrogen urate | Chronic diarrhoea, electrolytes and alkali loss, high urate concentration in urine, low phosphate intake, laxative abuse |
| Homogeneous rough. Color: whitish to beige | Poorly organized, or diffuse concentric layers. Color: whitish to beige | IVa1 | Carbapatite | Hypercalciuria and/or urinary tract infection |
| Embossed and varnished with small cracks. Glazed appearance. Color: homogeneous, pale brown-yellow to pale brown | Section made of compact alternated layers, thick brown-yellow and thin beige. Often, multiple nuclei (from collecting duct origin) | IVa2 | Carbapatite | Inherited or acquired distal renal tubular acidosis, Sjogren syndrome, chronic hepatitis |
| Heterogeneous, both embossed and rough with confluent superficial deposits. Heterogeneous color: cream to dark brown | Section made of irregularly alternating thick, whitish, and thin, brown-yellow layers | IVb | Carbapatite other calcium phosphates (±struvite) | Latent urinary tract infection, especially if they contain small amounts of ACCP, whitlockite or struvite-markers of urinary tract infection. Sometimes related to the minor defect in tubular acidification, or hypercalciuria (primary hyperparathyroidism), especially when they also contain weddellite |
| Homogeneous made of amalgamate crystals with blunt angles and edges | Crude radial crystallization. Color: whitish | IVc | Struvite | Urinary tract infection by urease producing bacteria |
| Finely rough or dappled. Color: whitish to beige | Radial crystallization with more or less visible concentric layers. Color: whitish to beige | IVd | Brushite | Hypercalciuria, primary hyperthyroidism, phosphate leak, medullary sponge kidney |
| Rough surface. Color: yellowish | Poorly organized, sometimes a radiating organization. Color: yellowish | Va | Cystine | Cystinuria |
| Smooth. Color: homogeneous, cream to yellowish | Concentric layers at the periphery, an unorganized core. Color: heterogeneous, cream (periphery) to yellowish (core) | Vb | Cystine | Cystinuria associated with inadequate diet and or medical management or urinary stasis |
| Homogeneous matrix soft stones. Color: cream to pale brown | Unorganized section. Color: cream to pale brown | VIa | Proteins | Urinary tract infection and chronic pyelonephritis |
| Heterogeneous, irregularly rough, locally scaled. Color: dark brown to black | Crude and diffuse foliated. Color: dark brown to black. Other components often present in these stones may alter the structure and the color | VIb | Proteins ± drugs or metabolic compounds | Proteins with metabolic components or drugs (quinolones, triamterene, atazanavir, …) |
| Homogeneous, smooth surface with clefts and scales. Color: dark brown | Dark brown protein shield surrounding a loose, unorganized light core containing whewellite crystals mixed with proteins | VIc | Proteins with whewellite | Typically seen, in end stage renal disease related to relatively high calcium concentration in the urine due to chronic calcium substitution and high vitamin D intake |
| Various morphologies and colors according to the stone composition (infrequent purines and drugs) | Variable organization and color according to the stone composition. | VII | Miscellaneous | Other aetiologies |
Illustrations of representative photomicrographs obtained during the assessment of kidney stone’s morphology under optic microscope (A, C and E) and their corresponding typical FTIR absorption spectra (B, D and F). (A) Type Ia and IIa mixed stones. Type Ia is characterized by a spherical form with a smooth and dark-brown surface seen in the patients with intermittent hyperoxaluria. The surface of type IIa is typically yellow or light-brown prickly, spiculated surface due to the presence of aggregated pyramidal crystals with very sharp angles and edges, their morphology characteristic of idiopathic hypercalciuria. (B) FTIR absorption spectrum of whewellite and weddellite mixed stone shown in (A). (C) Type IIIa stones. They have a smooth, typically orange surface and their section is characterized by concentric layers with radiating organization around very well-defined nucleus. (D) FTIR absorption spectrum of anhydrous uric acid identified in type IIIA stones shown in (C). (E) Type Va stones. They have a rough crystalline surface of yellowish color (their section is typically poorly organized, sometimes a radiating organization can be found, not shown in the picture). (F) FTIR absorption spectrum of cystine identified within type Va stones shown in (E).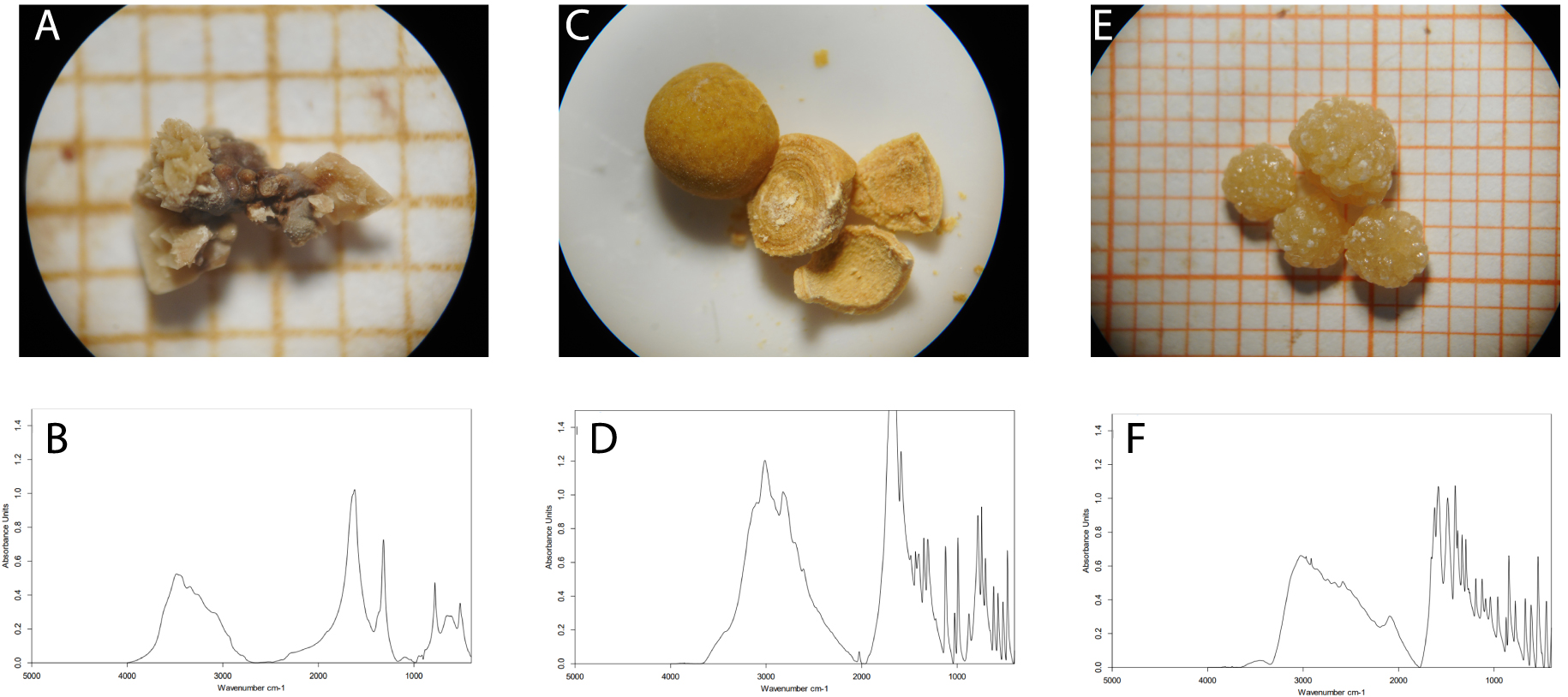
We recorded gender and age of all patients at the presentation, KS numbers in the submitted sample (single or multiple stones or fragments), and characteristics of the samples in terms of stone fragments or whole stones, morphological type according to the surface and section, composition (expressed in %) of each chemical component obtained by FTIR spectroscopy in global powder for all included stones.
