1. Introduction
The concept of green fuel that contributes to the protection of the environment emerged in the United States in the early 1980s. Chemical models for common fuel combustion must be established in order to improve fuel formulation. However, due to the complexities of the composition of these fuels, having kinetic models that include all of the compounds is nearly impossible. Several research groups have attempted to create similar fuel mixtures with a smaller number of constituents from the most organic groups found in fuels (alkanes, isoalkanes, cycloalkanes, aromatics, etc.) [1, 2, 3]. Alternative fuels should replicate a specific set of physico-chemical properties [4], allowing for a deeper comprehension of the primary effects of fuel formulation and their properties on combustion performances and gas emission when used in an internal combustion engine [5].
It is important to underline that the basic constituents of diesel, as well as their composition, are not constant, since several variables such as the place of origin of the source of crude oil, the refining operations, the standards adopted and even the season, and so on, may cause difference in the composition of the fuel, making it difficult to understand the engine’s overall emissions mechanism [6]. Many research reports in literature concern the optimization of surrogate formulations to emulate fossil diesel by using several selected properties including cetane number, density, calorific value, viscosity, distillation curve, boiling point, lower heating value (LHV), etc. [3, 6, 7, 8].
To reproduce the cetane number of fossil diesel, n-heptane was the typical single-component surrogate which was mostly used [9]. As a two-component surrogate, n-decane and a-methylnaphthalene showed a great agreement with a real fossil diesel fuel on ignition and emission characteristics [10].
Chen et al. [9] used iso-hexadecane and n-dodecane as a surrogate for real Fischer–Tropsch diesel by trying to reproduce its combustion-related physico-chemical properties and a skeletal oxidation model was developed to validate the ignition behavior.
Szymkowicz and Benajes [11] proposed a four-component surrogate that represented the primary four hydrocarbon classes of diesel fuel: normal-alkanes, isoalkanes, cycloalkanes and aromatics, it included n-hexadecane, heptamethylnonane, decahydronaphthalene, to represent the four classes respectively.
Sun et al. [12] used a four-component surrogate to develop a diesel combustion mechanism that was validated with experiment ignition delay time in shock tube, counter-flow configuration and marine engine.
Also, biofuels are considered a promising alternative to fossil fuels, either used in the pure form or in mixtures with ordinary fuels, in the context of large scale distribution. Biodiesel is usually used in mixtures to replace fossil diesel, especially if this biodiesel is second and third generation. Indeed, one of the most widely used second-generation biodiesel production method is the alcoholic transesterification of vegetable frying oils in order to recover this harmful waste from degrading the environment.
Several works have tried to optimize the operating conditions of this process, such as the temperature, the quantity and type of catalyst and the alcohol/oil ratio [13, 14]. Recent progress in this field has seen the use of nano-CaO catalyst [15, 16] and constant improvement in the green character of this production for the elimination of the co-product of the reaction, glycerol [17].
