1. Introduction
Gallbladder lithiasis is a common pathology, but its prevalence varies greatly in different regions of the world. It can affect 5–20% of the general population in Western countries, with women being 1.5 to twice as often affected as men of the same age [1, 2, 3]. Glambek et al. found that 41.3% of women aged more than 60 years presented with gallstones [3]. Most studies have observed a correlation with the age of patients, with the prevalence of cholelithiasis reaching up to 25% in subjects over 60 years old [4, 5, 6]. It appears to be less common in Asia [7, 8]. Differences by ethnic groups have been observed [9, 10], as in the USA where cholelithiasis seems very common (60–70%) in Indian populations, and less common in Black Americans than in Caucasian adults who are affected in 10–15% of cases [5]. Similarly, in China, cholelithiasis is twice as prevalent in the Uighur population (22.9%) than the Han population (11.6%) [11]. It very often remains asymptomatic (65–80% of cases), especially in young adults [4, 6, 12, 13]. Gallstones are more prevalent in obese and hypertriglyceridemic subjects and in multiparous women [2]. Assumed in Western populations to be mainly composed of cholesterol [5, 6], this lithiasis has, however, like its renal counterpart, a number of components actually suggesting not only diverse etiological factors of metabolic, genetic, dietary, but also infectious, origin. Basically, however, two compounds dominate the profile of cholelithiasis, on the one hand cholesterol, on the other pigment stones such as calcium bilirubinate [14, 15, 16, 17, 18]. Three main types of stones have been described: cholesterol stones, brown pigment stones and black pigment stones [6, 19]. The first are related to lipid imbalances, the second mainly to infectious contexts, and the third to haemolytic phenomena. Concerning stone analysis, this distinction is not always so clear cut because many mixed stones contain significant proportions of cholesterol and bile pigments, or even other calcium-rich compounds such as carbonated calcium phosphate apatite (carbapatite) or calcium carbonates [14, 15].
In sickle cell disease, vesicular lithiasis is a common complication of the disease [20, 21, 22, 23]. The nature of the stones is essentially related to haemolysis but the analysis of the calculi again shows a diversity of components that suggests the contribution of other factors to this lithogenesis. In addition, while all patients are exposed to haemolysis due to their haemoglobin abnormalities, not all develop stones suggesting that other factors than repeated haemolysis may contribute to lithogenic activity in these patients. The purpose of the study was to compare the morphological and compositional characteristics of sickle cell stones from adult patients with those of common gallstones.
2. Materials and methods
We had the opportunity to analyze 408 gallstones from adult patients, 75 of which came from patients with sickle cell disease. Among the latter, 56 were homozygous (ss) and 19 were heterozygous (sc). The analysis included morphological examination with a binocular magnifying glass (Stereomicroscope Olympus SZ51) and, for some calculi, Field Emission Scanning Electron Microscopy (FE-SEM) to specify the structural characteristics of the stones [24]. Samples were examined without coating through FE-SEM.
Then, the calculi were subjected to a sequential analysis by Fourier Transform Infrared (FTIR) spectroscopy from the core to the periphery to precisely define the composition and possible variations between the nucleation zone and the surface of the stone [25]. Infrared spectra were collected in the transmission mode on a Vector 22 FTIR spectrophotometer from Bruker Optics using the KBr pellet technique as described elsewhere [26, 27]. Statistical comparisons were made on the NCSS software using the Fisher’s exact test.
Ethical approval was obtained by the CPP of Tenon Hospital for this non-interventional patient study. Each sample was named only by a study number, without indicating the name of the patient or other potential identification data. The study was carried out as part of routine patient care without any specific sampling apart from the stone transferred to the laboratory for compositional analysis.
3. Results
The average age of sickle cell patients who underwent cholecystectomy is 14 years younger than that of patients operated on cholelithiasis of other causes (32.7 ± 13.5 years (extreme limits: 18.9 and 64.4 years) versus 46.8 ± 25.5 years (extreme limits: 17.8 and 95.7 years), p < 0.0001). Women with common bladder stones (n = 198) were more frequent than men (n = 135) but not in the case of sickle cell stones (women = 37, men = 38). The difference between groups was not significant (p = 0.1).
3.1. FTIR analysis
Of all 408 gallstones included in this series, FTIR analysis highlighted the preponderance of cholesterol and bile pigments as the most common and abundant constituents, but also the fact that many stones are mixed and have three or four components, or even more. In patients with sickle cell disease, the most common and abundant component was calcium bilirubinate. In addition, FTIR analysis revealed a great diversity of compounds in gallstones with more than 30 different components identified (Table 1).
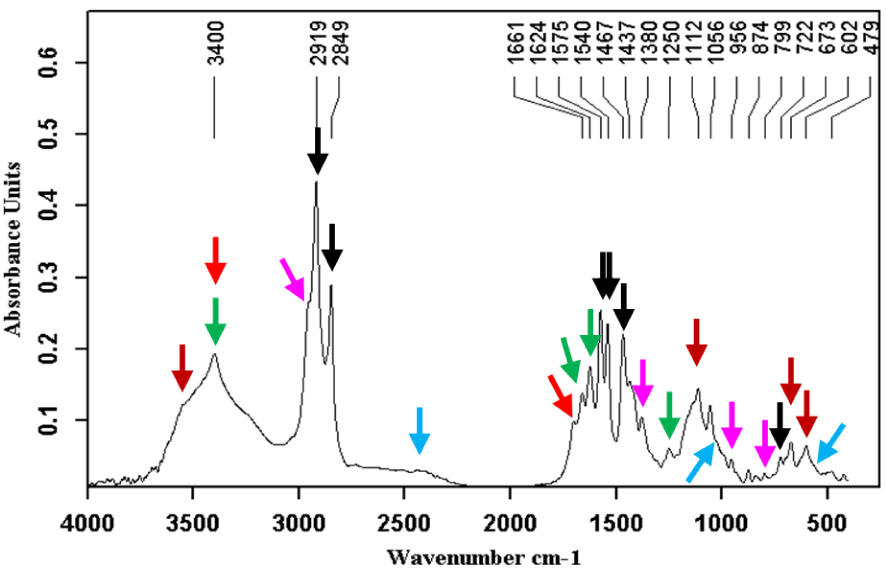
The spectra presented below illustrate this diversity. Even when a stone is composed of a single chemical species, it can contain several crystalline forms such as anhydrous cholesterol and monohydrate for cholesterol stones, neutral and acid calcium bilirubinate for pigment stones, or calcite and vaterite for calcium carbonate stones. In addition, sequential analysis from the core to the surface can reveal a change in composition suggesting the successive involvement of several factors in the formation of the stone. Finally, some components such as free bilirubin, which are abundant in gallbladder, can be incorporated into the stone without necessarily playing an active part in the lithogenic process. By contrast, polymers of bilirubin can be involved in stone formation [28]. Figures 1–16 illustrate the diversity of stone components and mixtures found in gallstones by FTIR analysis. Commonly, gallbladder stones are made of cholesterol (Figure 1), calcium bilirubinate (Figure 2) and/or calcium carbonate (Figure 3) and/or calcium phosphates (Figure 4) with various proportions of proteins (Figure 5). Calcium palmitate (Figure 6) or calcium stearate can also be frequently found. Exogenous compounds have also been identified such as drugs or surgical ligatures: they were found in 2% of stones in our series. Glafenine, dipyridamole, sulindac or indinavir were described more than 20 years ago [29, 30, 31, 32]. More recently calcium ceftriaxonate (Figure 7) and atazanavir (Figure 8) have been also reported in gallstones with a high frequency in patients receiving these treatments [33, 34, 35, 36, 37, 38, 39]. Gallstones can contain multiple components as shown in Figures 9–16. More than 43% of the stones contain at least five components and often more (Table 2).