We first expressed results as the main component accounting for more than 50% of the stone. Pure stone has been defined as a stone that contained less than 10% of other components. Secondly, we analyzed their distribution according to gender and age of KSF.
Our main aim was to analyze the epidemiology of stones according to the MCC approach and main physico-chemical component to establish their distribution according to age classes and gender and find the suggestive aetiologies.
2.1. Statistical analysis
The statistical analysis was performed with Statistica® (StatSoft Inc., Europe, GmbH, Hamburg, Germany). The distribution of data by gender and age intervals was tested by the Shapiro-Wilk test. The binary variables or categories are presented as a percentage. The comparison of proportions was analyzed using Pearson Chi2 or Fisher’s exact test for small sample size. A P-value lower than 0.05 was considered as statistically significant.
3. Results
Among 5480 samples we excluded 453 samples from analysis for the following reasons: urine samples without stones (71%), presence of filter material (9.7%), absence of stone (7.7%), undefined precipitation (5.3%), spurious stones (3%), tissue fibre (0.2%), tooth (0.2%) biliary stone (0.2%) or unidentified sample (0.8%).
Finally, we included all complete MCC and physico-chemical data of 5027 (96.7%) KS that fulfilled the inclusion criteria. Samples smaller than 0.5 mm were excluded.
3.1. Global distribution of kidney stones in Brussels cohort
The KS were provided from patients with first KS manifestation as well as during the follow-up. Single stones corresponded to 2783 (96.4%) and multiple stones to 437 (3.6%). Intact stones corresponded to 3226 (64.1%) and 1801 (35.9%) of samples corresponded to stone fragments. Stones were statistically more frequent from men (3554) than women (1473) (70.6% vs 29.4%, respectively).
We submitted the data of MCC for a total of 5027 stones provided by 4975 KSF for analysis by age intervals and gender (Table 2, Figure 2).
Global distribution of studied kidney stones (n = 4975) by age and according to the gender of kidney stone formers
| Age (years) | All stone formers (n = 4975) Number (%) | Women (n = 1426) Number (%) | Men (n = 3549) Number (%) | Men to women ratio | P-value |
|---|---|---|---|---|---|
| 0–9 | 54 (1.1) | 27 (1.9) | 27 (0.8) | 1.00 | 0.0005 |
| 10–19 | 102 (2.1) | 42 (2.3) | 60 (1.7) | 1.43 | 0.005 |
| 20–29 | 440 (8.8) | 161 (11.3) | 279 (7.9) | 1.73 | 0.0001 |
| 30–39 | 874 (17.6) | 244 (17.1) | 630 (17.8) | 2.58 | 0.59 |
| 40–49 | 1223 (24.6) | 329 (23.1) | 894 (25.2) | 2.72 | 0.12 |
| 50–59 | 1038 (20.9) | 274 (19.2) | 764 (21.5) | 2.79 | 0.07 |
| 60–69 | 760 (15.3) | 204 (14.3) | 556 (15.7) | 2.73 | 0.23 |
| >70 | 484 (9.7) | 145 (10.2) | 339 (9.6) | 2.34 | 0.57 |
Statistical analyses of distribution (women vs men) were performed using Pearson Chi2. Presented P-value reflect the significance of differences in the proportion (%) of kidney stones in studied groups considering the small number of kidney stones in some groups.
Global distribution of all analyzed kidney stones (n = 4975) according to gender and age classes.
Global men-to-women ratio was 2.4 (3549 men and 1426 women) and varied from 1.43 to 2.79 (ages 10 to 19 and 50 to 59-years respectively) except for patients younger than 10 years old where male-to-female ratio was 1.0.
The number of stones increased steadily with patient age in both genders, reaching a maximum between 40 to 49 years, and decreased thereafter. The lowest number of stones (n = 54, 1.09%) was recorded in the age group between 0 to 9 years (1.8% in girls and 0.7% in boys). The highest number of stones (n = 1223, 24.6%) was found in the group aged between 40 to 49 years in both genders.
3.2. Main morphological types of kidney stones
Furthermore, we analyzed the age distribution of 4635 kidney stones classified according to type I, II, III, IV and V of Daudon’s MCC. The lower number of stones included in this analysis is related to the fact that 362 out of 5027 KS were too small to provide a reliable classification (Tables 3 and 4). We found all morphological types in the surface and section.
