1. Introduction
Layer-by-layer (LbL) deposition of polyelectrolytes on inorganic supports imposed as a versatile and efficient method to obtain composite materials with active sites, which renders their possible use in many applications [1]. The deposition of polyelectrolytes is achieved by alternating immersion of the support material in aqueous solutions of complementary polyelectrolytes and can be performed by using different methods, such as dipping, spin-coating, spray-assisted deposition, or electrodeposition [2, 3, 4]. The assembly of the polyelectrolyte multilayers (PEMs) is the result of the electrostatic interactions, covalent bonds, hydrogen bonds, van der Waals forces, or hydrophobic interactions [5, 6]. LbL deposition of polyelectrolytes offers the important advantages of an easy fabrication procedure and the possibility of using different types of building-blocks: natural and synthetic polyelectrolytes [7, 8, 9], low molecular weight compounds [10], proteins [11, 12], nanoparticles [13] etc. It is also worth mentioning that the use of polyelectrolytes in the fabrication of multilayered composite materials allows the control of properties of the final material by changing the deposition conditions [2, 14], the LbL assembly of polyelectrolytes being dependent by a high number of factors such as: pH [15, 16, 17], ionic strength [18, 19, 20], temperature [21], as well as concentration [22], molecular mass [23, 24] or the charge density [25] of the polyelectrolytes used. The stability of the multilayered materials can be enhanced by cross-linking the polyelectrolyte chains [26]. The most used cross-linking methods are chemical treatment with reactive bifunctional organic compounds such as glutaraldehyde [27], epichlorohydrin [28], genipine [29], and thermal treatment [30]. The use of LbL deposition of polyelectrolytes for water/wastewater purification gained a lot of interest from the scientific community in recent years. As shown by many authors, the use of PEM (polyelectrolyte multilayer)-based materials as sorbents for emerging contaminants offers important advantages: enhanced stability of the sorbent, the possibility to reuse the material for several retention cycles, the improvement of sorption kinetics and better physical–chemical characteristics. Between the pollutants that were separated from water by using PEMs, can be mentioned drugs, dyes [31, 32], or metal ions [33, 34].
The increasing demand and use of medication, personal care products, disinfection, and cleaning agents lead to a very serious problem for human society: the reduction of drinking water supplies due to pollution. Compounds such as antibiotics, dyes, cosmetics, pharmaceutical products, metal ions, and pesticides are usually found in water and pose serious threats to human health [35]. The occurrence of new pollutants in the water supply, considered as emerging contaminants by environmental organizations and authorities, imposed the development of new techniques for wastewater treatment since traditional methods, such as coagulation–flocculation, oxidation, activated sludge treatment and filtration are insufficient to remove these pollutants [36, 37, 38, 39]. The increasing number of emerging contaminants found in water leads to the necessity of developing new materials and techniques for wastewater treatment. Sorption of micro-pollutants on composite materials with active sites is one of the water/wastewater treatment techniques of major interest for the scientific community, as demonstrated by the large number of materials fabricated for the separation of these pollutants, using building-blocks such as carbonaceous materials [40], polyelectrolytes [33], graphene [41] or magnetic compounds [42]. Between the composite materials studied for charged pollutants sorption, the core-shells composites obtained by LbL deposition of polyelectrolytes are distinguished as materials with high sorption capacity due to their large number of functional groups, and their flexible shell polymeric structure with responsiveness to external stimuli.
This study was conducted following the stages: (1) polycation/polyanion LbL deposition; (2) selective cross-linking of polycations layers; (3) polyanion extraction from the cross-linked multilayer and (4) sorption/desorption of Gallic Acid (GaAc) onto/from composites. Thus, first we obtained composite materials with a high number of active sites in a stable shell by LbL deposition of poly(ethyleneimine) with branched (PEIB) or linear (PEIL) structure, and poly(acrylic acid) (PAA) on Daisogel microparticles, followed by chemical stabilization by crosslinking and the subsequent extraction of PAA chains from the composite shell. The next step aimed to assess the feasibility of GaAc separation from simulated wastewater using LbL core/shell composite materials. GaAc, a trihydroxybenzoic acid, was used in this study as a model anionic pollutant due to the structural resemblance with humic acids which contain in their structure lots of phenolic and carboxylic groups [43, 44]. Even if they are of natural origin and are used as fertilizers, humic acids are considered water pollutants after exceeding certain values [45]. The composite microparticles were subsequently used for the sorption of GaAc from simulated wastewater in batch and column studies. To the best of our knowledge, this is the first study proposing the sorption of GaAc from wastewater by using LbL polyelectrolyte-based composite materials.
2. Materials and methods
2.1. Materials
Gallic acid (GaAc), PEIL (Mn = 1800 g/mol), PEIB (Mn = 10,000 g/mol), and glutaraldehyde (GA) were purchased from Sigma-Aldrich (Germany). PAA, Mv = 10,000 g/mol, was synthesized in the laboratories of “Petru Poni” Institute of Macromolecular Chemistry. All compounds were used as aqueous solutions prepared with deionized water (conductivity 0.055 μS/cm). The silica microparticles used as a template were Daisogel type (Daiso Co., Japan), with an average particle diameter of 40–60 μm, 100 nm pores, and pore volume of 0.95 mL/g.
2.2. Synthesis of Daisogel/(PEIL)n and Daisogel (PEIB)n
The construction of polyelectrolyte multilayers onto Daisogel microparticles was achieved by alternating deposition of PEIL or PEIB and PAA from 10−2 M (structural units) aqueous solutions (Scheme 1).
Daisogel/(PEI&PAA)n core/shell composite microparticles synthesis using LbL strategy.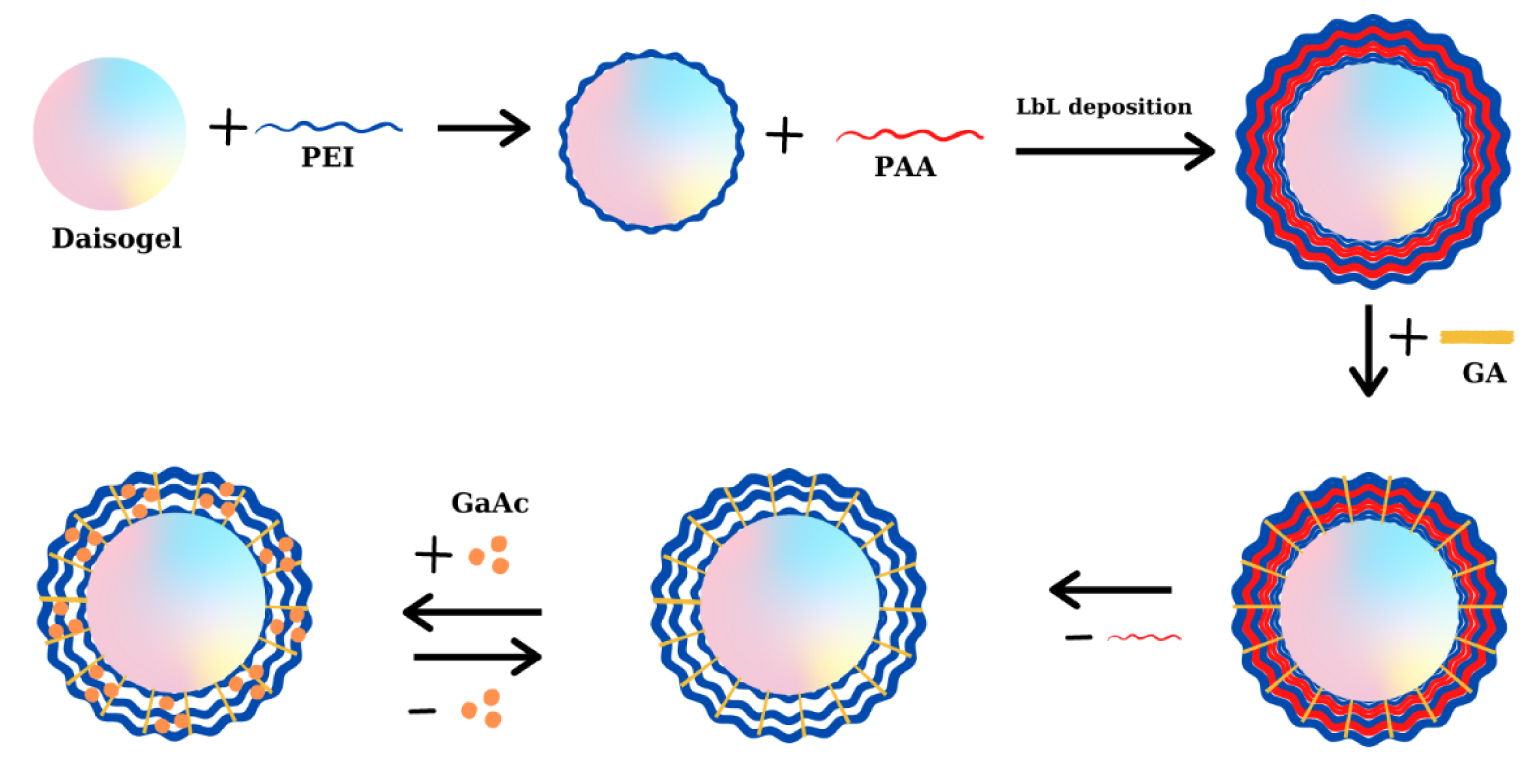