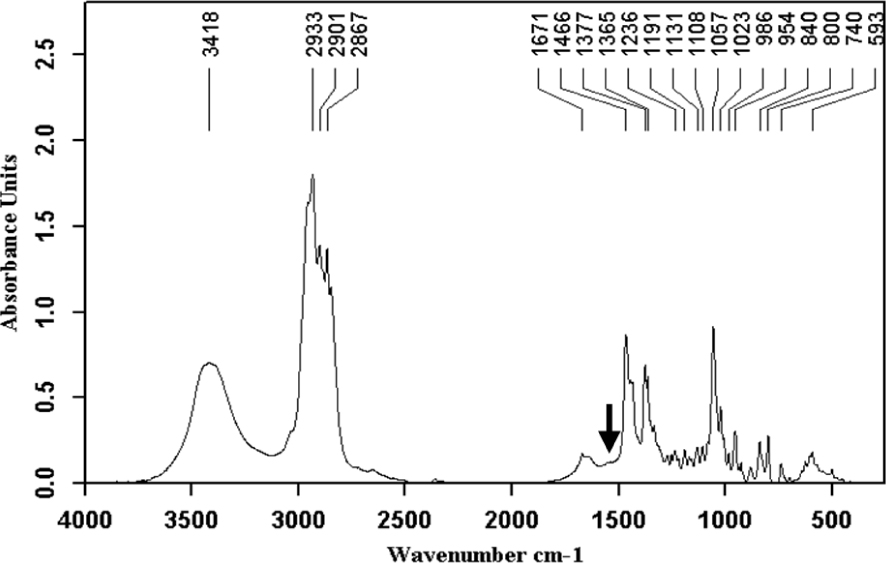
Cholesterol anhydrous and monohydrate with small proportions of proteins (black arrow).
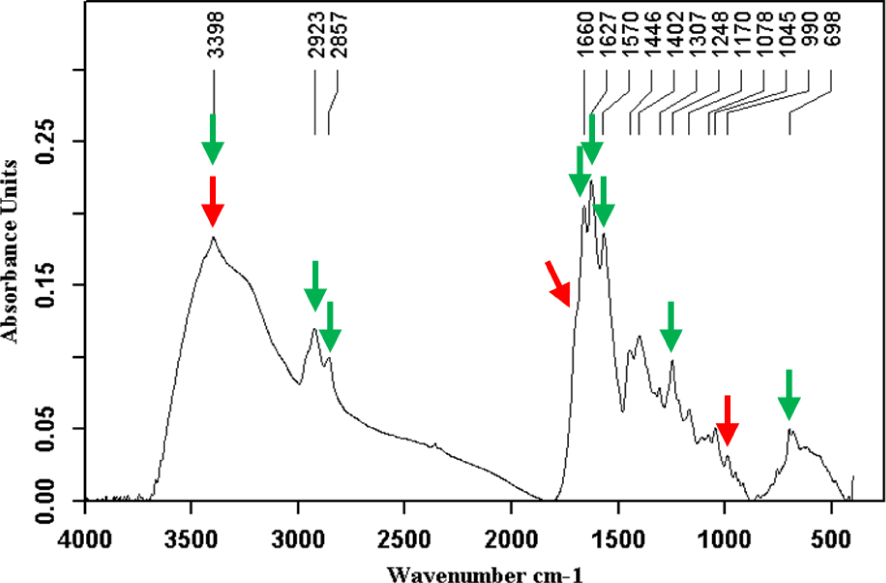
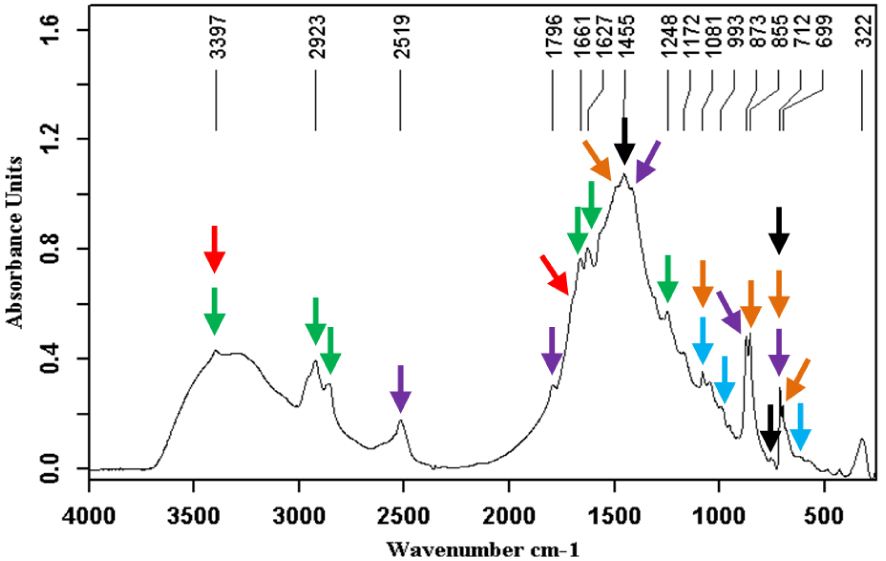
Calcium bilirubinates (green arrows) and free bilirubin (peaks at 3407, 1693 and 991 cm−1, red arrows). Two forms of calcium bilirubinate are often present in gallstones. The preponderant form is neutral calcium bilirubinate with its characteristic bands at 1664, 1627 and 1570 cm−1. The second form, more or less frequent and always less abundant than neutral bilirubinate resembles free bilirubin with peaks moved to 3395 and 1703 cm−1 that correspond to hydrogen bond and free carboxyl groups respectively. In the common cases where acid calcium bilirubinate and free bilirubin are mixed in the same stone, the peaks are slightly shifted to the intermediate position as shown at 3398 cm−1 in Figure 2. Masquer
Calcium bilirubinates (green arrows) and free bilirubin (peaks at 3407, 1693 and 991 cm−1, red arrows). Two forms of calcium bilirubinate are often present in gallstones. The preponderant form is neutral calcium bilirubinate with its characteristic bands at 1664, 1627 and ... Lire la suite
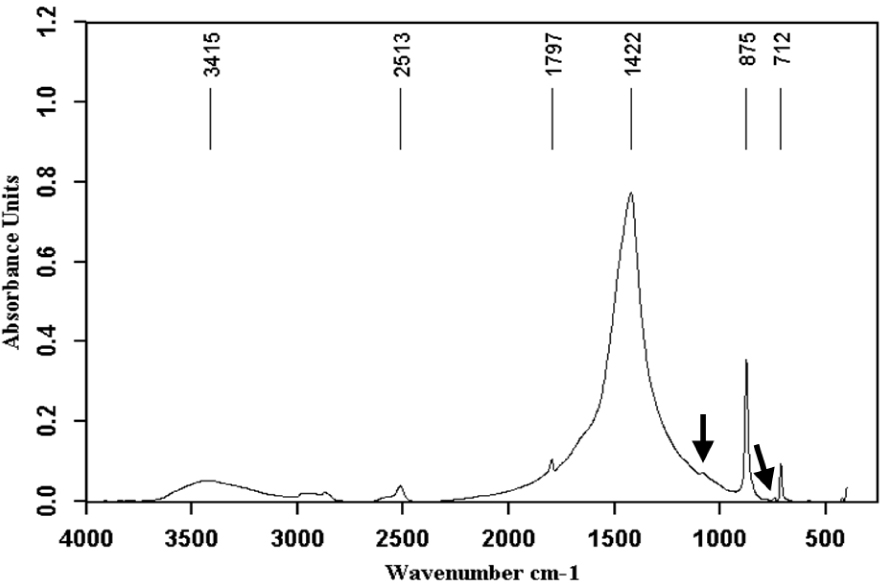
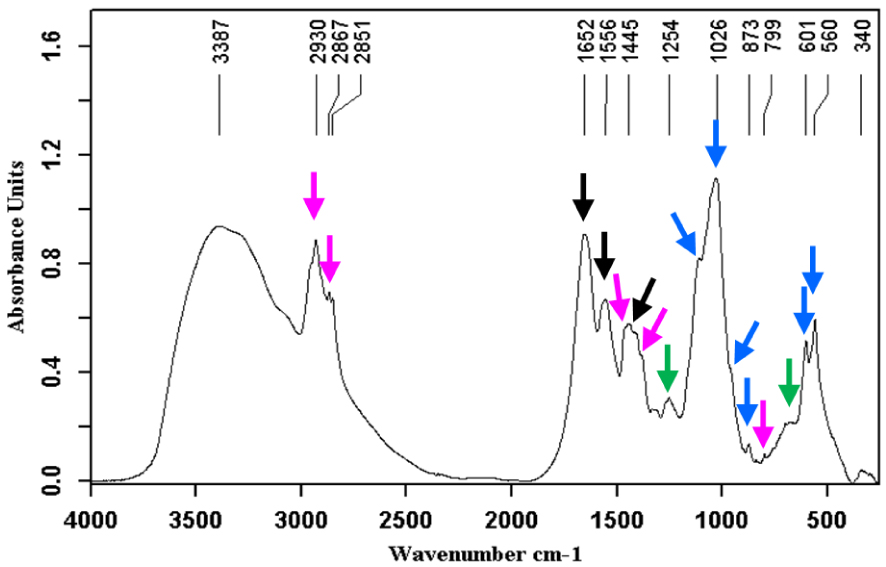
Calcite (calcium carbonate anhydrous crystallized in the rhombohedral system) + vaterite (also calcium carbonate anhydrous but crystallized in the hexagonal system) (black arrows).