Distribution of kidney stones according to the main morpho-constitutional type detected on the stone surface as a function of age (n = 4503)
| Main surface type | 0–9 years | 10–19 years | 20–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | > 70 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | ||||||||
| I | 9 (0.4) | 1.25 | 26 (1.13) | 1.60 | 142 (6.2) | 1.15 | 362 (15.7) | 2.41 | 622 (27.0) | 3.26 | 550 (23.9) | 3.09 | 365 (15.9) | 3.84 | 226 (9.8) | 2.75 | |||||||
| II | 15 (1.3) | 0.88 | 38 (3.3) | 1.31 | 173 (15.1) | 3.12 | 281 (24.5) | 4.65 | 297 (25.9) | 3.69 | 189 (16.5) | 3.48 | 103 (9.0) | 1.94 | 49 (4.3) | 1.45 | |||||||
| III | 4 (0.9) | 1.00 | 0 (0.0) | NA | 7 (1.6) | 1.33 | 15 (3.4) | 1.50 | 69 (15.5) | 2.29 | 106 (23.8) | 3.77 | 139 (31.2) | 2.97 | 106 (23.8) | 3.20 | |||||||
| IV | 19 (3.4) | 1.25 | 16 (2.9) | 1.67 | 53 (9.5) | 0.89 | 101 (18.1) | 0.91 | 117 (21.0) | 0.69 | 96 (17.3) | 1.04 | 83 (14.9) | 1.24 | 71 (12.8) | 1.26 | |||||||
| V | 1 (1.9) | 0.00 | 7 (12.9) | 0.75 | 11 (20.4) | 1.75 | 17 (31.5) | 7.50 | 4 (7.4) | 3.00 | 11 (20.4) | 0.57 | 2 (3.7) | 1.00 | 1 (1.9) | 0.00 | |||||||
Distribution of kidney stones according to the main morpho-constitutional type detected in the stone section as a function of age (n = 4637)
| Main surface type | 0–9 years | 10–19 years | 20–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | > 70 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | n (%) | M/F ratio | ||||||||
| I | 6 (0.2) | 1.00 | 32 (1.2) | 1.91 | 179 (6.7) | 1.33 | 435 (16.4) | 2.61 | 736 (27.7) | 3.60 | 628 (23.6) | 3.15 | 409 (15.4) | 3.79 | 409 (8.7) | 3.20 | |||||||
| II | 13 (1.4) | 0.44 | 36 (3.9) | 1.33 | 152 (16.6) | 4.07 | 234 (25.5) | 4.40 | 216 (23.6) | 3.49 | 143 (15.6) | 3.18 | 82 (9.0) | 1.65 | 82 (4.4) | 1.35 | |||||||
| III | 1 (0.2) | NA | 1 (0.2) | NA | 6 (1.4) | 2.00 | 15 (3.4) | 1.50 | 72 (16.5) | 2.79 | 102 (23.4) | 4.94 | 139 (31.9) | 3.48 | 139 (22.9) | 3.30 | |||||||
| IV | 16 (2.8) | 1.50 | 13 (2.3) | 1.60 | 58 (10.1) | 0.81 | 113 (19.6) | 1.17 | 123 (21.4) | 0.70 | 99 (17.2) | 1.23 | 82 (14.2) | 1.16 | 82 (12.5) | 1.37 | |||||||
| V | 1 (1.9) | 0.00 | 9 (17.3) | 0.80 | 11 (21.1) | 1.75 | 16 (30.8) | 7.00 | 3 (5.8) | NA | 10 (19.2) | 0.25 | 1 (1.9) | NA | 1 (1.9) | 0.00 | |||||||
Surface morphological type I accounted for 2302 KS, type II for 1145 and type III, IV and V for 466, 556 and 54, respectively.
However, the section morphological type I accounted for 2657 of KS, type II for 916 and type III, IV and V for 436, 576 and 52, respectively.
Type I composed of Whewellite (Wh) and type II composed of Weddellite (Wd) were the most frequent and predominant types in the age class 40–49 years.
Type III composed of UA progressively increased from the age class 40–49 years and reached the highest frequency after 60 years.
Type IV composed of CaP was mainly observed in age groups from 30 to 49 years and decreased slowly thereafter.
Type V composed of Cys was found mainly in age classes 20 to 29 and 30 to 39.
3.3. Distribution of KS according to morphological subtypes and the patient’s gender
According to the surface morpho-constitutional subtype the statistically significant higher proportion of subtype Ia (p < 0.0001), IIa (p = 0.0003) and IIIa (p = 0.007) was observed in men as compared with women (Table 5). However, a statistically significant higher proportion of subtypes IVa, IVb and IVc (p < 0.0001, p < 0.0001 and p = 0.006 respectively) was observed in women as compared with men. The most common Ia/IIIa combination in stone surface was found in women vs men (p < 0.0001) but Ia/IIa in men vs women (p < 0.0001).