Thus, first we obtained composite materials with a high number of active sites by LbL deposition of poly(ethyleneimine) (PEIB or PEIL) and PAA on Daisogel microparticles, followed by chemical stabilization and the subsequent extraction of PAA chains from the composite shell. Practically, 4 g of Daisogel microparticles were immersed in 200 mL PEIL or PEIB solution and gently mixed. After one hour, the microparticles were decanted, the aqueous solution was removed and the microparticles were washed 3 times with distilled water. The deposition of the PAA layers was achieved by suspending the Daisogel/PEIB or Daisogel/PEIL microparticles in 200 mL solution for one hour, followed by 3 washing steps. The deposition was repeated until multilayers with 2.5 and 4.5 double-layers were fabricated (namely 2 or 4 polycation–polyanion double layers followed by a half of double layer, PEIL and PEIB being always the last deposited layer), denoted as: Daisogel/ (PEIL&PAA)2.5, Daisogel/(PEIL&PAA)4.5, Daisogel/ (PEIB&PAA)2.5, Daisogel/(PEIB&PAA)4.5. In order to achieve maximum stability of the deposited shell, the composite microparticles were treated with a controlled volume of GA solution 2.5% which ensured a 1:10 ratio between the carbonyl functional groups of GA and the amino groups of PEI. To increase the flexibility of the cross-linked multilayers deposited on the Daisogel microparticles, the PAA was extracted using NaOH, 1 M. Similarly, the weakly bound PEI chains inside the cross-linked multilayer was removed using HCl, 1 M. After cross-linking and extraction of non-crosslinked chains, the core/shell composite have been denoted as Daisogel/(PEIL)3; Daisogel/(PEIL)5; Daisogel/(PEIB)3 and Daisogel/(PEIB)5. Before each experiment the microparticles were dried at 40 °C in a vacuum oven.
The quantity of organic material deposited on the microparticles was determined indirectly by polyelectrolyte titrations using a PCD 03 particle charge detector (Mütek GmbH, Germany). The polyelectrolyte sorbed amount at equilibrium (qPE, mg/g) was calculated using (1):
| (1) |
The potentiometric titrations were used to determine the point of zero charge (pzc), defined as the pH value where the measured potential is 0 mV. Potentiometric titrations were performed with the same particle charge detector (PCD 03, Mütek, Germany). The variation of pH between pH 3 and 10 was achieved using 0.1 and 0.01 M solutions of NaOH and HCl, respectively. The potential measurements were carried out with ∼10 mg solid composite suspended in 15 mL Millipore water at room temperature.
FTIR-ATR spectra were registered using an IRTracer-100 FT-IR spectrometer (Shimadzu Corporation, Japan) equipped with an ATR module, GladeATR (PIKE Technologies, USA).
The morphological analysis of the microparticles was performed by SEM using the Verios G4 UC scanning electron microscope (Thermo Fisher Scientific, Czech Republic) equipped with an EDAX analyzer (Octane Elect Super SDD detector, USA) and backscatter detector. To reduce the electrostatic charge and increase the conductivity of the samples, the microparticles were coated with a 10 nm platinum layer using a Leica EM ACE200 metallizer.
2.3. Batch and column sorption experiments of GaAc onto Daisogel composite microparticles
The batch sorption of GaAc onto the Daisogel/ (PEIL&PAA)n, Daisogel/(PEIB&PAA)n, Daisogel/ (PEIL)n and Daisogel/(PEIB)n followed two directions: (i) the quantification of the sorption capacity by the sorption isotherms and (ii) the sorption kinetics.
The quantity of GaAc retained onto each type of composite material was determined based on UV–Vis measurements at 𝜆 = 272 nm, using a UV–Vis spectrophotometer (UV-VIS SPEKOL 1300, Analytik Jena, Germany). A calibration curve in the range 5–20 μg/mLwas constructed. The GaAc sorbed amount at equilibrium or in each collected effluent fraction (qe, mg/g) was determined using (2):
| (2) |
The sorption isotherms were studied in the GaAc concentration range of 6.8–340 mg/L. The sorption kinetics were studied for the Daisogel/(PEIB)3, Daisogel/(PEIB)5, Daisogel/(PEIL)3, Daisogel/(PEIL)5 using 100 mg/L solution GaAc.
The GaAc dynamic sorption properties of Daisogel/(PEIB)3, Daisogel/(PEIB)5, Daisogel/ (PEIL)3 and Daisogel/(PEIL)5 composite microparticles were followed in a column study, using a fixed-bed OMNIFIT glass column, at room temperature, where ∼0.34 mg composite was uniformly packed inside. The GaAc molecule, partially negative charged in aqueous solution due to the weak carboxylic group, can interact through electrostatic interactions and H-bonding with the weak amino groups of PEIL or PEIB, which are partially positive charged at the pH of the GaAc solution. During all column experiments, a constant flow of 1 mL/min has been ensured by a Shenchen peristaltic pump without pH modification of GaAc influent. The GaAc sorbed amount has been calculated with the same formula as in the batch experiments (2), where initial and equilibrium concentration were replaced by influent and effluent concentrations. The GaAc influent concentration, 0.5 mM, has been kept constant during each experiment.
The exhausted column has been regenerated by 20 mL (13× bed volume) NaOH 0.1 M to remove GaAc from the composite, followed by 20 mL distilled water washing. Using NaOH solution with pH 14, the GaAc can be easily desorbed from core/shell composite surface due to the full ionization of carboxylic group which is more soluble in aqueous environment. Moreover, the electrostatic interactions between PEIB and GaAc are destroyed by deprotonation of ammonium groups and ionic strength of the environment. The composite reusability was tested only with the best performance composite (Daisogel/(PEIB)5) in 10 sorption cycles. The break-through time (tb, min), the exhaustion time (te, min) and the GaAc sorbed amount have been calculated from the breakthrough curves for each composite sample. The Thomas and Yoon–Nelson models have been applied to all breakthrough curves to determine the specific parameters of dynamic sorptions.
3. Results and discussions
The cross-linking degree is very important for the subsequent behaviour of core/shell particles in sorption studies; the strong cross-linked multilayers will not be able to sorb high amounts of pollutants due to their low number of available functional groups, while the weak cross-linked multilayers will be less stable in acidic/basic media. From our previous studies [36] we demonstrated that the optimum cross-linking of polycation with average molar mass deposited onto 50 μm silica particles is around 10:1 amino:aldehyde groups. Thus, the quantity of organic part deposited on the Daisogel microparticles was determined by polyelectrolyte titrations of the supernatant after each deposition step (Figure 1).