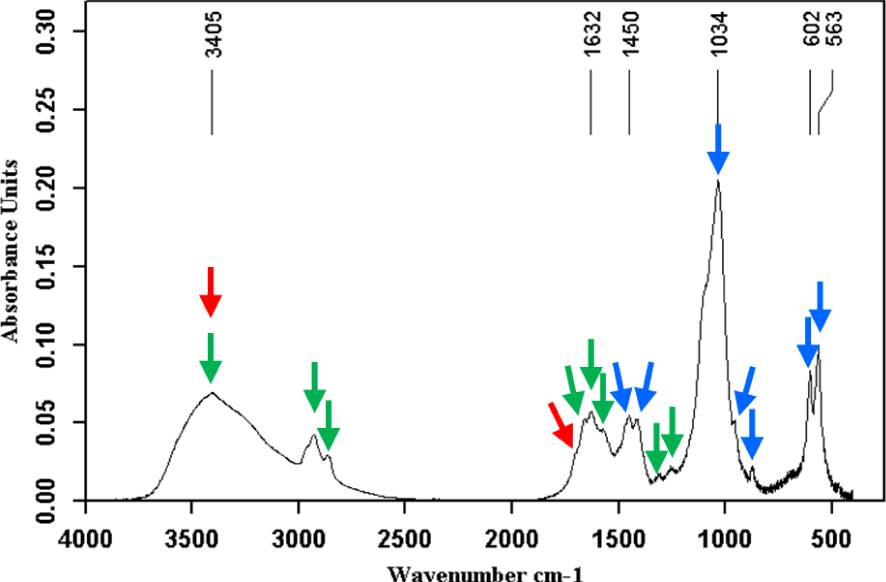
Calcium phosphate stone (carbapatite, blue arrows) also containing calcium bilirubinates (green arrows) and bilirubin (peak at 3405 cm−1, shoulder at 1698 cm−1, red arrows).
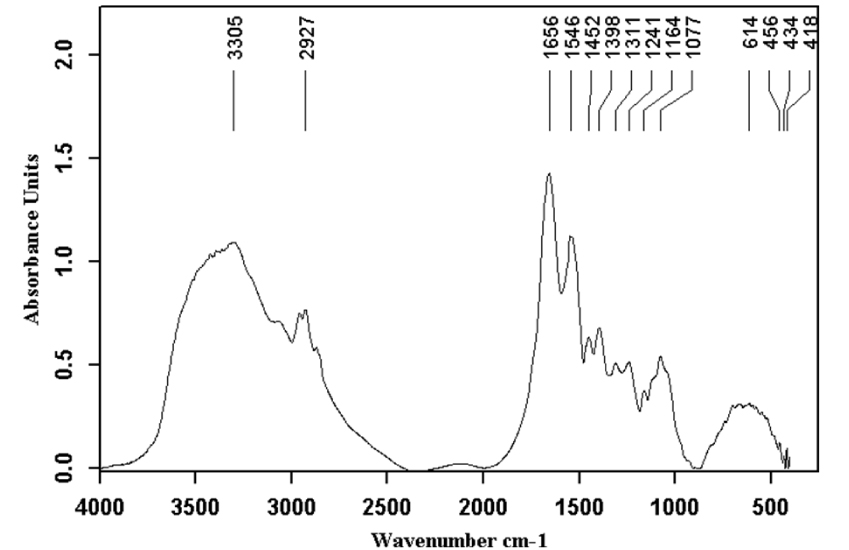
Proteins stone.
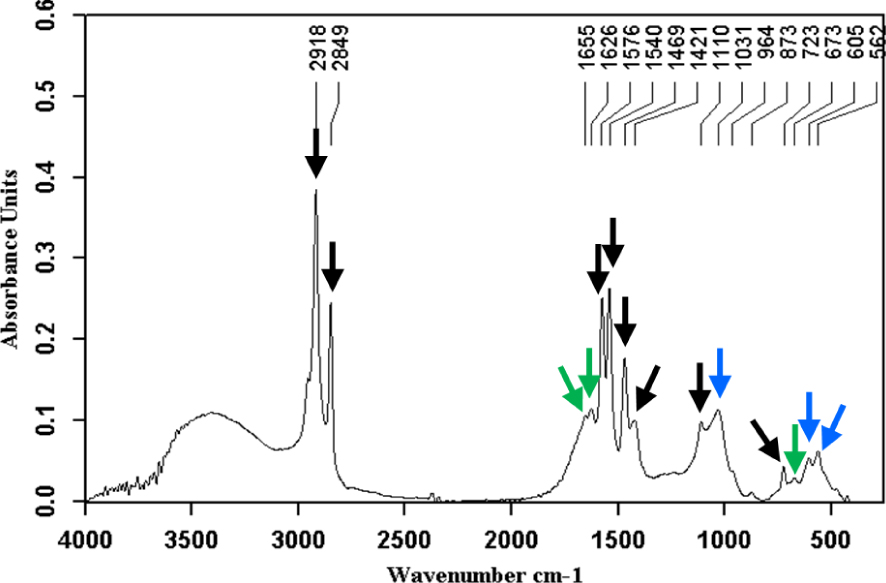
Calcium palmitate (black arrows) mixed with calcium bilirubinates (green arrows) and carbapatite (blue arrows).
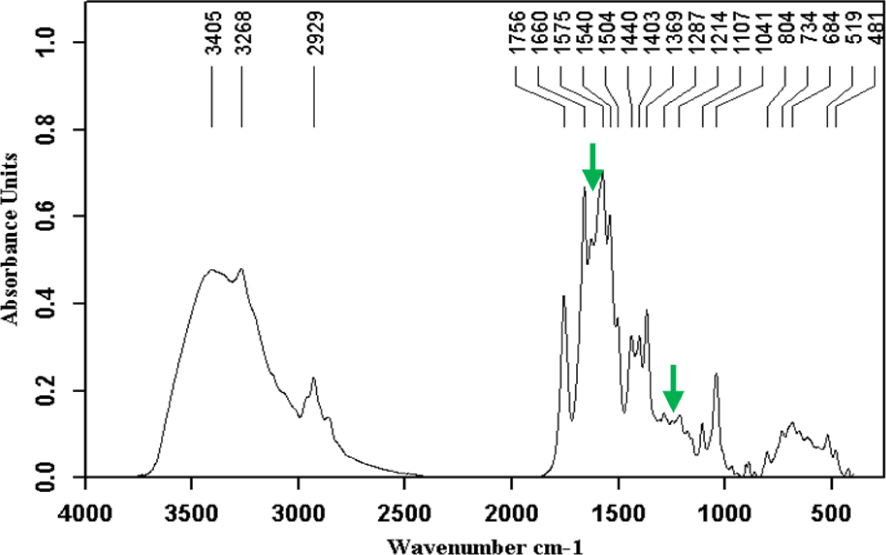
Calcium ceftriaxonate with small proportions of calcium bilirubinates (green arrows).