Transesterification reaction scheme.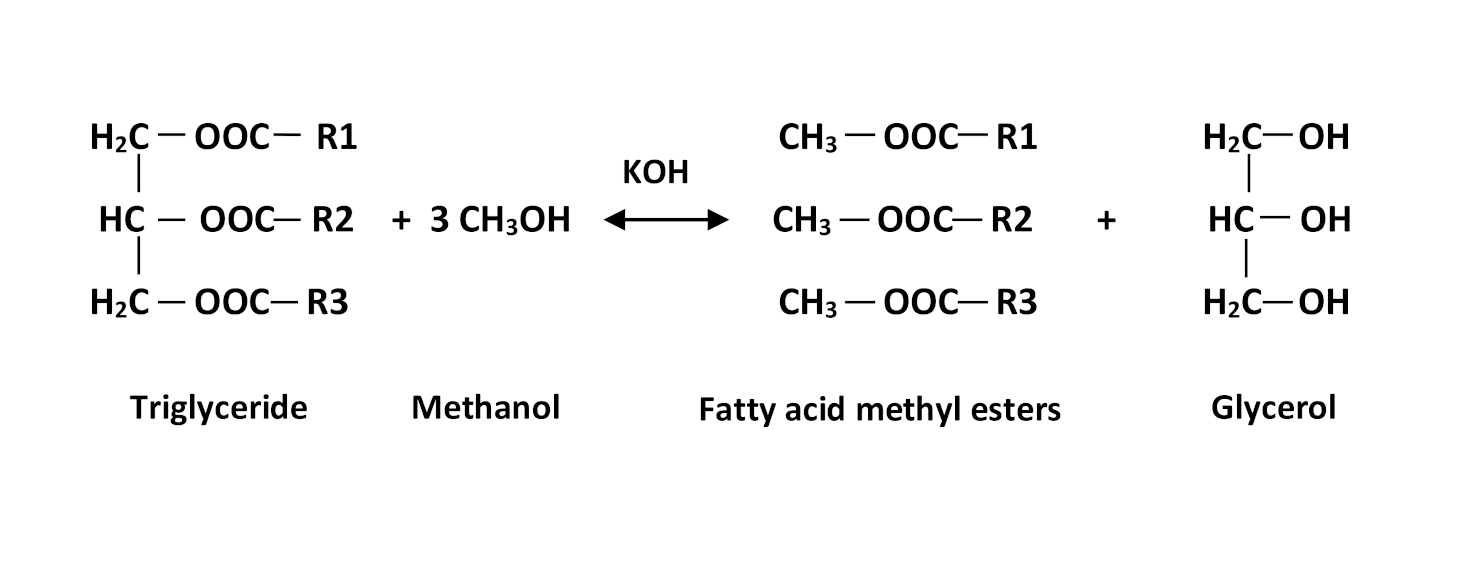
In fact, biodiesel is composed mainly of alkyl esters and contains less carbon than in diesel fuel, with 77.2 and 87% by weight, respectively, resulting in much less carbon-based emissions by biodiesel-powered engines, compared to those powered by diesel. Graboski and McCormick [8] investigated the efficiency and emissions of a diesel engine fueled by fossil diesel, biodiesel and several blends of diesel mixed with biodiesel at 10%, 20%, 30%, 50%, versus base diesel. The results showed that biodiesel–diesel mixtures reduced carbon monoxide, hydrocarbons, smoke, and polyaromatic hydrocarbon (PAH) emissions.
Research is increasingly focused on the formulation of fuels with the objective of reproducing the physical and chemical properties with a minimum of simple compounds that must be easy to handle experimentally [18].
Several fuels have been successfully imitated: diesel, gasoline, jet fuel as well as biofuels [1, 19, 20] and their usual blends with fossil fuels, such as gasoline–bioethanol and diesel–biodiesel [19, 21]. However, little work has focused on diesel–biodiesel blends over a wide range of blended percentages. The number of compounds in each proposed surrogate varied each time, ranging from two to twelve [1, 3, 18]. The chemical nature of surrogates also varied depending on the studied target fuel or studied blends and generally consists of n-alkane, isoalkanes, aromatics and cycloalkanes [18, 22]. For the surrogates that emulate biofuels and their blends with fossil fuels, in addition to these hydrocarbons, esters and oxygenates have often been used [19, 21] and proposed alternatives varied usually in number and nature of components.
In order to reproduce a fossil diesel in mixtures at different proportions with biodiesel, which was prepared by a transesterification reaction of local cooking oil wastes, in this work it is proposed to optimize a surrogate fuel considering density, viscosity and cetane number as the target properties. It is aimed at reproducing as closely as possible these properties of diesel, biodiesel and mixtures of the two fuels considering a large range in molar biodiesel percentages, namely 5%, 10%, 20%, 50% and 80%. To overcome the difficulty of the variety of number and nature of components of the proposed surrogates, the objective is to suggest surrogates with a minimum number of hydrocarbons and the same components that differ in composition to emulate all the studied target fuels and blends.
2. Methodology
Data collection from previous studies on surrogates as diesel alternatives was the primary method adopted [6, 7].
2.1. Palette components
Generally, in order to propose a surrogate fuel, a palette of chemical compounds should a priori be identified before any formulation optimization process is carried out [23]. The pure components used as palette database in this work were taken from some preceding studies and reviews that considered them as diesel surrogates, as shown in Table 1 which also presents their properties to constitute a database. Density at 15 °C was chosen instead of that at 20 °C, as used in previous work [24], and this was the most used one in literature and also in standard norms [25, 26]. The palette was also prepared using most recent references and the four organic classes are represented: n-alkanes, isoalkanes, cycloalkanes and aromatics.