Cumulative PEI and PAA deposited amount onto Daisogel microparticles determined from polyelectrolyte titrations.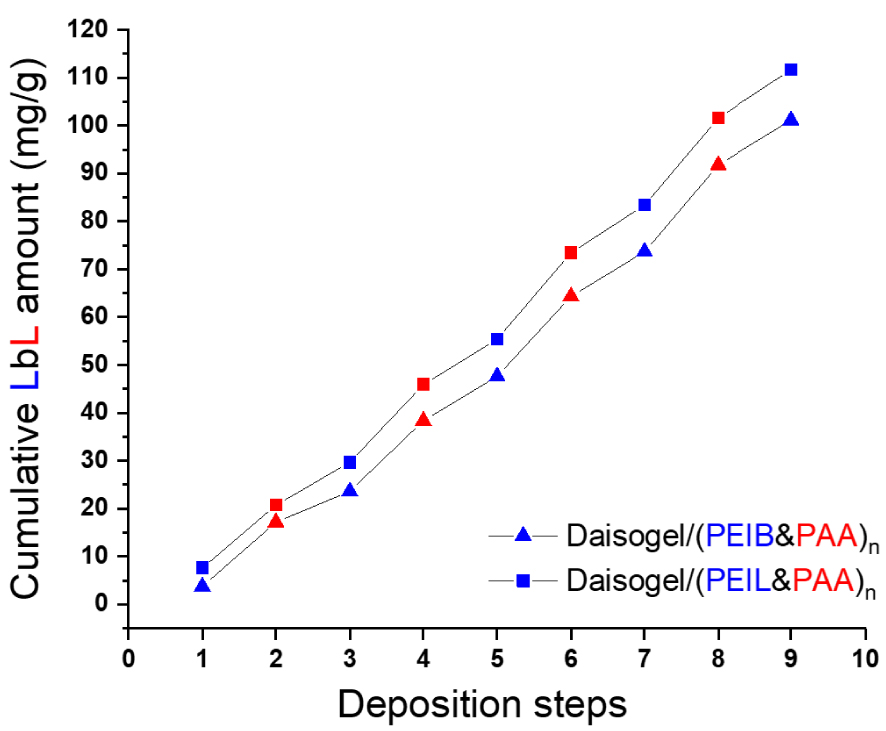
The cumulative amount of polyelectrolytes deposited was 101 mg/g for the composite microparticles fabricated with PEIB and 111 mg/g for the composite microparticles fabricated with PEIL (Figure 1). The PEIL chains, with lower molar mass than PEIB, are sorbed in higher amount onto porous Daisogel microparticles, since the smaller coils are more versatile and therefore, able to occupy the inner surface of 100 nm pores, as compared to the bigger PEIB coils. Nevertheless, only a small difference between PEIL and PEIB chains sorption during multilayer formation could be observed in Figure 1.
After the extraction of PAA from cross-linked PEI multilayers, only the polycation amount remains onto solid surface. Thus, 1 g Daisogel/(PEIB or PEIL)5 contained ∼38 mg PEIB and ∼46 mg PEIL, respectively. The extraction of PAA chains in strong basic medium from cross-linked shell has been investigated by potentiometric titration of Daisogel/(PEIL&PAA)4.5 and Daisogel/(PEIB&PAA)4.5 microparticles before and after extraction in NaOH aqueous solution (Figure 2).
Potentiometric titration of Daisogel, PEI, PAA and composite core/shell microparticles before and after PAA extraction from cross-linked multilayer.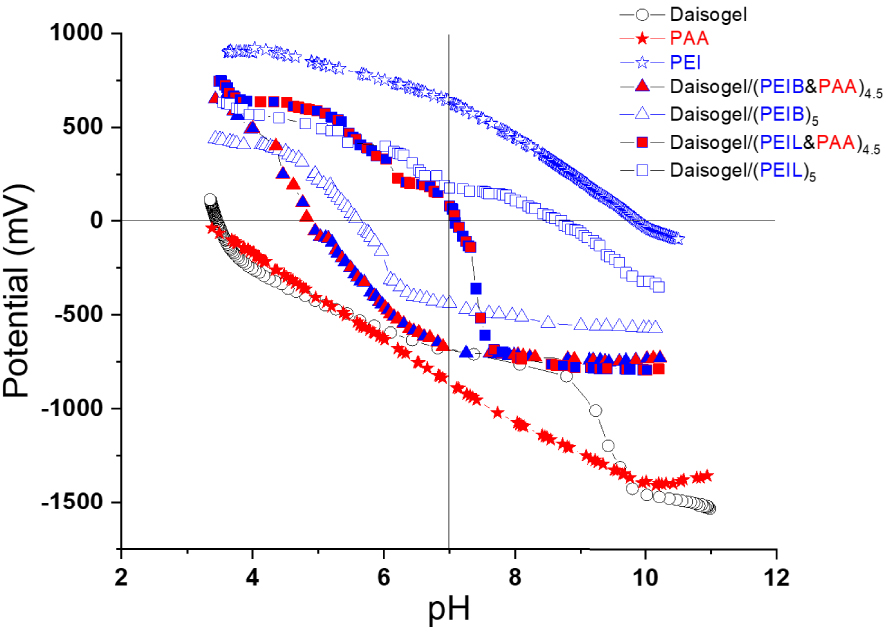
For pH < 10.5 all amino groups of polycation chains are in a protonated form, while carboxylic groups of PAA and silanol groups of Daisogel are negatively charged over a wide range of pH (pH > 3.0). Therefore, the pzc of Daisogel core/shell composites reflect the balance between all surface charges contributions. From Figure 2 it can be observed that pzc of each type of composite shifted to the right after the polyanion extraction from the shell. From these shifted pzc values, from 4.8 to 5.7 for Daisogel/(PEIB)5 and from 7.1 to 8.9 for Daisogel/(PEIL)5 we can observe that PAA chains have been extracted more easily from cross-linked (PEIL)5 multilayers than the PAA chains from (PEIB)5 multilayers, due to the linear structure and low molar mass of PEIL chains which allows the extraction. Also, during the cross-linking step of the composites with GA more imine cross-links have been formed between PEIB layers which makes the PAA chains difficult to escape from the cross-linked shell in the extraction step. Thus, after the extraction of PAA layers the ratio between positive and negative charges increased in both cases favouring the subsequent interaction of the composite surface with anionic species, such as GaAc.
The SEM images confirmed the spherical shape of all modified microparticles, demonstrating that the dry organic shell of each composite had an insignificant influence on surface morphology at this micrometric scale (Figure 3).