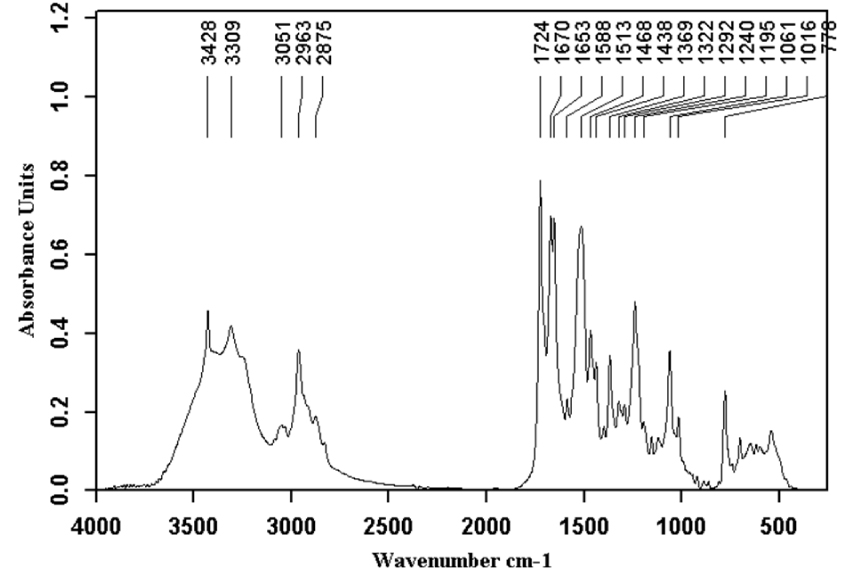
Atazanavir.
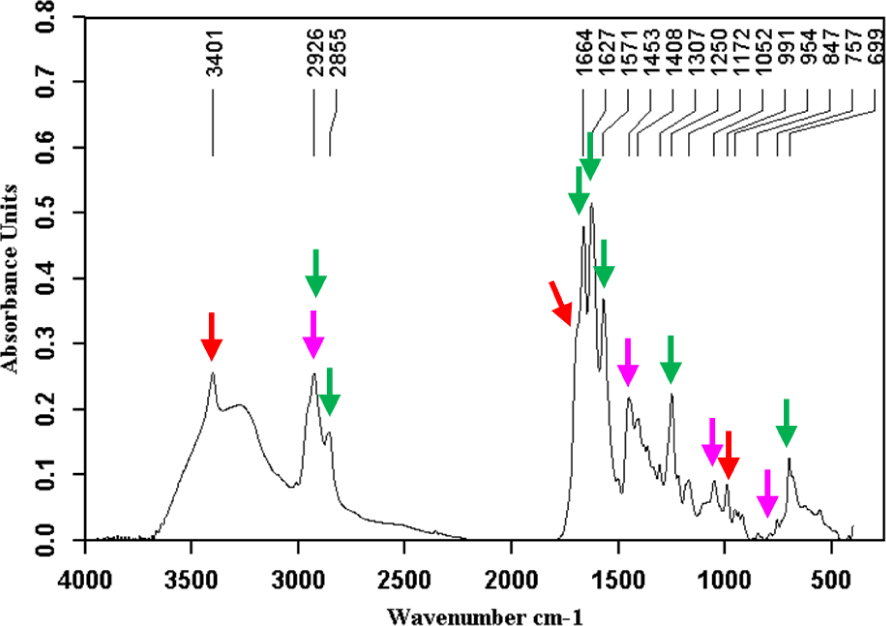
Calcium bilirubinates (green arrows) + cholesterol (pink arrows) + bilirubin (red arrows).
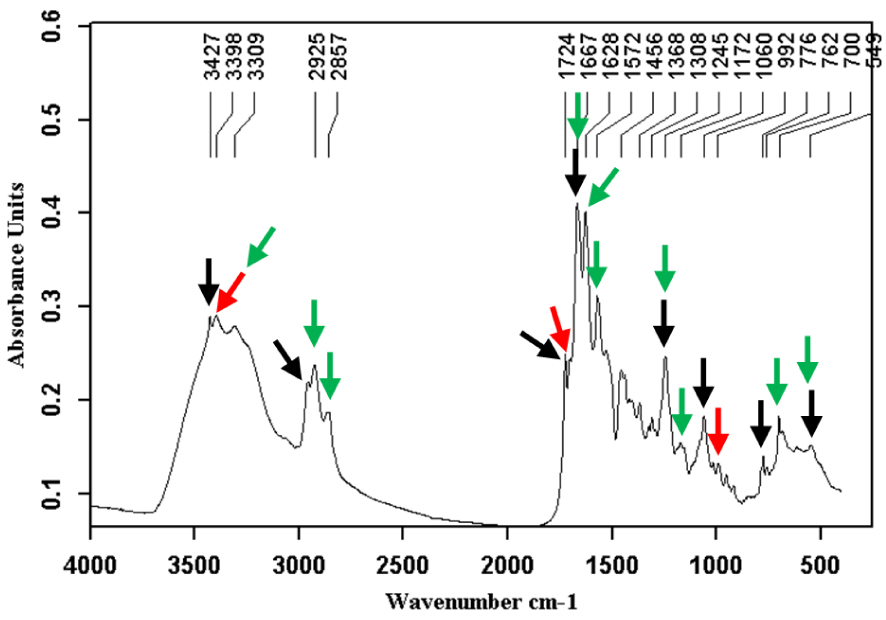
Pigment stone made of a mixture of calcium bilirubinates (green arrows), bilirubin (red arrows) and atazanavir (black arrows).
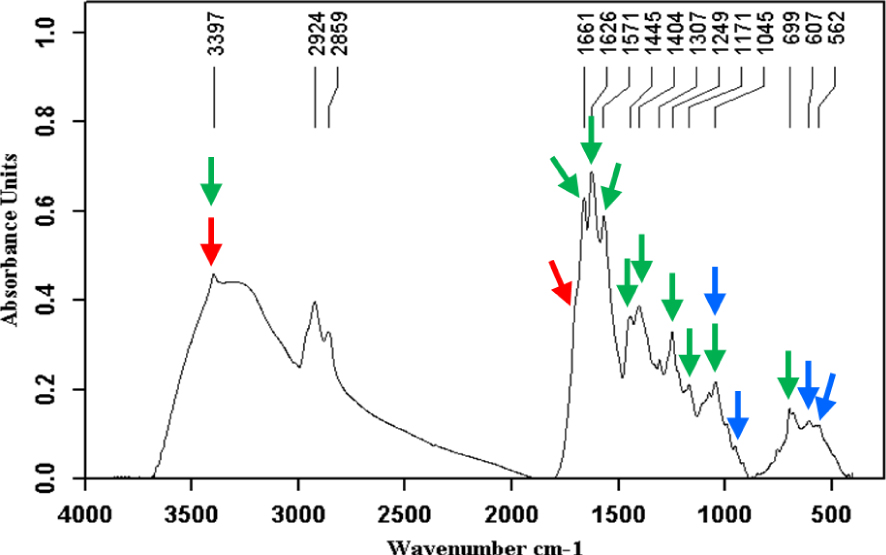
Calcium bilirubinates (green arrows) + bilirubin (red arrows) + carbapatite (blue arrows).
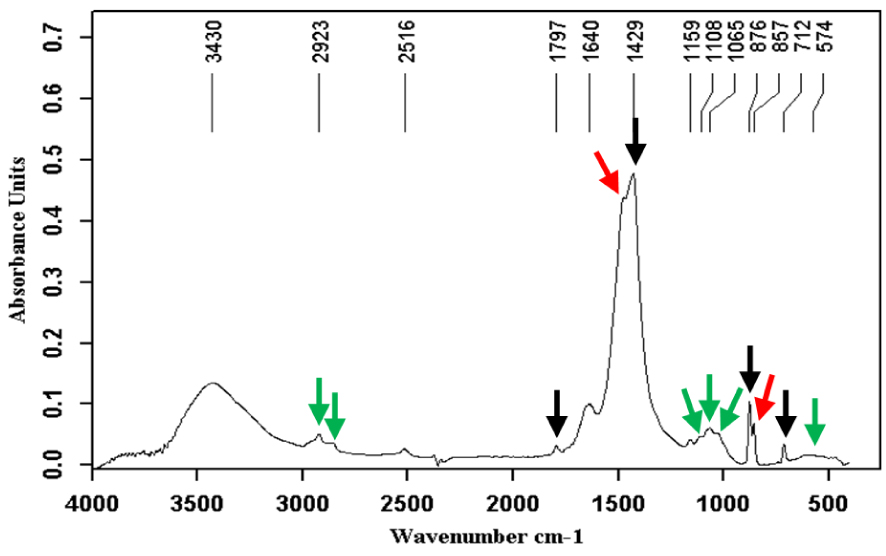
Calcite (black arrows) + aragonite (red arrows) + mucopolysaccharides (green arrows).