Global and gender distribution of analyzed kidney stones according to the main morpho-constitutional type detected in the surface of stones and as a function of gender (n = 4985)
| Stone surface type | Total (n = 4985) Number (%) | Women (n = 1431) Number (%) | Men (n = 3554) Number (%) | Men to women ratio | P-value |
|---|---|---|---|---|---|
| Type I: Calcium oxalate monohydrate (Wh) | |||||
| Ia | 2088 (41.9) | 530 (37.0) | 1558 (43.8) | 2.94 | <0.0001 |
| Ib | 113 (2.3) | 42 (2.9) | 71 (2.0) | 1.69 | 0.04 |
| Ic | 8 (0.2) | 1 (0.1) | 7 (0.2) | 7.00 | NS* |
| Id | 47 (0.9) | 18 (1.3) | 29 (0.8) | 1.61 | 0.14 |
| Ie | 23 (0.5) | 6 (0.4) | 17 (0.5) | 2.83 | 0.78 |
| Type II: Weddellite (Wd) | |||||
| IIa | 818 (16.4) | 192 (13.4) | 626 (17.6) | 3.26 | 0.0003 |
| IIb | 284 (5.7) | 77 (5.4) | 207 (5.8) | 2.69 | 0.54 |
| IIc | 9 (0.2) | 3 (0.2) | 6 (0.2) | 2.00 | NS* |
| Type III: Uric acid (UA) and urate | |||||
| IIIa | 222 (4.5) | 46 (3.2) | 176 (5.0) | 3.83 | 0.007 |
| IIIb | 194 (3.9) | 58 (4.1) | 136 (3.8) | 2.34 | 0.71 |
| IIIc | 5 (0.1) | 2 (0.1) | 3 (0.1) | 1.50 | NS* |
| IIId | 15 (0.3) | 5 (0.4) | 10 (0.3) | 2.00 | NS* |
| Type IV: Calcium and/or magnesium phosphates | |||||
| IVa | 237 (4.8) | 129 (9.0) | 108 (3.0) | 0.84 | <0.0001 |
| IVb | 179 (3.6) | 100 (7.0) | 79 (2.2) | 0.79 | <0.0001 |
| IVc | 66 (1.3) | 29 (2.0) | 37 (1.0) | 1.28 | 0.006 |
| IVd | 72 (1.4) | 21 (1.5) | 51 (1.4) | 2.43 | 0.93 |
| Type V: Cystine | |||||
| Va | 55 (1.1) | 21 (1.5) | 34 (1.0) | 1.62 | 0.12 |
| Type V: Protein | |||||
| VIa | 15 (0.3) | 7 (0.5) | 8 (0.2) | 1.14 | NS* |
| VIb | 14 (0.3) | 4 (0.3) | 10 (0.3) | 2.50 | NS* |
| Most frequently observed mixed stones | |||||
| Ia/IIa | 269 (4.6) | 54 (3.8) | 215 (6.1) | 3.98 | <0.0001 |
| Others | 239 (4.8) | 74 (5.1) | 165 (4.6) | 2.23 | 0.43 |
Statistical analyses of distribution (women vs men) were performed using Pearson Chi2 or * Fisher’s exact test for small group. Presented P-value reflects the significance of differences in the number of kidney stones in the studied groups.
According to the section morpho-constitutional subtype, a significantly higher proportion of subtypes Ia, IIa and IIIa was also observed in men as compared with women (p < 0.0001, p = 0.007 and p = 0.001 respectively). On the other hand, subtypes IVa, IVb and IVc were observed more frequently in women as compared with men (p < 0.0001, p < 0.0001 and p < 0.0001 respectively, Table 6).
Global and gender distribution of analyzed kidney stones according to the main morpho-constitutional type observed in the section of stones (n = 4985)
| Stone section type | Total (n = 4985) Number (%) | Women (n = 1431) Number (%) | Men (n = 3554) Number (%) | Men to women ratio | P-value |
|---|---|---|---|---|---|
| Type I: Calcium oxalate monohydrate | |||||
| Ia | 2499 (50.1) | 612 (42.8) | 1887 (53.1) | 3.08 | <0.0001 |
| Ib | 91 (1.8) | 32 (2.2) | 59 (1.7) | 1.84 | 0.17 |
| Ic | 3 (0.1) | 1 (0.1) | 2 (0.1) | 2.00 | NS* |
| Id | 32 (0.6) | 11 (0.8) | 21 (0.6) | 1.91 | 0.48 |
| Ie | 17 (0.3) | 4 (0.3) | 13 (0.4) | 3.25 | NS* |
| Type II: Calcium oxalate dihydrate | |||||
| IIa | 663 (13.3) | 161 (11.6) | 502 (14.1) | 3.12 | 0.007 |
| IIb | 236 (4.7) | 65 (4.5) | 171 (4.8) | 2.63 | 0.69 |
| IIc | 5 (0.1) | 2 (0.1) | 3 (0.1) | 1.50 | NS* |
| Type III: Uric acid and urate | |||||
| IIIa | 242 (4.9) | 47 (3.3) | 195 (5.5) | 4.15 | 0.001 |
| IIIb | 186 (3.7) | 48 (3.4) | 138 (3.9) | 2.88 | 0.37 |
| IIIc | 4 (0.1) | 2 (0.1) | 2 (0.1) | 1.00 | NS* |
| IIId | 1 (< 0.1) | 0 (0.0) | 1 (< 0.1) | NA | NS* |
| Type IV: Calcium and/or magnesium phosphates | |||||
| IVa | 203 (4.1) | 108 (7.6) | 95 (2.7) | 0.88 | <0.0001 |
| IVb | 163 (3.3) | 89 (6.2) | 74 (2.1) | 0.83 | <0.0001 |
| IVc | 140 (2.8) | 63 (4.4) | 77 (2.2) | 1.22 | <0.0001 |
| IVd | 69 (1.4) | 20 (1.4) | 49 (1.4) | 2.45 | 0.96 |
| Type V: Cystine | |||||
| Va | 52 (1.0) | 21 (1.5) | 31 (0.9) | 1.48 | 0.06 |
| Type VI : Protein | |||||
| VIa | 7 (0.1) | 5 (0.4) | 2 (0.1) | 0.40 | 0.02* |
| VIb | 9 (0.2) | 2 (0.1) | 7 (0.2) | 3.50 | NS* |
| Others | 363 (7.3) | 138 (9.6) | 225 (6.3) | 1.63 | <0.0001 |
Statistical analyses of distribution (women vs men) were performed using Pearson Chi2 comparison or * Fisher’s exact test for small group. Presented P-value reflects the significance of differences in the number of kidney stones in the studied groups.
The rare types Ic, Ie, IIId, IVa2 and V corresponded to 0.1%, 0.4%, 0.1% 0.3% and 1.1% respectively but suggested the involvement of a very specific lithogenesis process.
Within the type V, 55 stones represented the surface morphology of type Va and the morphology Vb was found in nine (0.1% of all stones).
3.4. Distribution according to the main physicochemical component (crystalline phase) of KS
Stones containing mainly Wh account for the majority of KS in the whole cohort (75.4%) (Table 7).