SEM micrographs and EDAX spectra of Daisogel/(PEIB/PAA)4.5 before (A) and after PAA extraction from cross-linked multilayer (B).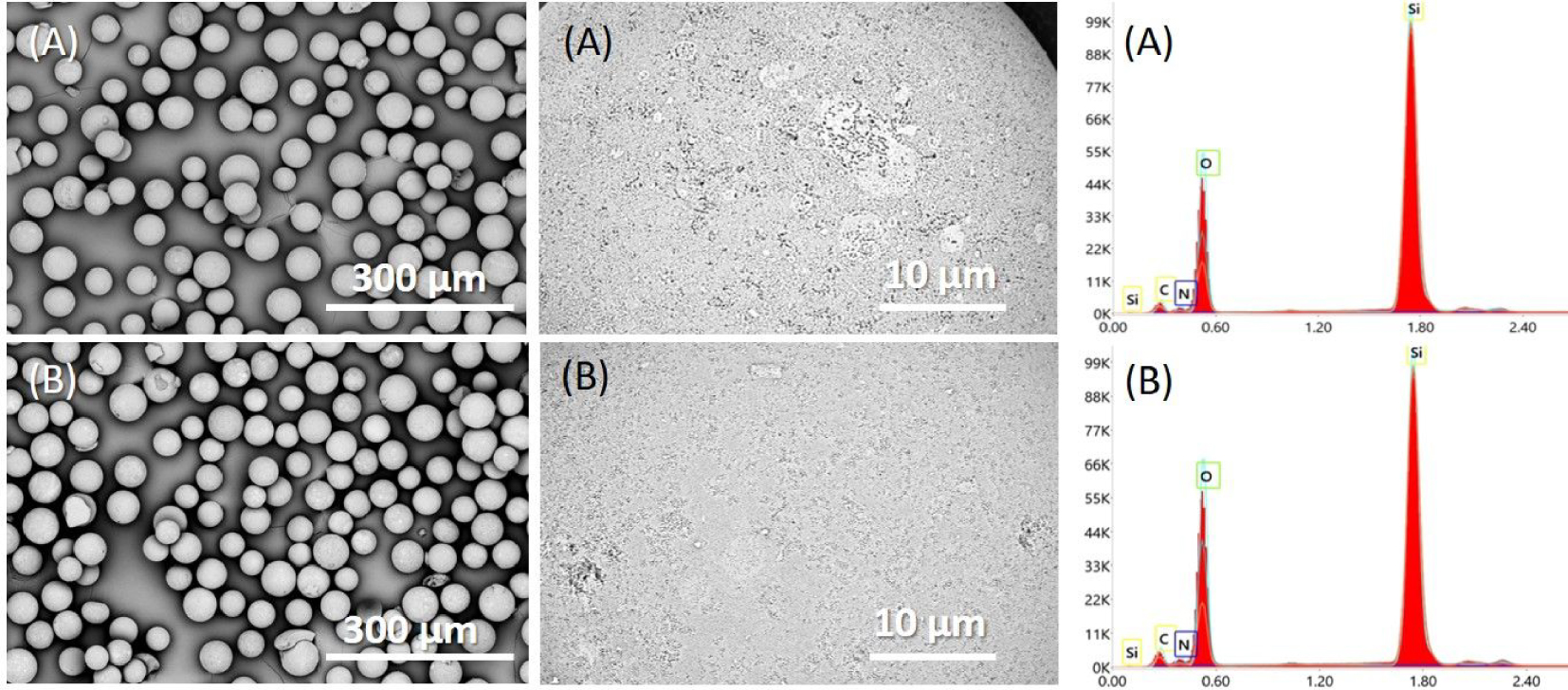
The deposition of PEIL, PEIB, and PAA onto Daisogel surface, and the GA cross-linking introduced considerable amounts of Nitrogen and Carbon onto SiO2 surface. To obtain information about the balance between polycation and polyanion chains inside the shell of the composite, before and after extraction, all composites were characterized by EDAX (Figure 3). In the EDAX spectra we can assume that C peak appeared from all polyelectrolyte chains, while N peak has been generated only by PEI chains. The specific signal for Daisogel substrate was attributed to the Si atoms from SiO2 core. Therefore, the amount of organic shell deposited onto SiO2 surface has been estimated by the N/Si and C/Si atomic ratios, while the balance between polycation and polyanion chains by the N/C atomic ratio (Figure 4).
Atomic ratios determined from EDAX spectra of Daisogel/(PEIB&PAA)4.5 before and after PAA extraction from cross-linked shell.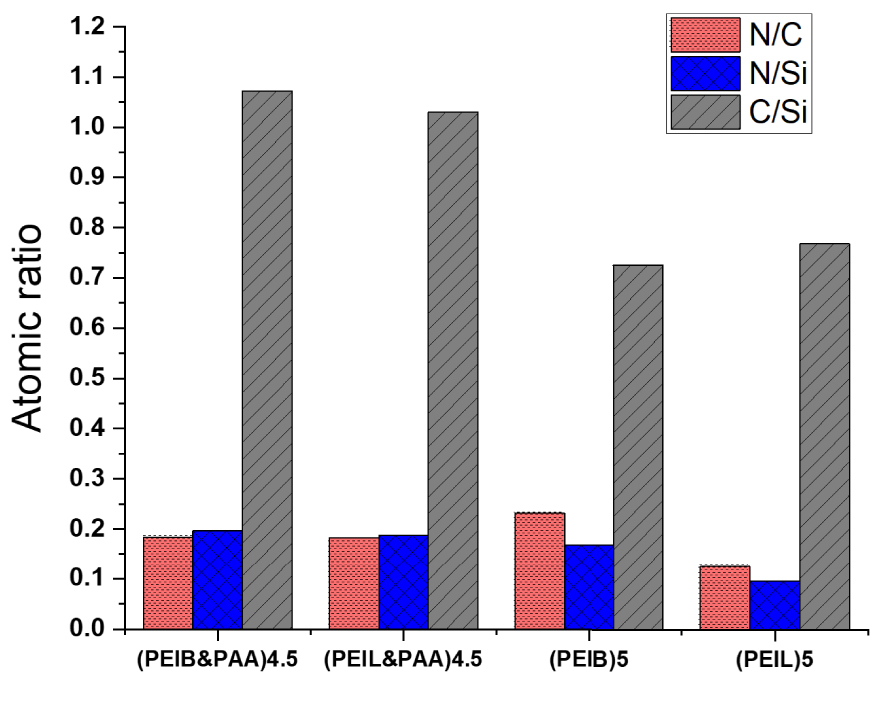
As shown in Figure 4, after the PAA extraction in basic media, the organic content decreased for both types of composites, Daisogel/(PEIB)5 and Daisogel/(PEIL)5, due to the PAA loss. Instead, the N/C atomic ratio increased for Daisogel/(PEIB)5 showing an enrichment of the cross-linked organic shell in PEIB chains. For Daisogel/(PEIL)5 composite particles, even if the N/C is higher than N/Si, at the same time, N/C is lower than the initial one, before the extraction. This fact has been attributed to the loss of PEIL together with PAA chains in the extraction/washing steps. This fact could be explained by the lower cross-linking density of the multilayer in the case of PEIL chains used in the fabrication of Daisogel/(PEIL)5. The higher PEIL deposited amount, determined from polyelectrolyte titrations, is more unstable onto the composite surface than PEIB amount, confirmed by EDA X method. Thus, the Daisogel/(PEIB)5 core/shell composite contains a higher and more stable amount of organic shell compared with Daisogel/(PEIL)5.
FTIR-ATR spectra recorded for the composite microparticles evidenced the LbL deposition of the polyelectrolytes on the Daisogel substrate and the successful extraction of the PAA chains from the cross-linked shell. Figure 5 presents the FTIR-ATR spectra of bare Daisogel, Daisogel/ (PEIB&PAA)4.5, cross-linked Daisogel/(PEIB&PAA)4.5, and Daisogel/(PEIB)5. The silica substrate is highlighted by the absorption bands located at 457 cm−1 and 1065 cm−1 (deformation and stretching vibrations of the Si–O–Si bonds in the Daisogel structure) and at 802 cm−1 (rocking vibrations of the Si–O bond). The infrared absorption bands corresponding to the substrate are also evidenced in the obtained composite materials, slightly shifted, showing that the substrate has not been affected by the deposition.