The palette used and component properties
| Properties | N°CAS | 𝜌 a (kg∕m3) at 15 °C | 𝜇 a (cp) at 40 °C | CN | References |
|---|---|---|---|---|---|
| n-decane | 124-18-5 | 735.1 | 0.6971 | 76.7 [27] | [28, 29, 30] |
| Isooctane | 540-84-1 | 697.1 | 0.4013 | 14 [22] | [21, 30] |
| Methylcyclohexane | 108-87-2 | 773.5 | 0.5631 | 20.8 [27] | [21, 30] |
| Toluene | 108-88-3 | 873.4 | 0.4623 | 7.4 [27] | [3, 29, 31] |
| n-hexadecane | 544-76-3 | 776.7 | 2.17 | 100 [22] | [6, 8, 32, 33] |
| Isocetane | 4390-0 4-9 | 880.2 | 2.089 | 15 [27] | [6, 28, 32, 33] |
| Cyclohexane | 110-82-7 | 782.4 | 0.7078 | 18.5 [22] | [19, 34, 35] |
| m-xylene | 108-38-3 | 870.4 | 0.4957 | 2.6 [22] | [28, 21] |
| Tetralin | 119-64-2 | 974.3 | 2.323 | 8.9 [22] | [28, 36, 21] |
| trans-decalin | 91-17-8 | 873.7 | 0.92 | 44 [22] | [29, 33, 21] |
| 1-methylnaphtalene | 90-12-0 | 1024 | 3.885 | 0 [22] | [6, 32, 33, 21] |
| n-dodecane | 112-40-3 | 753 | 1.051 | 82.5 [27] | [28, 37, 38, 39] |
| n-heptane | 142-82-5 | 686.7 | 0.3347 | 54.4 [40] | [7, 31] |
| n-butylcyclohexane | 1678-93-9 | 801.8 | 0.9484 | 48.8 [40] | [6, 33, 21] |
| n-butylbenzene | 104-51-8 | 865.1 | 0.7927 | 14.3 [40] | [6, 33, 29] |
| 1,2,4-trimethylbenzene | 95-63-6 | 882.5 | 0.6628 | 8.9 [22] | [22, 21] |
| n-octadecane | 593-45-3 | 786.2 | 2.925 | 116 [41] | [6, 28, 33, 36] |
| n-eicosane | 112-95-8 | 795.1 | 3.945 | 120 [41] | [28, 33, 36] |
Target properties of fossil diesel and its mixtures with biodiesel
| Property | Diesel | Biodiesel (B100) | 5% (B5) | 10% (B10) | 20% (B20) | 50% (B50) | 80% (B80) |
|---|---|---|---|---|---|---|---|
| Density at 15 °C (kg∕m3) | 835 | 888.4 | 837.51 | 840 | 845.16 | 860.87 | 877 |
| Kinematic viscosity at 40 °C (mm2∕s) | 3.2 | 6.89 | 3.34 | 3.49 | 3.80 | 4.83 | 6.01 |
| Cetane number | 55 | 51.33 | 54.81 | 54.63 | 54.26 | 53.16 | 52.06 |
2.2. Biodiesel production
The biodiesel considered was produced using base transesterification (Figure 1) of cooking oil brought back from the refectories of our university. Optimized conditions obtained from our earlier research was used to transform 50 g of soybean frying oil to biodiesel using 4.73 methanol/oil molar ratio and 2 wt% of KOH as a catalyst at a temperature of reaction fixed at 45 °C with agitation of the reaction medium at 300 rpm [13]. The biodiesel obtained was recovered and washed twice with distilled water, dried and characterized at NAFTAL (Algerian National Company for the Marketing and Distribution of Petroleum Products). Its target properties, used in the present work, are presented in Table 2.
2.3. Target fuels properties
Table 2 presents the properties of fossil diesel in conformity with the ASTM limits (considered at mid-range values) that match the most used diesel norms [25], the properties of the biodiesel produced in our laboratory that were characterized at NAFTAL and those of the studied blends that were calculated using the mixing rules used in this work.
2.4. Mixing rules for target property calculations
Three target properties were considered in this study: density (at 15 °C), viscosity (at 40 °C) and cetane number. The mixing rules used to calculate mixture properties are as follows.
2.4.1. Liquid density
It is an essential property of any combustible liquid because it directly influences the characteristics and performance of combustion engines [42]. This property, as defined by ASTM D4052, is introduced into the objective function so that the density of the optimized surrogate is as close as possible to the density of the target fuel. The density of a liquid mixture of n components was estimated as follows [21]:
| (1) |
2.4.2. Kinematic viscosity
The resistance of a liquid to flow is known as its kinematic viscosity. It influences the size of the fuel drop, the atomization quality, the spray characteristics, and the combustion quality [42]. ASTM D445 is used to determine the kinematic viscosity at 40 °C. The kinematic viscosity was calculated as follows [21]:
| (2) |
The dynamic viscosity of n hydrocarbons blend were estimated using the following mixing rule [42]:
| (3) |
2.4.3. Cetane number (CN)
It defines a fuel’s ignition quality under fixed conditions. Fuels with a higher cetane number contribute to a faster engine start-up and smoother combustion, as opposed to fuels with a lower one, which influences the combustion performance and thus results in much hydrocarbon (HC) and particulate matter (PM) emissions [43].