Calcite ( ) + aragonite (
) + aragonite ( ) + vaterite (
) + vaterite (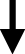 ) + calcium bilirubinates (
) + calcium bilirubinates ( ) + bilirubin (
) + bilirubin ( ) + calcium cholate (
) + calcium cholate ( ).
).
Carbapatite (blue arrows) + proteins (black arrows) + cholesterol (pink arrows) + calcium bilirubinates (green arrows).
Carbapatite (blue arrows) + calcium bilirubinates (green arrows) + calcium palmitate (black arrows) + bilirubin (red arrows).
Calcium palmitate (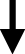 ) + calcium bilirubinates (
) + calcium bilirubinates ( ) + bilirubin (
) + bilirubin ( ) + gypsum (
) + gypsum ( ) + struvite (
) + struvite ( ).
).
3.2. Stone morphology
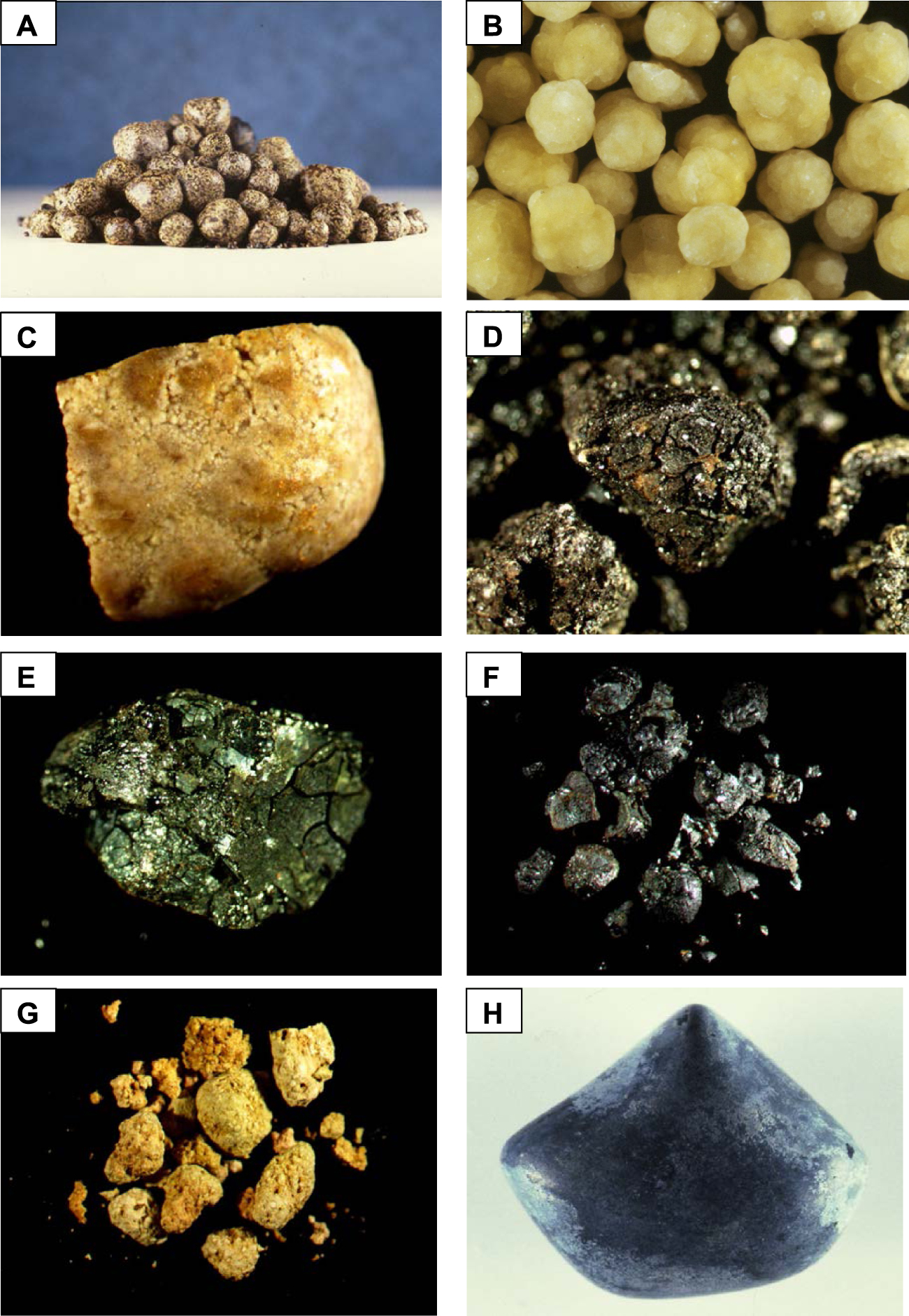
As with urinary stones, morphology can help classify stones according to their main component. However, morphological criteria based on the texture, internal organization, and colour of the calculi are much less relevant. Indeed, if a light colour on the surface or section suggests a predominance of cholesterol (Figures 17A–C and 18A,B), a dark brown or brown-black suggests pigment stones (or portions of stones), that is to say containing bilirubin derivatives (Figures 17D,E and 18D,E). Actually, as shown in the images below, the stones can be highly pigmented and yet be composed mainly of calcium carbonates, especially aragonite, or of calcium phosphates such as carbapatite (Figure 17F). Calcium carbonate-rich stones can be poorly organized or, by contrast, reveal a more or less compact concentric structure of different colours ranging from greyish to brown-black or, sometimes, light yellow-brown or red-brown. In some cases, a pure calcium carbonate shell can cover stones of a different type. In Figure 17H, the smooth surface of the stone was pure aragonite. The colour was dark grey with bluish reflections. In the case of calcium phosphate-rich stones containing various proportions of calcium bilirubinates as well, the inner structure is often poorly organized and the colour ranges from dark brown to black. When gallstones contain other compounds such as drugs, the morphology and colour are often unusual as in Figure 17G where the stones were made of nearly pure calcium ceftriaxonate. As seen in Figure 18, stone sections can reveal a succession of different structures, sometimes unorganized, sometimes concentric or radial, with different colours suggesting both a different composition and an evolution of lithogenic factors. For example, in Figure 18C the dark brown stone core is mainly composed of calcium bilirubinates with a small proportion of calcium palmitate while the surrounding yellowish layers are nearly pure cholesterol. Such a structure is highly suggestive of a lithogenic process related to infection with a secondary coating of cholesterol crystals as a consequence of lipid anomalies. Figure 18F shows a stone initiated by strands of non-absorbable ligations related to previous bile duct surgery. Calcium bilirubinate encrustations followed by accumulation of calcium bilirubinate deposits and finally calcium carbonate coating resulted in an obstruction of the bile duct requiring further intervention. Figure 18G illustrates a case of pigment stones mainly composed of calcium bilirubinates with a whitish core of calcium palmitate aggregates.