FT-IR spectra of Daisogel and Daisogel core/shell composite microparticles before and after the extraction of PAA from cross-linked shell.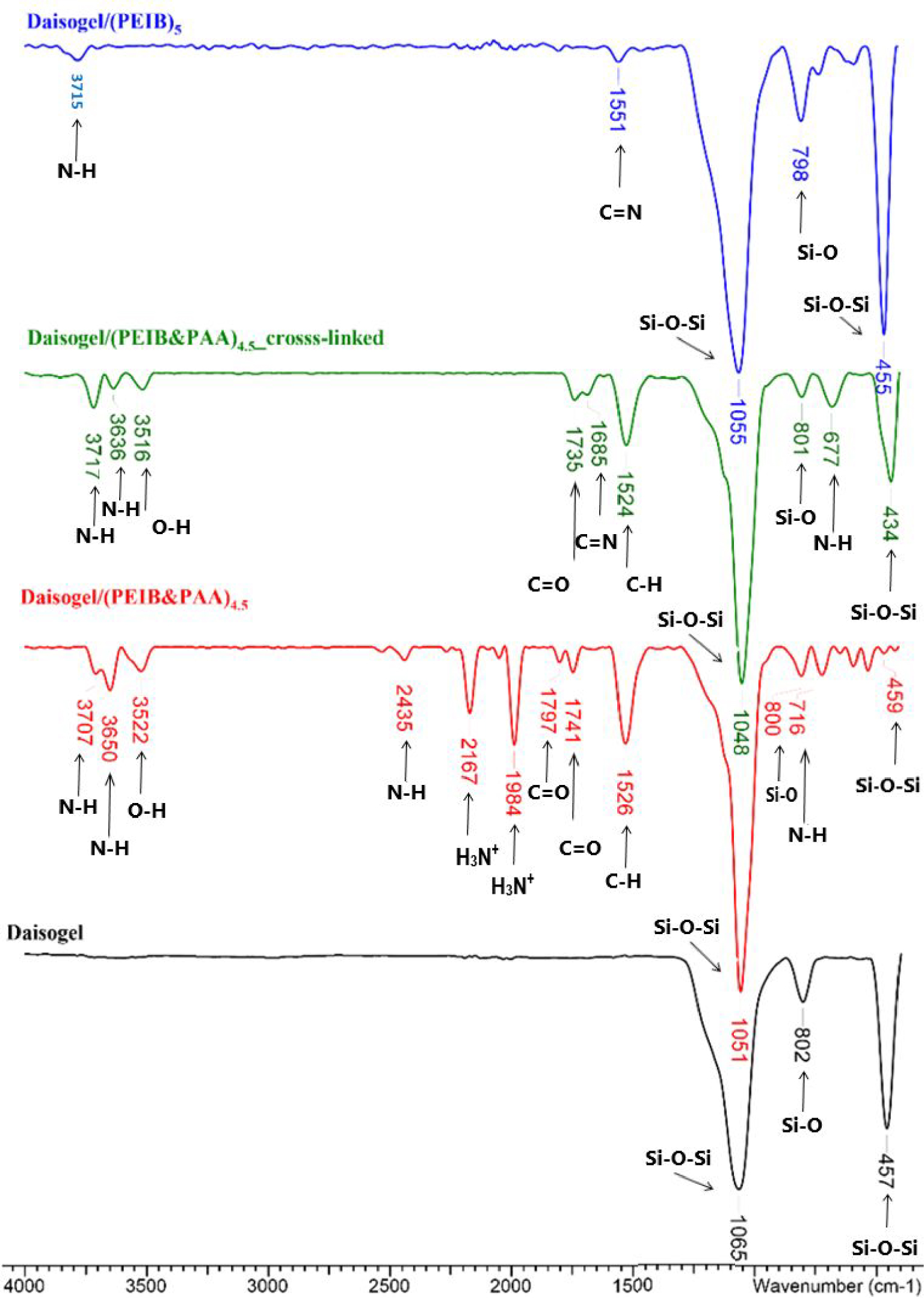
The deposition of PEI and PAA on the support is highlighted by the appearance of new bands. Thus, in the FTIR spectra of the composite materials, the characteristic bands of PEI are located at 677–716 cm−1 (out-of-plane bending vibration of N–H bond), 1524–1526 cm−1 (deformation vibrations of the CH bond of amines), 1984 and 2167 cm−1 (corresponding to the ionized amine groups, H3N+), 2435 cm−1 (asymmetrical stretching vibration of N–H bond), and 3636–3650 and 3707–3717 cm−1 corresponding to the NH bond. The absorption bands assigned to PAA (before extraction, represented by the red spectra) are located at ∼1735–1740 cm−1 and 1797 cm−1 (stretching vibrations of the C=O bond of the carboxylic group) and to ∼3516–3522 cm−1 (stretching vibrations of the OH bond in the carboxylic group). In the case of the cross-linked composites, an additional absorption band located at 1551 cm−1 and 1685 cm−1 appears, which can be attributed to the imine bond formed by cross-linking PEI chains. Their intensity is low, the quantity of cross-linker being small as compared to the amount of organic material deposited on the support.
To evaluate the capacity of the cross-linked Daisogel/(PEIB&PAA)n−0.5 and Daisogel/ (PEIL&PAA)n−0.5 composite microparticles in the sorption of GaAc, a series of batch experiments have been carried out on extracted and non-extracted samples (Figure 6).
The GaAc batch sorbed amount, qe, as a function of the number of polyelectrolyte layers deposited onto Daisogel microparticles before (full symbols) and after PAA extraction from cross-linked shell (empty symbols) (Ci of GaAc was 100 mg/L).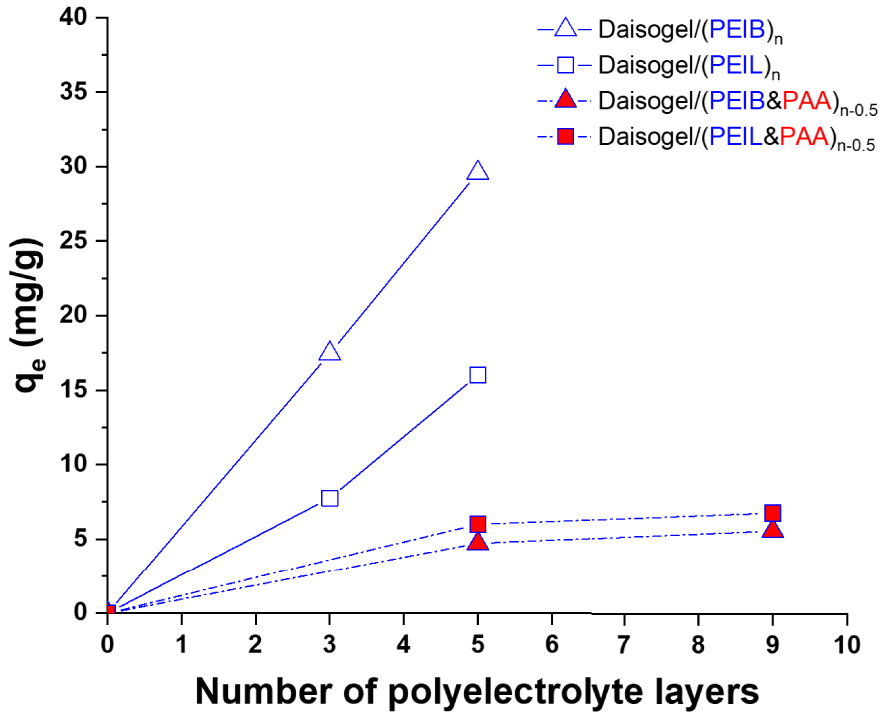
The quantity of GAc sorbed on/into each composite microparticles was assessed by using the UV–Vis spectrophotometric method, as an indirect method. The non-extracted microparticles (full symbols) retained lower quantities of GaAc as a result of three possible factors: (1) a large number of amino groups on the polycation chains are already compensated by the PAA carboxylic groups inside the organic shell; (2) due to the electrostatic repulsions between the carboxylic groups of PAA and carboxylic groups of GaAc, and (3) due to the packing density of the multilayer chains. Thus, in the case of the non-extracted multilayers, it is expected that GA will be adsorbed only on the outer surface of the composite shell and not inside the shell, this fact being demonstrated by the independence of GaAc sorption values on the number of deposited layers which form the shell. As it can be observed from Figure 6, the best results of GaAc sorption were obtained with the extracted microparticles due to the lack of PAA chains in the cross-linked shell. In this case, the sorbed amount of GaAc is much higher compared with non-extracted materials and increased linearly with the number of polycation layers. The highest GaAc sorbed amount, recorded for Daisogel/(PEIB)5, ∼30 mg/g, could be explained by the higher stability of the PEIB shell during PAA extraction step and by the higher number of accessible amino groups on the organic shell, compared with PEIL shell. Following these results the sorption isotherms and kinetics were studied (Figure 7).