The CN was chosen as the surrogate design property to quantify the ignition efficiency, and it was determined according to the procedure outlined in ASTM D6890. In the regression model, the CN of a mixture was considered to be equal to the sum of the volume fraction weighted CNs of all the components [6, 33, 44] according to the following expression:
| (4) |
2.5. Surrogate formulation optimization
The program performed for the optimization of a surrogate composition was developed using the Excel Solver and the numerical method of the GRG to optimize the objective function used:
| (5) |
Psurri was estimated using appropriate mixing rules expressed in terms of the component’s mass or molar fractions xi to optimize according to the following two constraints:
- 𝛴xi = 1
- The calculated properties must respect international norm values as presented in Table 2, and a deviation was calculated to evaluate the accuracy of the estimated property of the optimized surrogate as follows:
where is the deviation of property P, Ptarg is the target property of diesel to emulate and Psurr is the property of the blend of the optimized surrogate estimated using mixing rules.(6)
The measured target properties of the obtained biodiesel, those of target fossil diesel and the blends considered were implemented in the developed program. Figure 2 shows the key steps of the algorithm used.
Main steps of the algorithm used in the surrogate optimization program [24].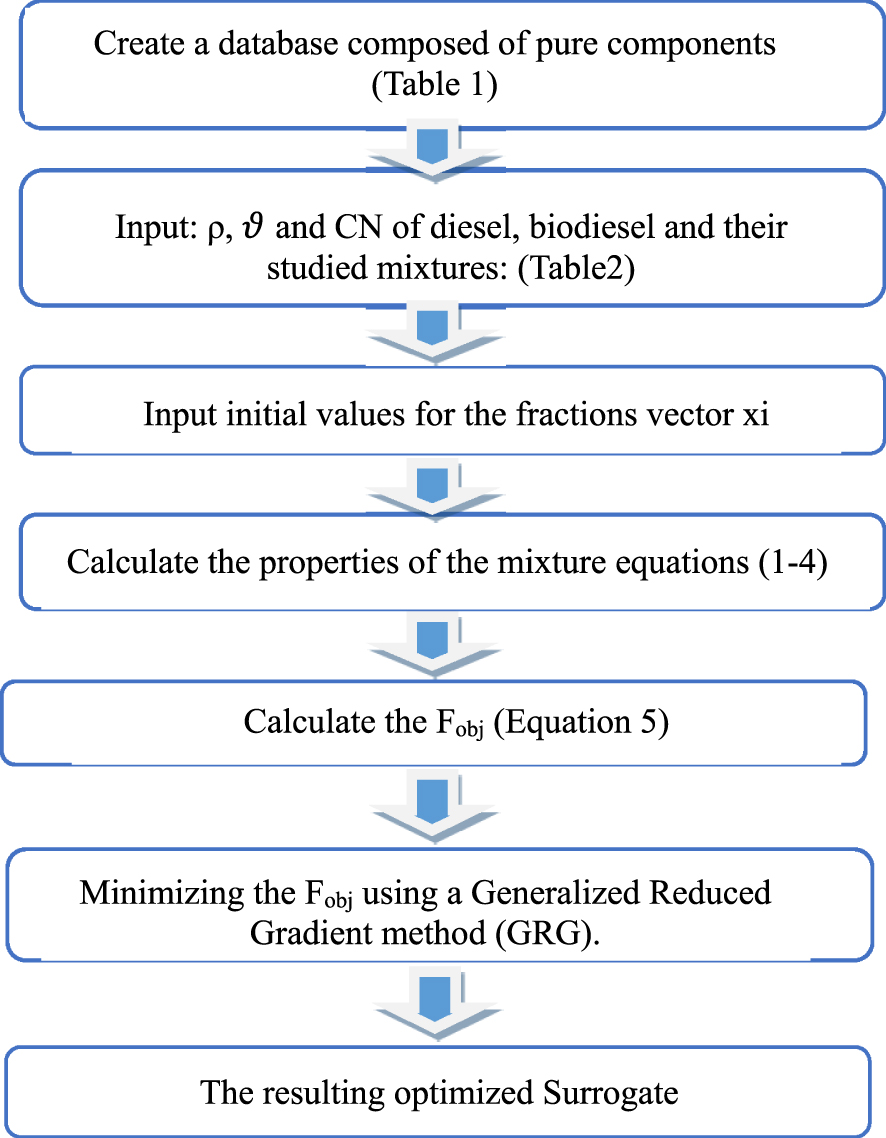
3. Results and discussion
3.1. Mixing rules validation
First of all, before using the mixing rules used in the adopted algorithm, the calculation methods used are validated by using three surrogates from the work of Mueller et al. [3], as presented in Table 3, and their measured mixture properties compared with the properties calculated with the mixing rules used in the present work. The results are presented in Figure 3 and show good agreement for density, viscosity and cetane number.