Global and gender distributions of analyzed kidney stones according to the main physicochemical component identified within the stones (n = 4985)
| Main physicochemical component | Total (n = 4985) Number (%) | Women (n = 1431) Number (%) | Men (n = 3554) Number (%) | Men to women ratio (2.5) | P-value |
|---|---|---|---|---|---|
| Calcium oxalate monohydrate | 2629 (52.7) | 669 (46.7) | 1960 (55.1) | 2.9 | <0.0001 |
| Calcium oxalate dihydrate | 1130 (22.7) | 271 (18.9) | 859 (24.7) | 3.2 | 0.0001 |
| Uric acid anhydrous | 457 (9.2) | 110 (7.7) | 347 (9.8) | 3.2 | 0.02 |
| Carbapatite | 338 (6.8) | 202 (14.1) | 136 (3.8) | 0.7 | <0.0001 |
| Magnesium ammonium phosphate | 106 (2.1) | 55 (3.8) | 51 (1.4) | 0.9 | <0.0001 |
| Cystine | 64 (1.3) | 24 (1.7) | 40 (1.1) | 1.7 | 0.12 |
| Dicalcium phosphate dihydrate | 62 (1.2) | 17 (1.2) | 45 (1.3) | 2.6 | 0.82 |
| Proteins | 44 (0.9) | 15 (1.1) | 29 (0.8) | 1.9 | 0.82 |
| Uric acid dihydrate | 35 (0.7) | 11 (0.8) | 24 (0.7) | 2.2 | 0.72 |
| Amorphous carbonated calcium phosphate | 30 (0.6) | 18 (1.3) | 12 (0.3) | 0.7 | 0.0001 |
| Octacalcium phosphate pentahydrate | 4 (0.1) | 2 (0.1) | 2 (0.1) | 1.0 | NS * |
| Miscellaneous | 86 (1.7) | 37 (2.6) | 49 (1.4) | 1.3 | 0.35 |
Statistical analyses of distribution (women vs men) were performed using Pearson Chi2 comparison or Fisher’s exact test (*) for small group. Presented P-value reflect the significance of differences in the number of kidney stones in studied groups.
Within 3759 CaOx stones, 2629 (52.7%) corresponded to Wh and 1130 (22.7%) to Wd. Among the 540 phosphate stones, 338 were made of CA, 106 of struvite, 62 were brushite, 30 amorphous carbonated calcium phosphate (ACCP) stones and only four octacalcium phosphate pentahydrate stones.
The UA stones accounted for 491 (9.87%) stones (UA anhydrous (UAA) 9.17 and UA dihydrate (UAD) 0.7%).
Stones containing cystine were rare and accounted for 1.2% (64 stones in whole cohort).
Protein composition was found in 44 (0.8%) of all stones. Only 86 stones were classified as miscellaneous (unknown composition). We did not detect drug(s) related stones or containing rare metabolic components such as dihydroxyadenine, xanthine, methyl-1 uric acid, ….
Within CaOx stones, Wh was identified as the main component of more than half of all analyzed samples (n = 2629) and was significantly more common in men than in women (55.1% vs 46.7%, p < 0.0001). The second most frequent crystalline phase was Wd corresponding to 1130 KS, also significantly more common in men than women (24.7% vs 18.9%, p = 0.0001).
The UAA corresponded to 457 KS mainly provided from men (9.7% vs 7.6%, p = 0.02).
In contrast, phosphate stones were less frequent (338 CA (6.8%) and 106 struvite stones (2.1%)) and were significantly more common in women as compared to men ((14.1% vs 3.8%, p < 0.0001) and (3.8% vs 1.4%, p < 0.0001) for CA and struvite respectively). Women submitted more ACCP stones than men (1.2% vs 0.3%, p = 0.0001).
3.5. Most common physico-chemical combination of kidney stones
In the constitutional analysis of 5027 samples, stones with a single component accounted for 2035 (40.4% pure stones), stones with two components for 1432 (28.4%) and stones with more than two components for 1560 (31%) out of the all KS. Note that minor protein content was not taken into account.
Wh was the main single component in 1621 stones and was followed by Wd and UAA (175 and 114 stones, respectively), which were observed mainly in men KSF (35.5% vs 25.1%, 3.9% vs 2.7% and 2.5% vs 1.7% respectively) (Table 8).
Global and gender related distribution of kidney stones according to the main physicochemical component and the most frequent combination of components as determined by the analysis of a global powder of stones by FTIR spectroscopy
| Main physicochemical component | Total (n = 4985) Number (%) | Women (n = 1431) Number (%) | Men (n = 3554) Number (%) | P-value |
|---|---|---|---|---|
| Wh | 1621 (32.5) | 359 (25.1) | 1262 (35.5) | <0.0001 |
| Wd + Wh + CA | 729 (14.6) | 237 (16.6) | 492 (13.8) | 0.01 |
| Wd + Wh | 620 (12.4) | 111 (7.8) | 509 (14.3) | <0.0001 |
| Wd + CA | 307 (6.2) | 93 (6.5) | 214 (6.0) | 0.53 |
| Wh + CA | 271 (5.4) | 154 (10.8) | 117 (3.3) | <0.0001 |
| UAA + UAD | 254 (5.1) | 58 (4.1) | 196 (5.5) | 0.03 |
| Wd | 175 (3.5) | 39 (2.7) | 136 (3.9) | 0.06 |
| UAA | 114 (2.3) | 25 (1.8) | 89 (2.5) | 0.11 |
| CA + ACCP | 89 (1.8) | 61 (4.3) | 28 (0.8) | <0.0001 |
| Miscellaneous | 805 (16.2) | 294 (20.5) | 511 (14.9) | <0.0001 |
Statistical analyses of distribution (women vs men) were performed using Pearson Chi2 comparison or * Fisher’s exact test for small group. Presented P-value reflects the significance of the difference in the number of kidney stones in the studied groups.
Abbreviations: Wh: whewellite; Wd: weddellite; UAA: uric acid anhydrous; CA: carbapatite; ACCP: amorphous carbonated calcium phosphate; UAD: uric acid dihydrate.
We found the most common combinations to be Wd + Wh + CA, Wd + Wh and Wd + CA (729, 620 and 307 stones).