(A) Multiple cholesterol stones. (B) Another example of multiple cholesterol stones. (C) Large gallbladder stone made of a mixture of cholesterol monohydrate and anhydrous. (D) Black pigment gallstone in a patient with sickle cell disease. (E) Another example of black-greenish pigment gallstone in a patient with sickle cell disease. One explanation for greenish-coloured reflections could be the abundant presence of calcium carbonate (calcite) mixed with calcium bilirubinates. (F) Multiple small black gallstones in a patient with sickle cell disease. Unexpectedly, the predominant crystalline phase is not calcium bilirubinate but carbapatite mixed with amorphous carbonated calcium phosphate. (G) Multiple gallstones mainly made of calcium ceftriaxonate. (H) Calcium carbonate gallbladder stone made of a mixture of calcium carbonates and calcium bilirubinates. The stone shell is made of nearly pure aragonite. Masquer
(A) Multiple cholesterol stones. (B) Another example of multiple cholesterol stones. (C) Large gallbladder stone made of a mixture of cholesterol monohydrate and anhydrous. (D) Black pigment gallstone in a patient with sickle cell disease. (E) Another example of black-greenish ... Lire la suite
(A) Section of a pure cholesterol gallstone. Colour is very light. The inner structure shows a diffuse radiating organization of the crystals. (B) Poorly organized section of a cholesterol gallstone containing a mixture of cholesterol anhydrous and monohydrate. The red colour is due to the presence of small proportion of calcium bilirubinate randomly distributed within the stone. (C) Another section of a cholesterol stone. Note that the core is dark brown because the stone was initiated from calcium bilirubinate crystals. (D) Dark brown stone made of calcium bilirubinates in a patient with sickle cell disease. The surface is rough and the section red-brown is unorganized. (E) Another example of poorly organized section of a pigment stone in a patient with sickle cell disease. Colour is dark brown to brown-greenish. Photograph E corresponds to the section of the stone shown in Figure 17E. (F) Example of a pigment gallstone initiated from the threads of a surgical ligation. The inner part of the stone is mainly composed of calcium bilirubinates while the peripheral layers are made of calcium carbonates (aragonite and calcite). (G) Gallbladder black pigment stone initiated from whitish lamellar crystals of calcium palmitate. Masquer
(A) Section of a pure cholesterol gallstone. Colour is very light. The inner structure shows a diffuse radiating organization of the crystals. (B) Poorly organized section of a cholesterol gallstone containing a mixture of cholesterol anhydrous and monohydrate. The red ... Lire la suite
When examined under FE-SEM, the characteristics of the crystals and their organization within the stone can be ascertained at the mesoscopic scale. Figure 19 presents various images of gallstones from patients without sickle cell disease. In Figures 19A and B, two different cholesterol stone organisations can be seen, the first of lamellar crystals stacked on top of each other and the second of crystals organized in perpendicular spans. Figures 19C and D illustrate the appearance of the surface of the pigment stones, one smooth and cracked, the other rough with irregularly arranged holes. Figure 19E shows the poorly organized interior of another pigment stone. Figure 19F reveals calcium carbonate spheres mixed with an unorganized and rough structure made of calcium bilirubinate. In Figure 19G, lamellar crystals of calcium palmitate are observed in the core of a pigment stone; in Figure 19H, aggregated rod atazanavir crystals are mingled with calcium bilirubinate.
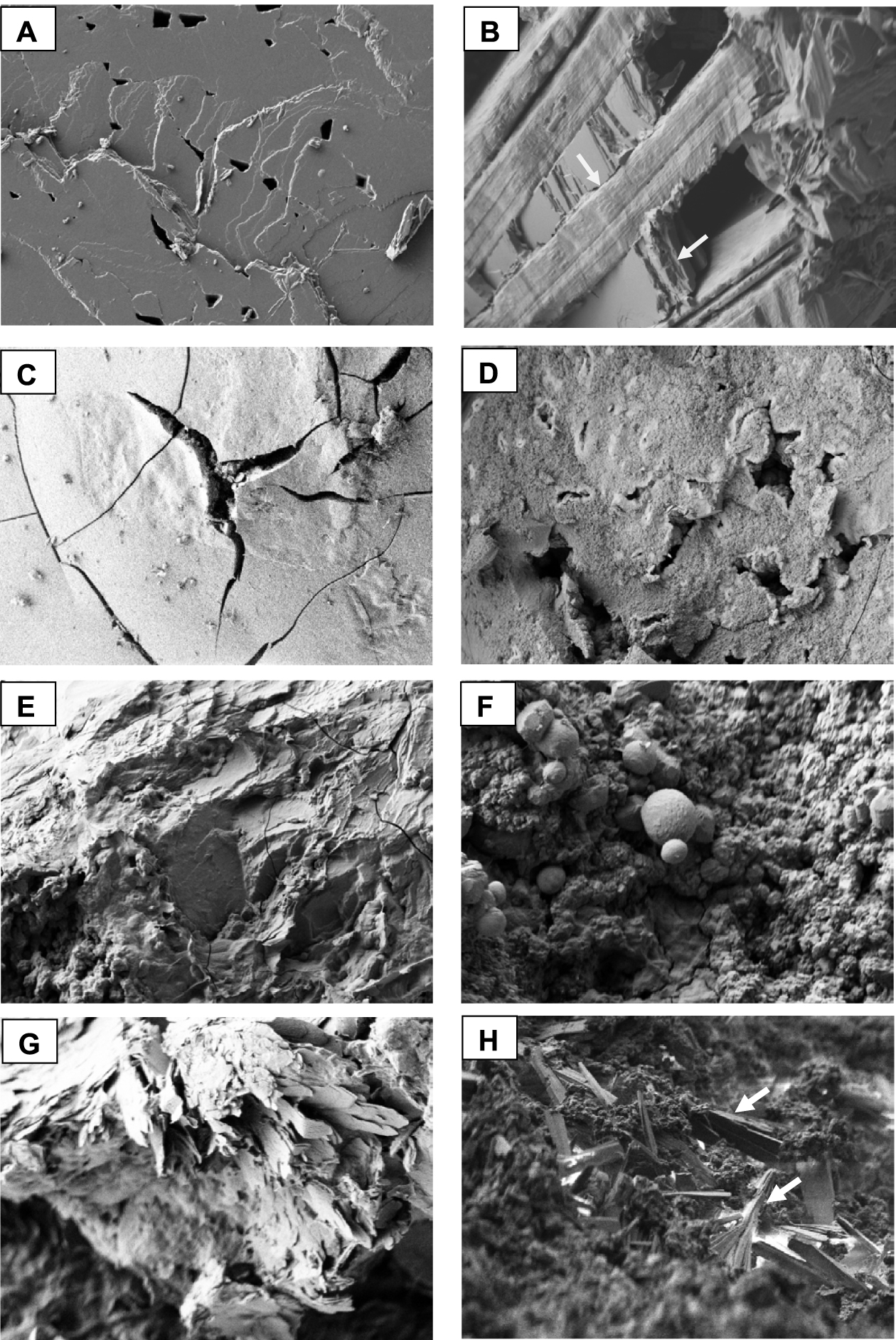
Scanning electron microscopic (SEM) images of gallstones. (A) Peripheral layers of a cholesterol gallstone. Note that large cholesterol plaques are stacked on top of each other. (B) Another gallstone made of a mixture of cholesterol anhydrous and cholesterol monohydrate. The inner organization shows that the cholesterol plaques stacked on top of each other are organized alternately in two orthogonal directions (arrows). (C) Smooth, cracked surface of a black pigment stone mainly composed of calcium bilirubinates. (D) Rough and lacunar surface of another pigment stone mainly composed of calcium bilirubinates. (E) Unorganized section of a black pigment gallstone, made of nearly pure calcium bilirubinate. (F) Spheres of calcite in a pigment stone predominantly composed of calcium bilirubinates. (G) Core of a pigment gallstone with lamellar crystals of calcium palmitate. (H) Unorganized section of a pigment gallstone made of a mixture of calcium bilirubinates and atazanavir. Atazanavir rod crystals are grouped into small asymmetric aggregates (white arrows). Masquer
Scanning electron microscopic (SEM) images of gallstones. (A) Peripheral layers of a cholesterol gallstone. Note that large cholesterol plaques are stacked on top of each other. (B) Another gallstone made of a mixture of cholesterol anhydrous and cholesterol monohydrate. The ... Lire la suite
Figures 20A–H illustrate microscopic aspects of gallstones from patients suffering sickle cell disease. In Figures 20A and B, two different pigment gallstones surface types can be observed, with cracks in Figure 20A and a smooth aspect in Figure 20B. Figure 20C illustrates the rough structure of a pigment stone similar to that in Figure 19E for patients without sickle cell disease. In Figures 20D and E, needle-shaped bilirubin crystals organized as asymmetric aggregates can be seen. Figure 20F illustrates the association of a rough and cracked structure composed of calcium bilirubinate with multiple calcite spheres. Figure 20G shows a less common “crossed rod” calcite crystal morphology. Finally, in Figure 20H, another type of spheres can be seen, this time made of carbapatite.