Composition in mole fraction of diesel surrogates optimized by Mueller et al. [3]
| Surr1 | Surr2 | Surr3 | |
|---|---|---|---|
| n-hexadecane | 27.8 | 0 | 2.7 |
| n-octadecane | 0 | 23.5 | 20.2 |
| n-eicosane | 0 | 0 | 0 |
| heptamethylnonane | 36.3 | 27.0 | 29.2 |
| 2-methylheptadecane | 0 | 0 | 0 |
| n-butylcyclohexane | 0 | 0 | 5.1 |
| 1,3,5-triisopropylcyclohexane | 0 | 0 | 0 |
| trans-decalin | 14.8 | 0 | 5.5 |
| perhydrophenanthrene | 0 | 0 | 0 |
| 1,2,4-trimethylbenzene | 0 | 12.5 | 7.5 |
| 1,3,5-triisopropylbenzene | 0 | 0 | 0 |
| tetralin | 0 | 20.9 | 15.4 |
| 1-methylnaphthalene | 21.1 | 16.1 | 14.4 |
Surrogate diesel properties: (a) density, (b) viscosity, (c) cetane number, as measured by Mueller et al. [3] and calculated using mixing rules used in the present work.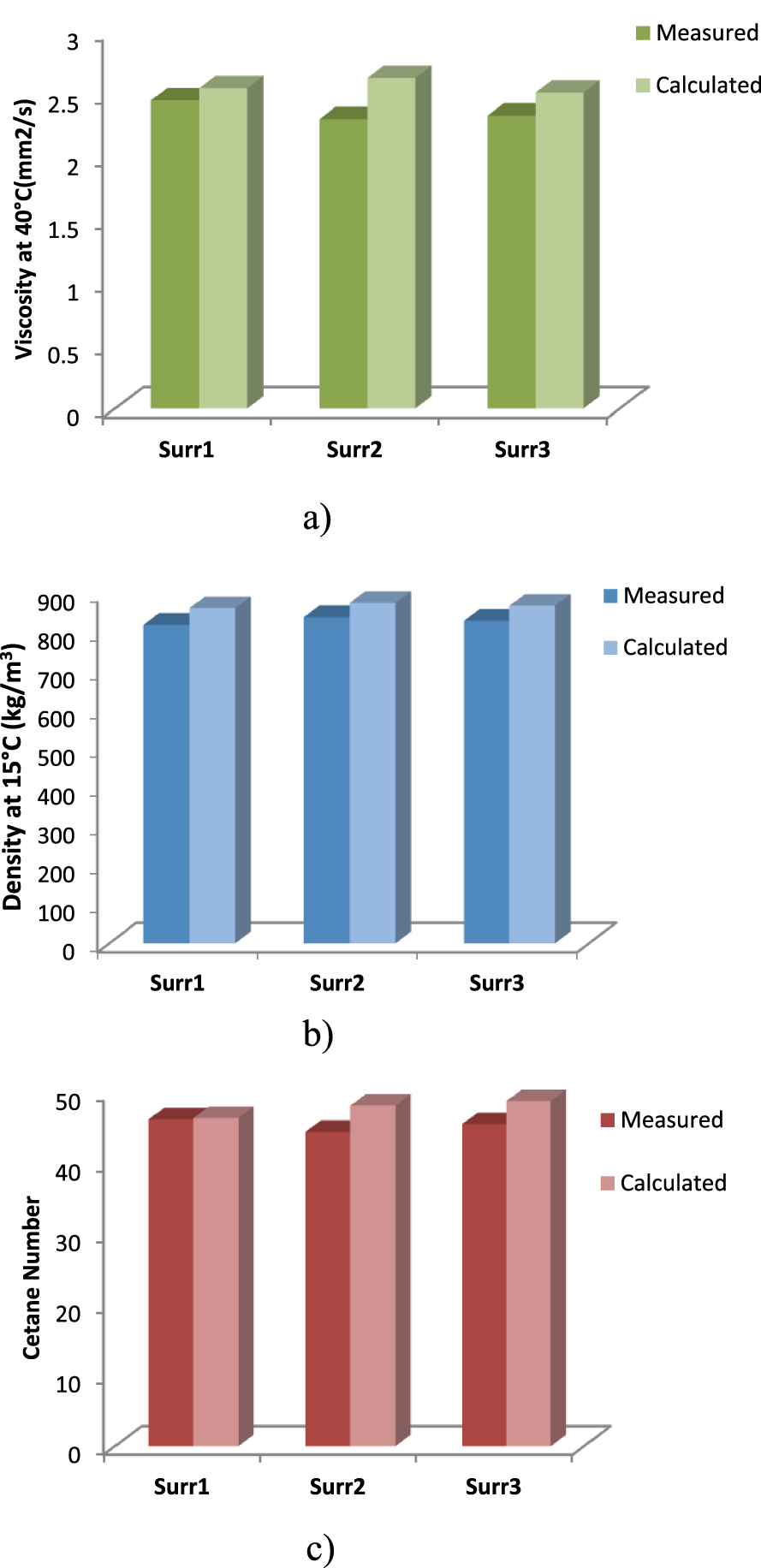
3.2. Optimized surrogate for target fossil diesel
Once validated using experimental results from literature, we use the mixing rules and the methodology summarized in Figure 1 to generate the optimized surrogates for each target fuel and target blends.