The combinations Wd+Wh were significantly higher in men than in women (14.3% vs 7.8%, p = 0.0001) contrasting with the higher distribution of combinations Wd + Wh + CA, Wh + CA and CA + ACCP in women as compared with men (16.6% vs 13.8%, p = 0.01; 10.8% vs 3.3%, p < 0.0001 and 4.3% vs 0.8%, p < 0.0001, respectively).
3.6. Distribution of main crystalline phase types according to patients’ age and to gender
3.6.1. Calcium oxalate kidney stones
Whewellite. The distribution of Wh stones (type I) increased steadily with patient age in both genders (Figure 3A). Lowest distribution was recorded between 0 and 9 years (0.3%) and highest distribution between 40 to 49 years (27.7%); patients aged more than 70 years accounted for 8.6%. Men submitted more Wh stones than women in all age classes (the men-to-women ratio varied from 1.0 to 3.6).
Kidney stone distribution by gender and age intervals according to the main physical component (crystalline phase). (A) Whewellite, (B) Weddellite, (C) Uric acid, (D) Carbapatite.
Weddellite. Similarly, the distribution of Wd stones (type II) increased steadily with patient age in both genders, reaching a maximum earlier between 30 and 39 years and decreasing thereafter with male predominance in all age groups except for the age classes from 0 to 9. Peak distribution was observed between 40 and 49 years for women and 10 years earlier in men (Figure 3B). Men submitted more Wd stones than women in all age classes (the men-to-women ratio varied from 1.1 to 4.6, except for patients less than 10 years old where M/F ratio was lowest at 0.6).
3.6.2. Uric acid stones
The distribution of UA (type III) increased steadily with patient age in both genders. In our cohort, lowest distribution was recorded between age 10 to 19 years and reached a maximum between 60 to 70 years (Figure 3C). Men accounted for more KSF than women in all age classes (men-to-women ratio varied from 1.3 to 3.7).
3.6.3. Calcium and magnesium phosphate stones
In our patients, CA was the most common crystalline phase of CaP stones. CA stones (type IV) were found in 6.8% of all stones and predominated in women (14.1% vs 3.8% in men, p < 0.0001) (Figure 3D). The number of KS containing CA increased in patients older than 20 years and reached a plateau from 30 years (Figure 3D). Except for the age interval 10 to 19 years, women submitted more CA stones than men (men-to-women ratio < 1.0).
In our cohort 117 stones (2.3%) contained struvite. This crystalline phase was more frequent in women KSF as compared to men (3.8% vs 1.4%, p < 0.0001).
Among phosphates, whitlockite was found in 19 cases (0.4%). In all cases, whitlockite was found admixed with more abundant species, mainly CA (63.2% of cases).
4. Discussion
To our knowledge, we report the largest Belgian cohort of stone types according to Daudon’s MCC and we provide a statistical analysis of their demographic distribution in relation to age classes and gender.
Within both gender categories, the overall distribution of KS increased with age, peaking at age 40 to 50 years, and decreasing thereafter as reported in Germany and France [55, 56] but later than reported in Nepal (peak of prevalence 2nd and 4th decades) [57] and occurring earlier as compared with the cohort from Mayo Clinic and Northwestern University of Chicago (45.6% of age more than 60) [58, 59]. The peak age interval is shifted ten years earlier as compared with the data from the Liège region in the North-East region of Belgium [60]. The differences could be linked to a characteristic of the studied population, the patients from Liège region were older.
As reported by others [41, 55, 60], men submitted more stones than women and we observed male predominance for CaOx and UA stones, and female predominance for CaP stones [3, 52, 61, 62, 63]. The predominance of oxalate stones within mixed stones (83%) and the prevalence of UA stones (54%) in “pure” kidney stones as analyzed by FTIR spectroscopy has been recently reported also in the European part of the Russian Federation [64]. In China, CaOx corresponds to 65.9% of new-onset male KSF [65].
The increase in the proportion of calcium stones mainly in women has been reported in a retrospective study of 1516 patients followed from 1980 to 2015 in the USA [66]. Talati and coworkers reported that as stone formers age, the men-to-women ratio decreased significantly from 2005 to 2015 (from 1.8 to 1.08) concomitantly with the alarming increase in the frequency of obesity in American women [59]. Additionally, Taiwanese women with stones exhibited a higher risk of chronic kidney disease (CKD) as reported by multivariate analysis (ORs 5.31; 95% CI: 3.3–13.7) [67]. These data are alarming and invite a broader revision of current management of KSF with additional focus on the prevention of CKD.
More than half of the stones mainly contained calcium oxalate: 79.8% in men and 65.6% in women. Wh accounted for 55.1% of stones in men and 46.7% in women while Wd accounted for 24.7% in men and 18.9% in women. As previously reported [26, 50, 68], subtype Ia indicated intermittent or moderate hyperoxaluria whereas types IIa and IIb were suggestive for hypercalciuria, whether associated or not with hyperoxaluria of dietary origin or inadequate water intake.
This hypothesis is supported by preliminary data from the retrospective cohort including 112 recurrent KSF that provide the urinary metabolic lithogenic risk factors and KS composition in adult patients from the Brussels region [69]. The physico-chemical analysis of 53 stones demonstrated that Wh has been more prevalent than Wd. In addition, subtype Ia has been found in a higher proportion than subtype IIa suggesting hyperoxaluria and hypercalciuria of dietetic origin as a main urinary abnormality. Indeed, this presumption has been confirmed by the data of 24 h urine collection performed during the first metabolic assessment. Hyperoxaluria followed by idiopathic hypercalciuria has been the most frequent direct lithogenic abnormality, in addition to the high frequency of hypernatriuresis (an indirect promoter of lithogenesis) that has been highlighted. The authors conclude that the leading metabolic disorders involved in the formation of KS in recurrent KSF in Brussels are dietary hyperoxaluria followed by idiopathic hypercalciuria in correlation with high salt intake. These conclusions corroborate the aetiologies suggested by applying the MCC of stones to our cohort.