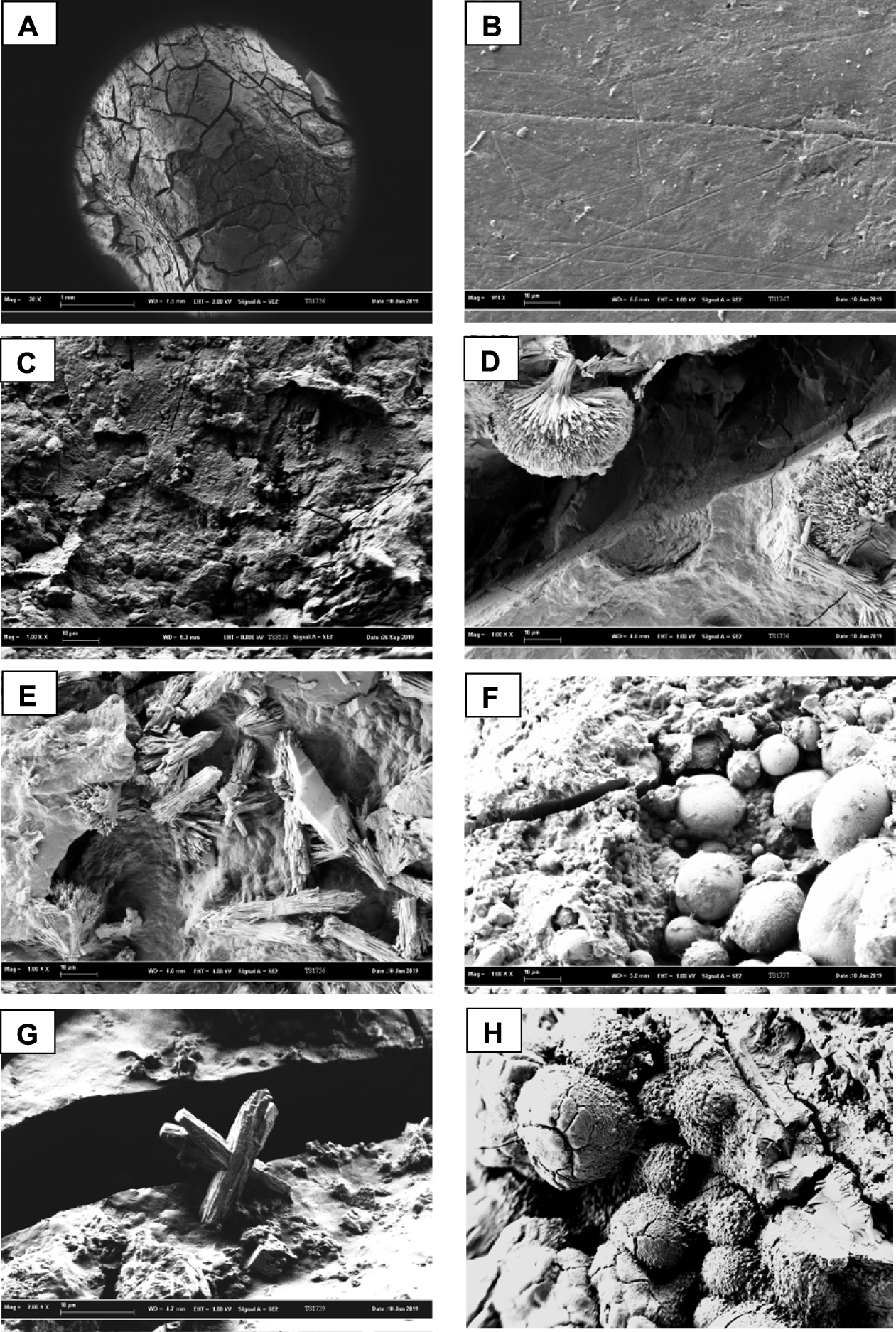
SEM photographs of stones from patients with sickle cell disease. (A) Cracked surface of a black pigment stone that contains 75% calcium bilirubinates, 20% cholesterol and 5% free bilirubin. (B) Smooth surface of a black-brown pigment stone made of 85% calcium bilirubinates, 12% carbapatite and 3% of free bilirubin. (C) Rough unorganized section of a black pigment stone composed of 55% calcium bilirubinates, 30% calcite, 7% carbapatite, 4–5% proteins and 3–4% cholesterol. (D) Asymmetric aggregates of free bilirubin within the black pigment stone of A. (E) Other aggregates of bilirubin crystals inside the same pigment stone as in D. (F) Numerous spheres of calcite crystals within a pigment gallstone mainly made of calcium bilirubinates (55%) + calcite (20%) + vaterite (10%) + aragonite (10%) + free bilirubin (5%). (G) Other morphology of calcite crystals as aggregated rods in another pigment stone shown in C. (H) Spheres of carbapatite in the core of a black pigment stone made of calcium bilirubinates (⩾65%) + carbapatite (25%) + calcite (5%) + bilirubin (⩽5%). Masquer
SEM photographs of stones from patients with sickle cell disease. (A) Cracked surface of a black pigment stone that contains 75% calcium bilirubinates, 20% cholesterol and 5% free bilirubin. (B) Smooth surface of a black-brown pigment stone made of 85% calcium ... Lire la suite
Excluding sickle cell disease, the morpho-constitutional analysis of gallstones occurring in France shows essentially four categories: cholesterol stones (60.1%), pigment stones (32.1%), calcium carbonate stones (4.7%) and atypical stones (3.1%). The latter group can include various compounds: bile pigments, cholesterol, calcium carbonates, calcium phosphate, but also drugs such as calcium ceftriaxonate or antiproteases such as indinavir or atazanavir. Of 75 sickle cell patient stones, we found the following distribution: cholesterol stones: 6.7%; bile pigments: 74.7%; calcium carbonates: 14.6%; atypical: 4%. Of note, no sickle cell stones contained any drug deposits.
3.3. Stone composition
The composition of gallstones shows a great diversity of mineral and/or organic phases: more than 30 different species have been identified (Table 1). In addition, gallstones are rarely pure in a single crystalline species (1.3%). Up to nine different compounds have been identified within a single stone. As shown in Table 2, a quarter of the stones in our series contained four different compounds and 43% had at least five components belonging to several chemical families.
Components identified in gallstones
| Components | Frequency (%) |
|---|---|
| Biliary pigments | |
| Calcium neutral bilirubinate | 83.6 |
| Calcium acid bilirubinate | 58.2 |
| Bilirubin | 14.3 |
| Steroids | |
| Cholesterol anhydrous | 69.9 |
| Cholesterol monohydrate | 13.3 |
| Cholesterol palmitate and/or stearate | 7.0 |
| Calcium desoxycholate | 0.8 |
| Calcium taurocholate | 0.5 |
| Calcium glycocholate | 0.5 |
| Sodium cholate | 0.3 |
| Calcium chenodesoxycholate | 0.3 |
| Fatty acids and triglycerides | |
| Calcium palmitate and/or stearate | 28.0 |
| Triglycerides (tripalmitine, tristearine …) | 13.5 |
| Calcium carbonates | |
| Calcite | 25.7 |
| Aragonite | 12.2 |
| Vaterite | 3.4 |
| Calcium phosphates | |
| Carbapatite | 21.8 |
| Amorphous carbonated calcium phosphate | 3.6 |
| Whitlockite | 0.8 |
| Octacalcium phosphate pentahydrate | 0.3 |
| Magnesium phosphates | |
| Struvite | 0.3 |
| Calcium sulfates | |
| Gypsum | 0.3 |
| Proteins | 64.7 |
| Drugs | |
| Atazanavir | 0.8 |
| Calcium ceftriaxonate | 0.5 |
| Indinavir | 0.3 |
| Glafenic acids | 0.3 |
| Others | |
| Ligatures | 0.8 |
| Metal staples | 0.5 |
| Monosodium urate monohydrate | 0.3 |
| Phospholipids | 0.3 |
Number of components/stone
| Frequency (%) | |
|---|---|
| Pure stones (only one component) | 1.3 |
| Two-component stones | 9.7 |
| Three-component stones | 20.0 |
| Four-component stones | 25.8 |
| Five-component stones | 20.2 |
| Six-component stones | 13.4 |
| Seven-component stones | 7.5 |
| Eight-component stones | 1.3 |
| Nine-component stones | 0.7 |
Many of the compounds described above have been identified in minor proportions within a limited number of calculi. If we look at the main components of gallstones (Table 3), we note, as might be expected, a significant difference in the distribution of the main phases detected within the stones associated with sickle cell disease compared to other groups of cholelithiasis. For example, calcium bilirubinates accounted for 74.7% of cases in sickle cell patients versus 22.5% in other conditions (p < 10−6). Similarly, calcium carbonates (mainly calcite) were more frequent in sickle cell patients (14.6% versus 3.9%, p < 0.001). By contrast, cholesterol stones accounted only for 6.7% of gallstones in the case of sickle cell disease while they accounted for 67.6% of stones in other patients (p < 10−6). Of note, calcium phosphate stones were found with a similar occurrence in both groups.