Dependence of GaAc sorbed amount on equilibrium concentration (a) and time (Ci,GaAc = 100 mg/L) (b). The experiments were performed in duplicate on 25 mg composite at room temperature.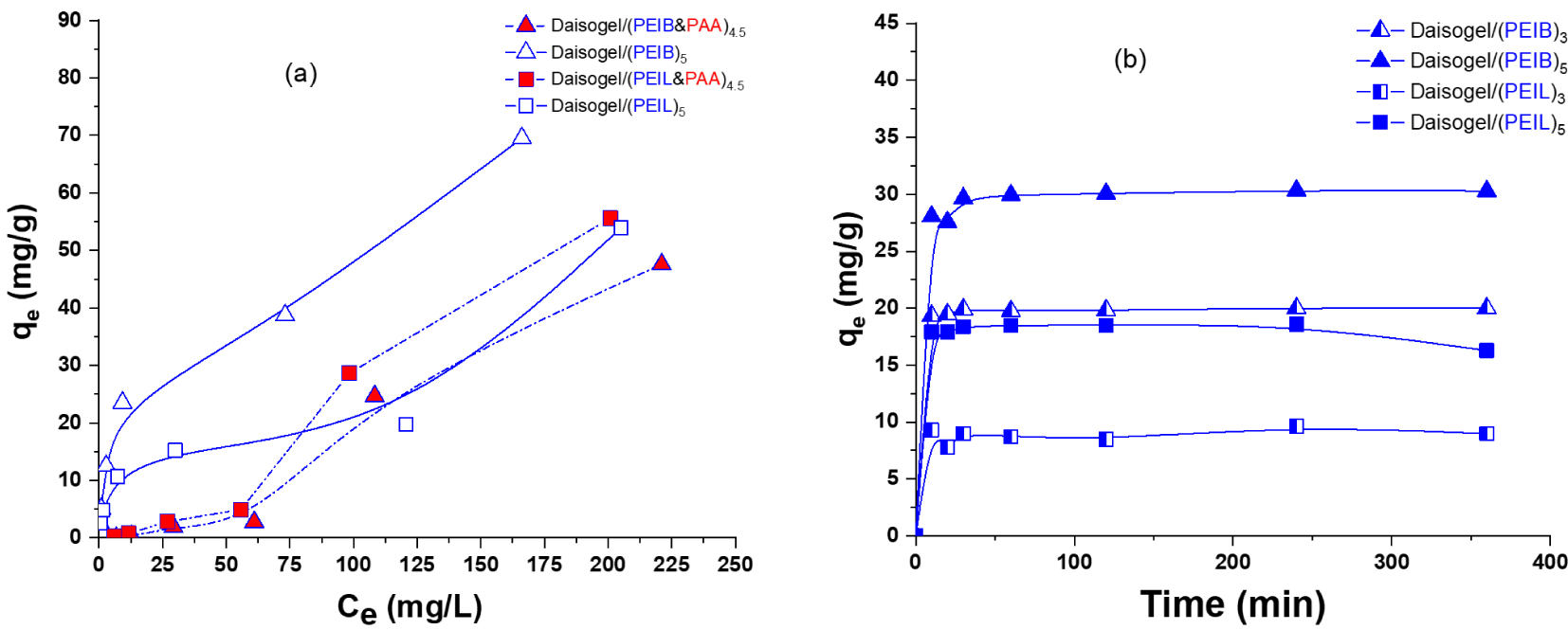
The extracted composites, Daisogel/(PEIB)5 and Daisogel/(PEIL)5, presented higher sorption capacity toward GaAc as compared with non-extracted samples, because the removal of PAA chains from the cross-linked shell allowed the negatively charged GaAc molecules to electrostatically interact with the accessible free amino groups. At higher initial concentrations of GaAc ( >100 mg/L), an increase in the sorbed amount of GaAc onto non-extracted samples has been observed. This increase could be the result of conformational changes in the organic shell due to the decrease of pH when increasing the GaAc concentration in solution. At lower pH values, the number of protonated amino groups increased in the cross-linked shell, therefore more repulsions between polycation chains will take place. These repulsions create more space in the multilayer and more GaAc molecules will be able to interact with the charged amino groups. For surface water treatment, where the targeted pollutants are in low concentrations, this type of materials are more suitable for the sorption processes, as we can see from Figure 7a, at low concentration of model pollutant (GaAc). The highest sorbed amount of GaAc has been recorded for extracted Daisogel/(PEIB)5 composite microparticles. Instead, all extracted Daisogel composite microparticles present fast sorption processes (<20 min), independent of the organic shell amount (Figure 7b). This fact showed the lack of long time diffusion for any extracted composite. Combining the isotherms with kinetics, it can be observed that this type of composite microparticles, with thin cross-linked organic layer on the surface and without polyanion chains, are very suitable for high and fast sorption of negative charged species, such as emerging pollutants. The experimental data obtained for GaAc sorption isotherms of extracted core/shell composites did not fitted the Langmuir, Freundlich and Dubinin–Radushkevich isotherm models (data not shown) for the indicated range of GaAc concentrations due to the dynamic change of the multilayer morphology with the pH of sorbate solution. This fact was attributed to all weak charged functional groups present on solid composite surface which dictates the electrostatic repulsions inside the composite shell.
Column experiment designed for GaAc removal from a simulated aqueous solution (Ci,GaAc = 0.5 mM) is presented in Figure 8. The GaAc aqueous solution has been introduced in the column (0.34 g sorbent) with a flow of 4.0 mL/min using a peristaltic pump.