The recognition of the existence of Wh and Wd crystalline conversion process is crucial for the clinician. The crystalline conversion from Wd to Wh defines a specific situation when the contradiction appears between the FTIR spectroscopy information about the presence of Wh and morphological findings of bipyramidal crystallites related to Wd [26]. It is admitted that Wh indicates hyperoxaluria and Wd is related to hypercalciuria [26, 48], therefore Wh and Wd indicate distinct aetiologies and thus a very different treatment. Bazin et al. [70] underlined that if crystalline conversion occurs, the clinician needs to focus on the stone’s morphology rather than on the FTIR spectrum as the major focus for assessment of urinary abnormalities. Indeed, he demonstrated that in the case of crystalline conversion from Wd to Wh the FTIR spectra are related to the formation of amorphous whewellite. In this case, hypercalciuria suggested by the stone morphology needs to be considered as a primum movens for stone formation instead of hyperoxaluria suggested by FTIR analysis.
Although composed of Wh, the particular morphology of subtypes Ic and Ie, suggests a very distinct lithogenic process [48]. In case of Ic stone, primary hyperoxaluria (an inborn metabolic disease leading to severe systemic complications and kidney failure) should be systematically sought [45] and in the case of type Ie stone, heavy hyperoxaluria of enteric origin related to increased gut oxalate absorption (short bowel syndrome, pancreatic insufficiency, etc.) should be considered. Types Ib and Id subtypes, although composed also of Wh, are related to precise conditions such as low urine output and urinary stasis, associated or not, with kidney and urinary tract abnormalities. They were not as common in our series as reported by others [60, 69].
Type III (UA) progressively increased with age from 40 and reached the highest frequency after age 60, mainly in men as reported in France, in Tunisia and USA [56, 62, 66]. High temperature and humidity have been reported to impact UA stone formation in Florida [7]. Any relationship between serum UA and KS has been found in a logistic regression of individual data of 6398 KSF from the UK biobank [71], corroborating the postulate of Sakhaee et al. [18, 21, 32] suggesting the major role of urinary pH in AU stone formation.
Low urine pH, recognized as a key factor of UA composed stones, has frequently been reported in observational studies of type 2 diabetic patients and overweight subjects with insulin resistance, as a factor in forming stones. Recently genetic data has reinforced this association [72, 73]. During the metabolic work-up of UA stone formers the evaluation of insulin resistance should be a rule and indicate the risk of existence of metabolic syndrome. Early therapeutic interventions should be set up to prevent progression to diabetes and related cardiovascular complications [74].
The pathogenesis of type IV stones is mixed, combining hypercalciuria and alkaline urine pH higher than 6.0 [50]. The type IV stones were submitted mainly by women aged more than 30-years and accounted for 12%, similar to that reported in France (15%) [75]. A plausible hypothesis could be vitamin D and high calcium supplementation to prevent postmenopausal osteoporosis [50, 51].
The subtype IVa was the most frequent in both genders and suggests the pathogenic role of hypercalciuria and/or hyperparathyroidism and/or UTI [25, 75]. The subtype IVa2 accounted for 0.3% of stones and pointed to the very specific clinical entities involved in CaP formation such as alkaline urinary pH especially related to renal distal tubular acidosis whatever its origin, inherited, autoimmune, of iatrogenic origin or related to medullary sponge kidney [52, 75, 76].
The stones of subtype IVb indicate past or latent UTI with abnormally high alkaline urine and predominate in women, correlating with the evidence of a higher rate of UTI in women than in men [75, 77]. Women with infectious stones have almost twice as high risk for postoperative fever, indicating longer antimicrobial therapy in the postoperative period [78].
Only 66 stones (1.3%) were classified as type IVc and were mainly seen in women (2.0% vs 1.4%, p < 0.0001) correlating with French data (1.2%) [48]. This type unambiguously results from UTI by urea splitting bacteria. Within our cohort, 106 stones (2.13%) contained struvite as a main component and were mainly seen in women. In all analyzed stones and fragments the overall frequency of struvite accounted for 2.3%, similar to the frequency reported in Canada (2%), China (2.7% to 3%), Argentina (3%), Iraq (3%), Poland (3%) Italy (3.5%), but lower than the frequency reported in France (7.2%), or in Brazil (8.3%) [65, 79, 80, 81]. The discrepancy between our data findings and data from Pakistan (18%) or India (23%) or data from paediatric patients from Morocco (18%) could be attributed to the differences in epidemiological characteristics of the populations studied, especially a low human development index and related low access to healthcare (late diagnosis and/or delay in accurate treatment of UTI) rather than temperature and humidity [81]. It was recently reported that neurological bladder and CAKUT contributed to 38% of infectious stones in pediatric populations [25].
The carbapatite stones admixed with struvite exhibit a high content of carbonate ions revealing UTI by urea-splitting bacteria [82]. Indeed, in analysis of the physico-chemical composition of stones the calculation of the content of carbonate ions within the carbapatite (carbonation rate, CR) is helpful to assess UTI as an aetiological factor involved in the stone’s formation. Carpentier et al. [82] reported a close relationship between the carbonation rate of carbapatite (the amounts of carbonate ions () in relation to the amount of phosphate ions () and the number of bacterial imprints within 39 urinary idiopathic hypercalciuric stones but without struvite. This data needs more attention as identification of bacterial imprint could be helpful in controlling stone formation, in case of negative results of urine culture [83].
In the cross-sectional study including 107 KSF in tertiary hospitals in Nepal, Escherichia coli was the major non-urease producing organism isolated in the preoperative urine culture [57]. Recently the presence of whitlockite structures detected by SEM and synchrotron radiation has been proposed as a new criterion to identify the infectious origin of stones [84]. In our cohort only 0.3% contained whitlockite as a physical signature of non-urea splitting bacteria involvement in stone genesis.
In the absence of carbonation rate measurements, our data underestimate the prevalence of infectious stones within our cohort as we defined the infectious aetiology stones based on the presence of struvite. Indeed, it has been proposed that low CR value (<10%) in type IV stones suggest a metabolic origin without the participation of UTI; on the contrary CR > 15% indicates the involvement of past or actual UTI in the lithogenesis [82].
The association between the presence of struvite identified by FTIR analysis and type II morphology was suggestive for both UTI by urea-splitting bacteria and hypercalciuria in the patient. Interestingly 54% of paediatric patients (n = 111) with infectious stones (22%) presented hypercalciuria [25]. Indeed, evidence suggest that oral vitamin D supplementation may induce hypercalciuria among children under five years of age [85].