First, we started by optimizing a surrogate formulation for 100% fossil diesel, the composition obtained is shown in Table 4.
Optimized surrogate composition and target properties for fossil diesel
| Optimized formulation for a surrogate for 100% diesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| n-hexadecane | 0.0859 | |
| Isocetane | 0.5200 | |
| 1-methylnaphtalene | 0.157 | |
| n-eicosane | 0.236 | |
| Sum | 1.00 | |
| Target properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 855.26 | 0.024 |
| 𝜗 at 40 °C (mm2∕s) | 3.19 | 2.971 × 10−3 |
| CN | 55.14 | 2.544 × 10−3 |
Clearly the results show that the optimized surrogate is composed only of four components and reproduce with a good agreement the target properties of the fossil diesel: density, viscosity and the cetane number that are very close to those of the target fossil fuel, which enhances the capability of the alternative fuel to emulate the characteristics and performance of combustion engines, the atomization quality, the spray characteristics, and the combustion quality and also the ignition quality of the target diesel.
Concerning the nature of the surrogate components, Reiter et al. [21] proposed four alternative fuels to emulate fossil diesel properties, with eight to ten components and three components from the composition of our optimized surrogate were present in their surrogates: Isocetane, 1-methylnaphthalene and n-eicosane.
Mueller et al. [3] proposed four optimized diesel surrogate fuels using eight pure components with two compounds in common with our surrogate diesel composition: Isocetane and 1-methylnaphtalene.
Al-Esawi and Al Qubeissi [19] obtained surrogates for diesel fuel composed of between three and six hydrocarbons and n-hexadecane was present in all of them.
The surrogate obtained by Reiter et al. [21] with eight hydrocarbon composition had three components in common with our optimized formulation: the eicosane, the isocetane, and the n-hexadecane.
Wang and Chen [45], on the other hand, obtained an alternative diesel composed of only four pure hydrocarbons, namely: isocetane, 1,2,4-trimethylbenzene, n-hexadecane and trans-decalin.
So, the surrogate obtained for fossil diesel meets the objective of using small number of hydrocarbons with four components and emulates well the target properties.
3.3. Optimized surrogate for target biodiesel (B100)
The following results concern the formulation of the obtained optimized surrogate that emulates 100% biodiesel (B100). Table 5 shows the composition in mole fraction and the calculated properties of the surrogate obtained.
Optimized surrogate composition and properties for biodiesel
| Optimized formulation for 100% biodiesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| 1-methylnaphtalene | 0.56838121 | |
| Isocetane | 0.33258609 | |
| n-eicosane | 0.30088512 | |
| Sum | 1.20185252 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 888.5 | 1.26 × 10−4 |
| 𝜗 at 40 °C (mm2∕s) | 6.5 | 0.057 |
| CN | 52 | 0.013 |
For this case it can be seen that a surrogate composed of only three compounds can emulate the required properties of the biodiesel studied, and this result therefore makes it possible to achieve the objective of finding a surrogate with a reduced number of components which will facilitate kinetic models and reaction mechanisms, the study of emissions and engine performance.
However, we note that the sum of the fractions in this case, compared to the formulation of the fossil diesel, is greater than 1 and it was difficult to find an optimum for which the sum is equal to 1. This can be explained by the fact that it is difficult to imitate biodiesel, which is composed mainly of esters [46], by a hydrocarbon surrogate. Indeed El Esawy et al. and Reiter et al. [19, 21] had introduced esters in the palette to propose a formulation of surrogate of biodiesel and diesel–biodiesel blends.
To overcome the problem of a composition that exceeded 1 (Table 5), we performed further iterations with additional constraints to achieve the target properties with a composition equal to 1 (Table 6). This problem has been encountered with blends from a percentage of 50% biodiesel, hence the use of iterations for the B100, B80 and B50. The final results for the B100 are presented in Table 6.
Optimized surrogate composition and properties for biodiesel with constrain optimization
| Optimized formulation for 100% biodiesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| 1-methylnaphtalene | 0.4778008 | |
| Isocetane | 0.27218158 | |
| n-eicosane | 0.25001862 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 889 | 6.754 × 10−4 |
| 𝜗 at 40 °C (mm2∕s) | 3.75213712 | 0.455 |
| CN | 52 | 0.013 |
As presented in Table 6, we always get a surrogate with the same three compounds, with different composition and a sum of 1, but the target properties of the density and cetane number are obtained with better precision than the viscosity. This is explained by the fact that the target viscosity is higher than that of the constituents of the palette used. This difficulty to reproduce viscosity of target fuels with higher viscosity was encountered in several works [21]. Components of high kinematic viscosity have higher boiling points and have not been widely used as surrogates.