Sorbed amount (%) of GaAc onto Daisogel/(PEIB)n and Daisogel/(PEIL)n composite microparticles using column sorption setup (influent: 0.5 mM GaAc).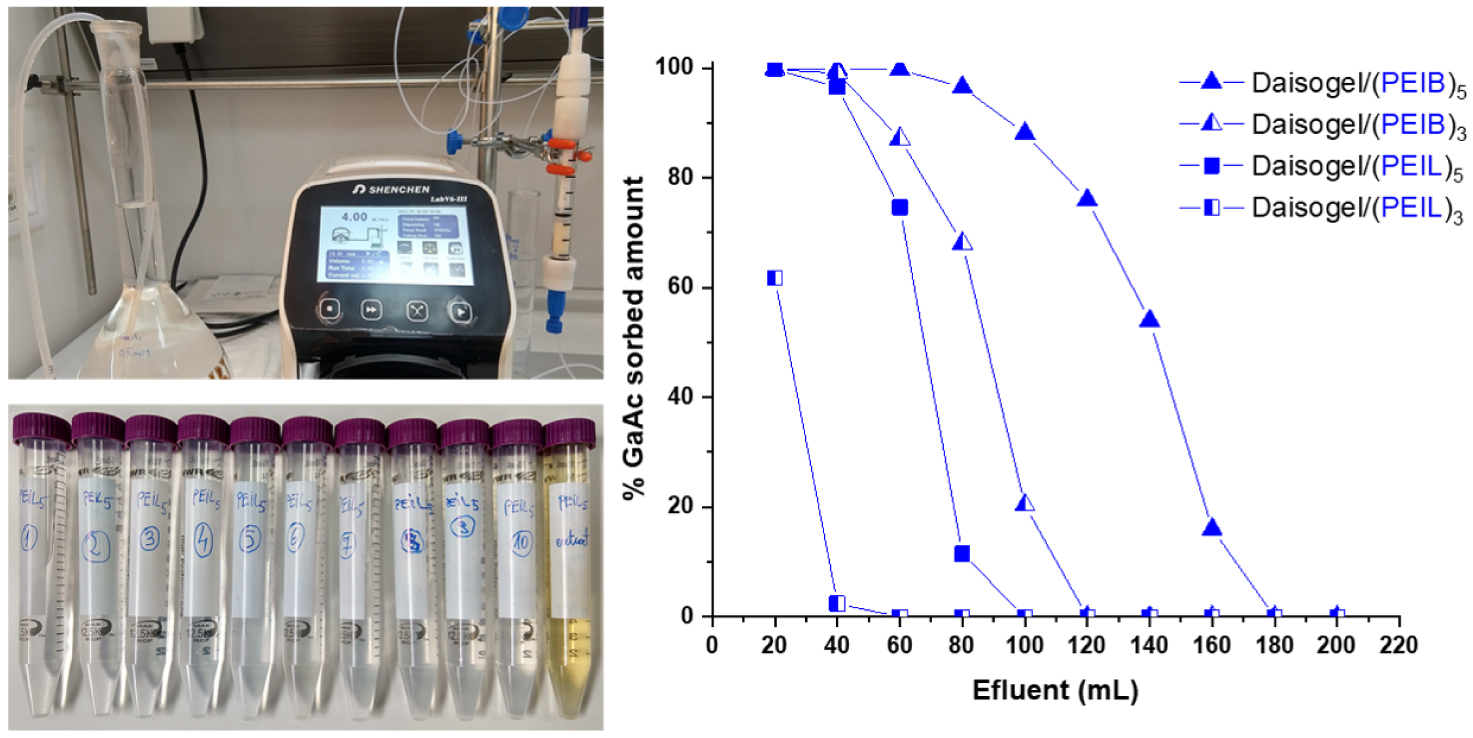
By using the UV–Vis measurements after each 20 mL effluent to determine the removal efficiency of GaAc by composite microparticles, two main aspects have been identified: (i) the dynamic sorption capacity of each composite depends on the shell organic amount and (ii) the complete GaAc extraction and regeneration of column after each sorption test. Based on the experimental data obtained in dynamic experiments, it was demonstrated that solid core/shell support interacts mainly electrostatically with the anionic weakly charged GaAc molecules. From the calculated maximum sorption capacities of each composite (i.e., qe = 27 mg/g for Daisogel/(PEIB)5) it was shown that the composites exhibited the same sorption capacity as in batch experiments. The difference between dynamic and static experiments is that the retention efficiency reached ∼100% value if the composites are used as column fillers and this efficiency depends strongly on the deposited PEI amount on Daisogel surface, as it can be seen in Figure 8. The breakthrough time (tb), which represents the time when Ce reached 5% from the initial concentration of sorbate, increases in order Daisogel/(PEIL)5 (41 min) < Daisogel/(PEIB)3 (47 min) < Daisogel/(PEIB)5 (82 min), with Daisogel/(PEIL)3 exception. It was observed that tb depended on the amount of organic part deposited onto core/shell composite. Also, the exhaustion time, te, which represents the time when Ce reached 95% from the initial concentration, increased in the same order: Daisogel/(PEIL)3 (38 min) < Daisogel/(PEIL)5 (90 min) < Daisogel/(PEIB)3 (114 min) < Daisogel/ (PEIB)5 (173 min) showing the same dependence of the sorption saturation with the organic shell amount of composite microparticles. The experimental breakthrough curves of Daisogel/(PEIL)5, Daisogel/(PEIB)3 and Daisogel/(PEIB)5 were fitted to the Thomas and Yoon–Nelson models [46] by linear regression (Figure 9).
Linear plots of (a) Thomas and (b) Yoon–Nelson models for various Daisogel composites.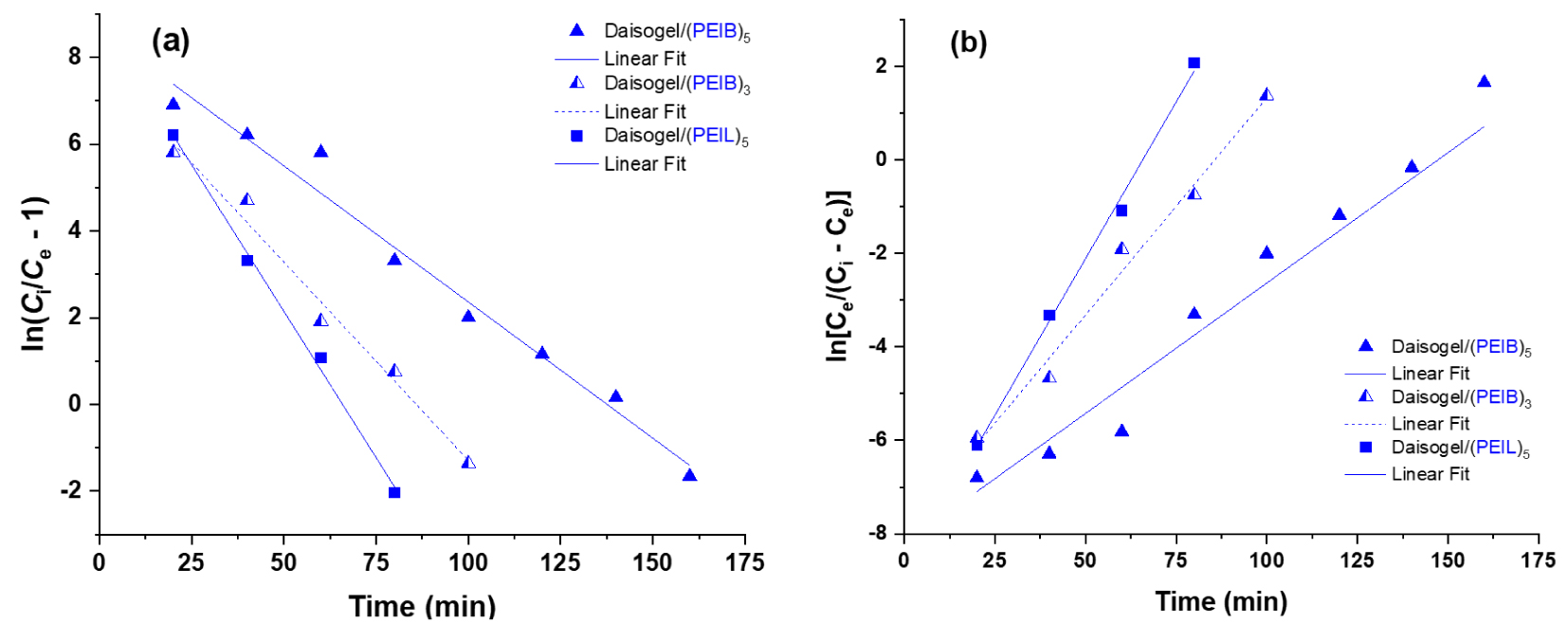
The Thomas model has the following linear equation:
| (3) |
The linear form of the Yoon–Nelson model is presented in (4):
| (4) |
The model constants kYN and 𝜏 have been calculated using linear regression analysis. All model parameters, calculated with (3) and (4) are summarized in Table 1. The values for R2 situated between 0.958 and 0.996 provided an excellent fit to the experimental data.
Thomas and Yoon–Nelson models parameters for dynamic sorption of GaAc onto Daisogel composite microparticles
| Sorbent | Thomas model | Yoon–Nelson model | |||||
|---|---|---|---|---|---|---|---|
| kTH (mL/(min⋅mg)) | qmax (mg/g) | R2 | kYN (1/min) | 𝜏 (min) | R2 | ||
| Daisogel/(PEIB)5 | 0.739 | 34.38 | 0.9781 | 0.0558 | 147 | 0.9582 | |
| Daisogel/(PEIB)3 | 1.075 | 21.47 | 0.9835 | 0.0928 | 85 | 0.9862 | |
| Daisogel/(PEIL)5 | 1.588 | 16.46 | 0.9964 | 0.1339 | 65 | 0.9961 | |
The kYN explains the diffusion characteristics of the mass transfer zone (MTZ), defined as efficiency of certain sorbent as the length of the sorption zone in the column. MTZ is calculated by following equation:
| (5) |
The relative MTZ close values, 10.52 mm, 11.75 mm and 10.88 mm calculated for Daisogel/(PEIB)5, Daisogel/(PEIB)3 and Daisogel/(PEIL)3, respectively, showed the lack of GaAc diffusion inside the composites thin shells during sorption process. In dynamic conditions, high 𝜏 values (Table 1) indicates that more sorbate can be bound by a specific sorbent, such as, Daisogel/(PEIB)5 composites. Also, the theoretical sorption capacity, qmax, calculated based on Thomas constant, kTH, showed a perfect correspondence with the values determined from sorption isotherms, where the Daisogel/(PEIB)5 had the highest sorbed amount per unit mass of composite.