The cumulative frequency of stones containing carbapatite, struvite, brushite, ACCP was 10.8% in our study, corroborating the average cumulative frequency of all calcium and magnesium phosphate stones (9%) analyzed in the smaller series of stones from different countries (n = 1204) as recently reported by Halinski et al. [80].
Stones with type IVd morphology accounted for 1.4% and 62 stones (1.24%) were composed mainly of brushite in our experience. Type IVd has been reported in 14% of patients with primary hyperthyroidism in some series [86].
Distinctive morphology of V allowed us the diagnosis of cystinuria [87, 88]. The subtype Va is pathognomonic of a heavy cystinuria.
In our series, the subtype Vb was less common, and its frequency was similar to that reported by Daudon et al. (0.1% vs 10%). The type Vb stones suggest uncontrolled cystinuria associated with inadequate diet and/or medical management or urinary stasis.
We recognize the limitations of our study, including the absence of concomitant 24 h urine collection data to correlate the urinary abnormalities with the aetiologies evoked as suggested by MCC. Such correlation has been previously reported for Wh and Wd stones. Lithogenic factors identified in the 24 h urine collection correlate with the main stone composition identified by physical methods, although the morphological type of KS is unknown in those reports [89, 90, 91]. The discordance between the findings from 24 h urine collection and aetiologies evoked by MCC occurs mainly in case of intermittent hyperoxaluria and hypercalciuria, especially if the patient already performed diet and/or hydration habit modifications. Unfortunately, in every day clinical practice this is by far the most frequent clinical scenario as the patient undergoes metabolic evolution at least two months after the acute manifestation or urological intervention (according to current guidelines). In this context, the identification of prior urine metabolic abnormalities involved in initiation and growth of stones could be revealed only by adequate morphological and physicochemical analysis of stones as described by Daudon [2], which should integrate a metabolic work-up guidelines as previously proposed.
Astonishingly, there is discrepancy between the propositions of the “best” stone classification. Thongprayoon Ch and coworkers [42] proposed a classification of kidney stones according to major chemical composition into the following seven mutually exclusive groups: calcium oxalate (if majority), hydroxyapatite (if majority), uric acid (if any), struvite (if any), brushite (if any), cystine (if any) and others (including stones composed of drugs). However it has been admitted that classification based on chemical analysis is too inadequate to accurately recognize KS components, and could even fail to detect certain elements, such as rare purine stones and drug-induced stones [92].
Recently Williams and coworkers [93] have reported the value of micro-CT technique alone to successfully identify majority of the apatite, brushite, uric acid, and struvite stones and additionally the three-dimensional nature of micro-CT also allows the visualization of surface features in stones, which is valuable for the study of stone formation. The limitation observed by the authors concerned the detection of small quantities well below 1% of minor minerals, such as apatite in CaOx or calcium salts in UA stones.
Our results highlight the need for field-specific standardization of clinical protocols in terms of stone assessment procedures, as well as in defining clinically relevant metadata including MCC type and physical composition of stones considering the initiative of European Renal Stone Network for a common database for observational research [94, 95].
Currently the Daudon’s MCC remains a gold standard method in KSF assessment [52, 96].
4.1. Conclusions
According to Daudon’s MCC, the leading metabolic disorders involved in lithogenesis in the Belgian population are intermittent hyperoxaluria (Ia), hypercalciuria (IIa), hypercalciuria ± hyperoxaluria ± hypocitraturia (IIb), low urine pH or stasis (IIIa) and diabetes with metabolic syndrome (IIIb). Rare morphologies, types Ie, Id, IIId and IVa2 point to precise clinical entities such as enteric hyperoxaluria, urinary tract abnormalities, hyperuricosuria with diarrhoea and distal tubular acidosis respectively. In a few cases, very distinctive morphology such as Ic and V allowed the rapid diagnosis of primary hyperoxaluria type 1 and cystinuria respectively, both severe inborn disorders of metabolism.
Based on our and other experiences, we emphasize that international guidelines for the assessment and management of KSF need to be actualized and should strongly highlight the contribution of Daudon’s MCC to the clinical reasoning of physicians.
Abbreviations
| ACCP | amorphous carbonated calcium phosphate |
| CA | carbapatite |
| CaP | calcium phosphate |
| CKD | chronic kidney disease |
| CAKUT | congenital anomalies of the kidney and/or urinary tract |
| CaOx | calcium oxalate |
| Cys | cystine |
| FTIR | Fourier Transform Infrared (Spectroscopy) |
| KS | kidney stones |
| KSD | kidney stones disease |
| KSF | kidney stones formers |
| MAP | magnesium ammonium phosphate hexahydrate |
| MCC | morpho-constitutional classification |
| UA | uric acid |
| UAA | uric acid anhydrous |
| UAD | uric acid dihydrate |
| UTI | urinary tract infection |
| Wd | weddellite |
| Wh | whewellite |
Conflicts of interest
None declared.
Authors’ contributions
AP: Contributed substantially to the conception and design of the study, the acquisition of data, or the analysis and interpretation. Supervised the research, drafted or provided critical revision of the article, provided final approval of the version to publish;
AH: Contributed substantially to the conception and design of the study, the acquisition and the analysis and interpretation, drafted or provided critical revision of the article, provided final approval of the version to publish;
JR: Performed bioinformatic analyses. Drafted or provided critical revision of the article, provided final approval of the version to publish;
TR: Provided the data;
FW: Provided the data, contributed substantially to the analysis and interpretation of data, provided critical revision of the article;
FC: Contributed substantially to the conception and design of the study, the acquisition of data, or the analysis and interpretation, provided critical revision of the article.
Funding
No funding supported this study.
Acknowledgments
The authors would like to acknowledge Dr. Khashayar Sakhaee and Dr. Naim Maalouf (University of Texas Southwestern Medical Center, Dallas, Texas, USA) for helpful discussions during the preparation of this manuscript.




 CC-BY 4.0
CC-BY 4.0