3.4. Optimized surrogates for diesel–biodiesel blends
Once the surrogates for fossil diesel and biodiesel have been obtained, formulations are optimized to emulate the properties of the blends of the two, as shown in Table 2.
Tables 7, 8 and 9 show the formulation of the optimized surrogates and their properties for the target fuel B5, B10 and B20 respectively. The results show the same constituents as before with similar fractions and a good representation of the properties of the two surrogates in comparison to the properties of target B5, B10 and B20.
In comparison with previous works on biodiesel blends, the number of components of the optimized surrogate is reduced compared to that obtained by Reiter et al. [21] for B20, which was composed of eight hydrocarbons (with other fatty acid methyl esters). Also, the optimized surrogate obtained by Reiter et al. [21] for a B20 contained, among other components, isocetane, 1-methylnaphtalene, and n-eicosane with different mole fractions.
Optimized surrogate composition and properties for 5% biodiesel (B5)
| Optimized formulation for 5% biodiesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| 1-methylnaphtalene | 0.11444286 | |
| Isocetane | 0.58392385 | |
| n-eicosane | 0.30163329 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 838 | 5.766 × 10−4 |
| 𝜗 at 40 °C (mm2∕s) | 3.30004934 | 0.013 |
| CN | 56 | 0.022 |
Optimized surrogate composition and properties for B10
| Optimized formulation for 10% biodiesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| 1-methylnaphtalene | 0.16249074 | |
| Isocetane | 0.54221574 | |
| n-eicosane | 0.29529353 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 858.908468 | 0.022 |
| 𝜗 at 40 °C (mm2∕s) | 3.29999999 | 0.056 |
| CN | 55 | 6.723 × 10−3 |
Optimized surrogate composition and properties for B20
| Optimized formulation for 20% biodiesel | ||
|---|---|---|
| Components | Xi (molar fraction) | |
| 1-methylnaphtalene | 0.17931062 | |
| Isocetane | 0.51159544 | |
| n-eicosane | 0.30909394 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 860.962739 | 0.019 |
| 𝜗 at 40 °C (mm2∕s) | 3.35608557 | 0.118 |
| CN | 56.8973601 | 0.049 |
On the other hand, the results shown in Table 10 and Table 11 present the optimized surrogates for B50 and B80 respectively. The same three pure components are obtained with different fraction compositions and with good agreement of density and cetane number as target properties but with a large deviation for viscosity. It is also noticed that this deviation increases with the increase in percentage of biodiesel in the blend.
Optimized surrogate composition and properties for 50% biodiesel (B50) (using constraints)
| Optimized formulation for 50% biodiesel | ||
|---|---|---|
| Components | Xi | |
| 1-methylnaphtalene | 0.17020343 | |
| Isocetane | 0.54938129 | |
| n-eicosane | 0.28041528 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 861 | 1.479 × 10−4 |
| 𝜗 at 40 °C (mm2∕s) | 3.27647193 | 0.322 |
| CN | 53.2 | 2.165 × 10−4 |
Optimized surrogate composition and properties for 80% biodiesel (B80) (using constraints)
| Optimized formulation for 80% biodiesel | ||
|---|---|---|
| Components | Xi | |
| 1-méthylnaphtalène | 0.35287043 | |
| Isocetane | 0.38859399 | |
| n-eicosane | 0.25853514 | |
| Sum | 1 | |
| Properties | Property | Deviation |
| 𝜌 at 15 °C (kg∕m3) | 877 | 2.058 × 10−4 |
| 𝜗 at 40 °C (mm2∕s) | 3.54880295 | 0.41 |
| CN | 52 | 3.751 × 10−3 |
To summarize, Figure 4 represents the composition of all the diesel–biodiesel mixtures obtained with the optimized compositions. All surrogates are composed of three components namely: 1-methylnaphthalene, isocetane and n-eicosane. The results show similar fractions in n-eicosane and differences depending on the percentage of biodiesel. It is also noted that the methylnaphthalene fraction increases with the increase in the percentage of biodiesel in the mixture.
Yuanqi Bai [47] developed a skeletal mechanism for tri-component diesel surrogate fuel: N-hexadecane/iso-cetane/1-methylnaphthalene, this mixture was found to be a good diesel surrogate fuel. The skeletal mechanism was validated with various fundamental experiments using several Homogeneous Charge Compression Ignition (HCCI) engine conditions. In comparison with our results, we obtained the n-eicosane instead of n-hexadecane and as shown in the palette (Table 1), the n-hexadecane and n-eicosane as alkanes have similar properties with high cetane number and moderate viscosity and density. The three components obtained in the present work were validated by several previous works as components of optimized surrogates [6, 28, 32, 21, 33, 36].