Scheme 2 shows the main interactions that occur in the sorption of gallic acid in the extracted crosslinked multilayers, which support the results obtained above.
Schematical representation of the main interactions that occur at the sorption of GaAc in the extracted crosslinked multilayers of Daisogel/(PEIB)5.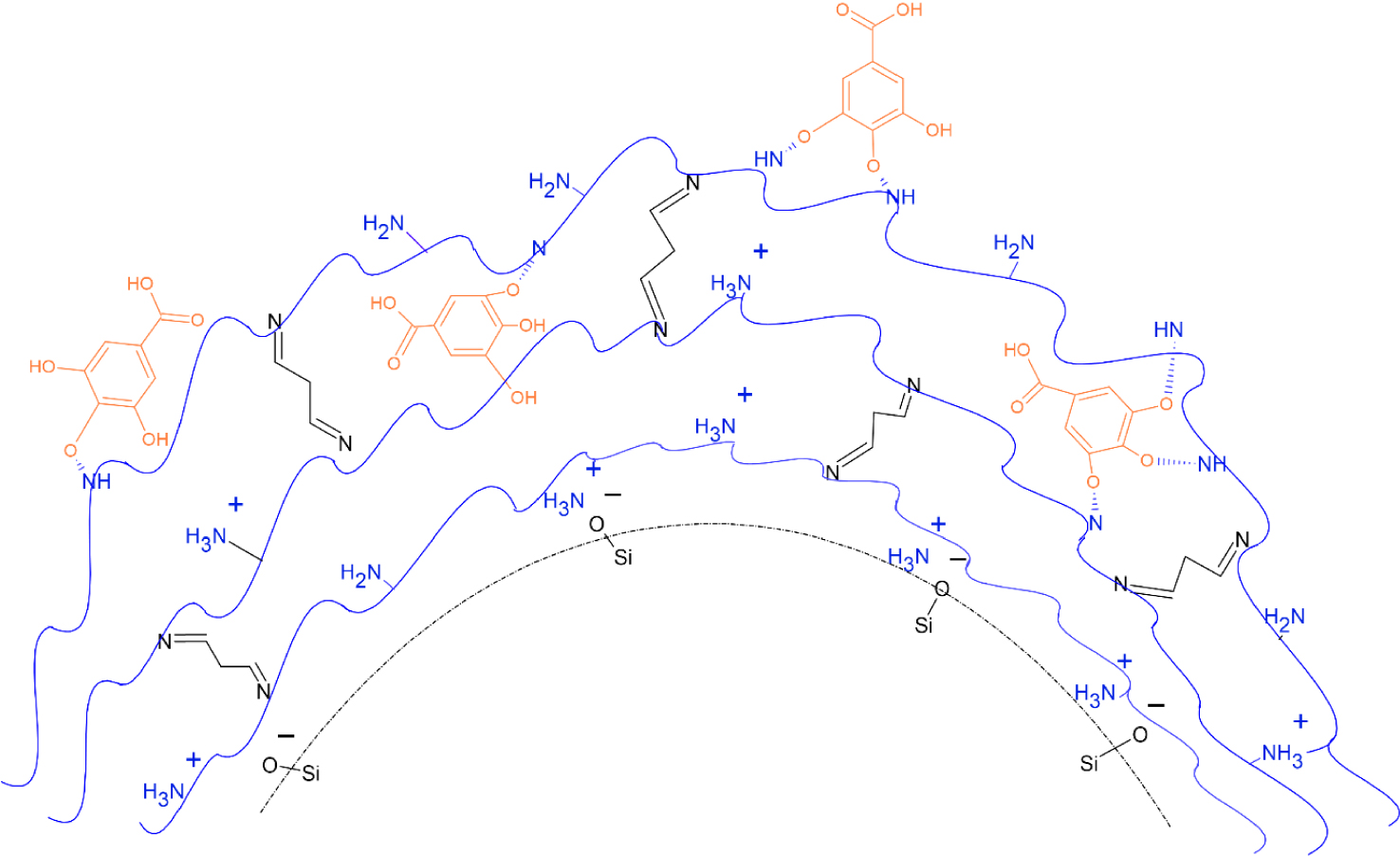
The crosslinked and unextracted multilayers adsorb small amounts of gallic acid due to the fact that: (1) a large number of amine groups on the polycation chains are already neutralized by the carboxylic groups on the PAA chains; (2) due to the electrostatic rejections between the carboxylic groups of GaAc and PAA and (3) the packing density of the multilayer chains. Thus, in the case of these multilayers, it is expected that the GaAc will adsorb only on the surface of the composite and not in the multilayer, which would explain the small amount of active substance absorbed. In the case of extracted multilayers (Scheme 2), the amount of GaAc absorbed is higher than in the case of similar non-extracted materials. Higher amounts of GaAc are adsorbed in multilayers obtained with PEIB as a result of the specific conformation that the polymer adopts and which exposes a maximum number of amino groups, accessible for interaction with the GaAc.
The exhausted column, fully loaded with GaAc, was treated with a minimum volume of NaOH 0.1 M (∼20 mL) to extract the sorbate and to regenerate the column. To demonstrate the reusability capacity of this type of sorbent, a number of 10 multiple sorption/desorption cycles has been carried out using only Daisogel/(PEIB)5 microparticles, without significant loss (<3%) of the sorption capacity determined in the first cycle (27 mg GaAc/g composite). The results obtained in dynamic conditions for multiple sorption/desorption cycle’s recommend this type of core/shell composites in different water/wastewater treatment processes.
4. Conclusions
The aim of this study was to obtain stable core/shell composite sorbents based on an inorganic silica core, named Daisogel and weak cross-linked polycations as shell, capable of controllable interactions with anionic charged species dissolved in aqueous systems. The stability of the organic shell is ensured by the cross-linking reaction with GA and its flexibility, by the extraction of PAA chains, used as template. The static and dynamic sorption experiments demonstrated that the composite materials obtained by LbL deposition of PEIB or PEIL and PAA on Daisogel microparticles are stable in different media only after the extraction of polyanion from the cross-linked shell. The best sorption results in terms of GaAc retention were obtained for the composites synthesized using PEIB which offers the advantage of a specific conformation that ensures the deposition of a flexible polymer network that can easily interact with negatively charged compounds. The calculated parameters of Thomas and Yoon–Nelson models confirmed the experimental data obtain in batch conditions, where the GaAc sorbed amount increased with the deposited shell amount onto Daisogel microparticles. The Daisogel/(PEIB)5 composite, with the highest and fast GaAc sorbed amount, was tested in at least 10 multiple sorption/desorption cycles in dynamic conditions to demonstrate the versatility of this type of composite in different water treatment strategies. The use of weak polyelectrolytes with high content of functional groups, as “shell” in combination with inorganic components, as “core”, could results in a versatile composite material with tunned properties in aqueous pollutant retention. Therefore, this type of “core/shell” composite polyelectrolytes could be very promising sorbents for the elimination of charged inorganic/organic pollutants from water or wastewater.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgements
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI–UEFISCDI, project number PN-III-P4-ID-PCE-2020-1199, within PNCDI III, contract PCE 56/2021, “Innovative and sustainable solutions for priority and emerging pollutants removal through advanced wastewater treatment processes” (SUSTINWATER).




 CC-BY 4.0
CC-BY 4.0