Composition of the all surrogates obtained for the diesel–biodiesel surrogates studied.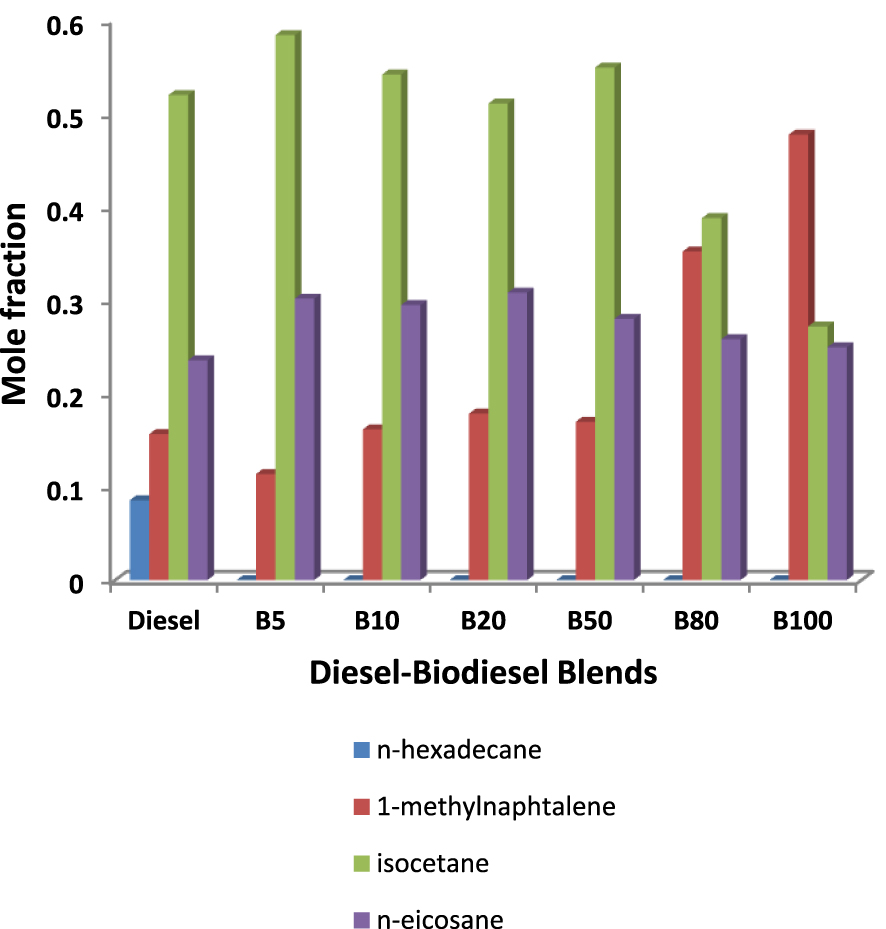
Therefore, with only three and the same constituents as surrogates for diesel–biodiesel blends, this result enables a better study and control of engine efficiency and the quality of emissions. It contributes to facilitate the kinetic model development for the target fossil diesel and its blends with biodiesel, which will enhance biodiesel use as biofuel. However, this surrogate can accurately mimic target properties down to less than 50% of biodiesel, at which point viscosity estimation becomes less accurate.
4. Conclusion
The findings of this study showed that it is possible to optimize alternative fuel formulations that emulate the required properties of a target fossil fuel, as well as those of several diesel–biodiesel blends, considering a large range in molar biodiesel percentages, as 5%, 10%, 20%, 50% and 80%.
The objective of minimizing the number of components in the optimized surrogates was achieved using the same three components for all the target fuels studied: Isocetane, 1-methylnaphthalene and n-eicosane. This result allows managing increased complexity when developing kinetic models.
The optimized formulations had properties that were similar to the targeted fuels and the mixtures of diesel and biodiesel studied, especially for density and cetane number. However, the viscosity estimation was less accurate when the percentage of biodiesel in the target blend was higher. That is why the surrogates obtained are recommended for blends with less than 50% in biodiesel.
Also, the biodiesel percentages in the studied blends affect the composition of the proposed surrogates and also the accuracy of its target property estimation.
Future work will focus on the preparation and analysis of the surrogates obtained on a laboratory scale, as well as an enhancement of the palette and its enrichment with the addition of esters and additional target properties. Furthermore, the program developed is adaptable, allowing for the expansion of the database and target properties for the same and other fuels.




 CC-BY 4.0
CC-BY 4.0