Main components identified in gallstones related to sickle cell disease or to other etiological situations
| Main component | Sickle cells disease | Other contexts | p |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Biliary pigments | 56 (74.7) | 75 (22.5) | <10−6 |
| Calcium bilirubinates | 56 (74.7) | 75 (22.5) | <10−6 |
| Calcium carbonates | 11 (14.6) | 13 (3.9) | <0.001 |
| Calcite | 10 (13.3) | 8 (2.4) | <0.001 |
| Aragonite | 1 (1.3) | 5 (1.5) | NS |
| Cholesterol | 5 (6.7) | 225 (67.6) | <10−6 |
| Cholesterol anhydrous | 5 (6.7) | 220 (66.1) | <10−6 |
| Cholesterol monohydrate | 0 | 5 (1.5) | — |
| Calcium phosphates | 3 (4.0) | 8 (2.4) | NS |
| Carbapatite | 3 (4.0) | 7 (2.1) | NS |
| Amorphous carbonated calcium phosphate | 0 | 1 (0.3) | — |
| Proteins | 0 | 8 (2.4) | NS |
| Drugs | 0 | 4 (1.2) | — |
| Calcium ceftriaxonate | 0 | 2 (0.6) | — |
| Atazanavir | 0 | 1 (0.3) | — |
| Indinavir monohydrate | 0 | 1 (0.3) | — |
| Total | 75 | 333 |
Of the 408 stones, 30 cores were lost due to significant fragmentation during extraction. Of the remaining 378 calculi, infrared analysis of the selectively collected nucleation zone shows that 118 nuclei (31.2%) have a different composition than that observed later on the entire stone. The proportion was similar (41.1%) for stones associated with sickle cell disease and for those from other aetiologies (29%, NS). Thus, about 1∕3 of the calculi are initiated by a mechanism other than the one responsible for their subsequent growth. In sickle cell patients, 60% of the stones were initiated by calcium bilirubinate and only 1.5% by cholesterol. On the contrary, in other situations, cholesterol was the component of the nucleus of stones in 51.6% of cases and calcium bilirubinate in 31.6% of cases.
Table 4 shows the distribution of the stones according to the main component and the component of the nucleus that initiated the calculus. It shows that, for cholesterol stones and those composed mainly of bile pigments, about 70% had a nucleus of the same nature. This was even clearer for calcium phosphate stones, since 100% of them were formed from a calcium phosphate nucleus. On the other hand, calcium carbonate calculi were initiated in 69.2% of cases from another chemical phase. However, it still seemed to be a calcium salt, mainly calcium bilirubinate. An interesting point was an unusual prevalence of calcium phosphates in the nuclei of stones from sickle cell patients which appeared significantly higher than that observed in other conditions (23.5% versus 5.5%, p < 0.0001). Moreover, when a distinction was made between homozygous and compound heterozygous subjects, it appeared that the prevalence of phosphate nuclei was very high in the first group (31.4%) and not different from other bile stone contexts in the second group (5.9%, p < 0.01). No other significant difference was found in stone composition among homozygous and heterozygous sickle cell patients.
Composition of stone nucleus according to the main component of the stone
| Main component (number) | Component of the nucleus | Number (%) |
|---|---|---|
| Sickle cell disease | ||
| Calcium bilirubinate (n = 49) | Calcium bilirubinate | 33 (67.4) |
| Carbapatite | 11 (22.4) | |
| Calcium palmitate/stearate | 3 (6.1) | |
| Calcium carbonate | 1 (2.0) | |
| Unknown component | 1 (2.0) | |
| Calcium carbonate (n = 11) | Calcium carbonate | 3 (27.3) |
| Calcium bilirubinate | 4 (36.3) | |
| Carbapatite | 2 (18.2) | |
| Calcium palmitate/stearate | 2 (18.2) | |
| Cholesterol (n = 5) | Cholesterol | 1 (20.0) |
| Calcium bilirubinate | 4 (80.0) | |
| Carbapatite (n = 3) | Carbapatite | 3 (100) |
| Sickle cell disease excluded | ||
| Cholesterol (n = 215) | Cholesterol | 158 (73.5) |
| Calcium bilirubinate | 36 (16.7) | |
| Calcium palmitate/stearate | 11 (5.1) | |
| Carbapatite | 7 (3.3) | |
| Calcium carbonate | 2 (0.9) | |
| Ligature | 1 (0.5) | |
| Calcium bilirubinate (n = 76) | Calcium bilirubinate | 53 (69.7) |
| Calcium palmitate/stearate | 11 (14.5) | |
| Carbapatite | 6 (7.9) | |
| Foreign material (ligature, staple) | 3 (3.9) | |
| Cholesterol | 2 (2.6) | |
| Proteins | 1 (1.3) | |
| Calcium carbonate (n = 15) | Calcium carbonate | 5 (33.3) |
| Calcium bilirubinate | 9 (60.0) | |
| Calcium palmitate/stearate | 1 (6.7) | |
| Carbapatite (n = 4) | Carbapatite | 4 (100) |
4. Discussion
The varied nature of gallstones must, as with urinary stones, point to different aetiologies. However, several crystalline species can signal the same etiological process. For example, the presence of anhydrous cholesterol and cholesterol monohydrate points to lipid disorders. Conversely, the presence of neutral calcium bilirubinate, calcium hydrogen bilirubinate, or even free bilirubin, points to a very different lithogenic mechanism related to overproduction of bilirubin or a lack of conjugation thereof. Similarly, calcium carbonate stones, whether calcite, aragonite or vaterite, can point to local metabolic imbalances involving pH, calcium, and carbonate homeostasis.
As expected, the gallstones of sickle cell patients were composed mainly of bile pigments resulting from increased haemolysis in these patients (74.7% versus 22.5% for all gallstones excluding sickle cell disease, p < 10−6). However, a quarter of the stones were composed mainly of another crystalline species, in particular calcium salts (18.6%), such as calcium carbonate (14.6%) or carbapatite (4%). Despite the haemolytic background, about 7% of stones were mainly made of cholesterol, suggesting that abnormalities in lipid balance were also present in some patients and might actively participate in lithogenesis, even if cholesterol was rarely the cause of the stone (1.5%). In addition, analysis of the stones by SEM suggests that some of them might be related to a bile duct infection. These stones, few in number (n = 4∕75, or 5.3%), were pigment stones and black in colour on the periphery with a lighter brown-rust center, in which imprints of bacteria have been observed.
Not all sickle cell patients are susceptible to lithiasis. The lithogenic risk mainly arises from unconjugated forms of bilirubin which can then precipitate in the form of calcium salts. It is therefore necessary to question the participation of factors other than haemolysis in lithogenesis, in particular an alteration of glucuronoconjugation which can significantly increase the proportion of free bilirubin. Mutations of the hepatic UGT1A1 gene, which is able to ensure glucuronidation of bilirubin, could be an important factor in pigment gallstone formation. UGT1A1 gene polymorphisms with mutations in the TATA box promoter region were associated with serum level of unconjugated bilirubin and the risk of cholelithiasis in various populations, including sickle cell patients [40, 41, 42, 43, 44, 45, 46, 47]. It was shown that the TATA box region of the UGT1A1 gene contains a group of TA repeats, with four alleles that differ in the number (from 5 to 8) repeats [42]. As reported for thalassemia patients, homozygous mutations TA(7)/TA(7) of the UGT1A1 gene were associated with a high probability of the prevalence of gallstones, in comparison with thalassemia patients exhibiting TA(6)/TA(6) UGT1A1 gene: OR = 3.88 (95% CI: 1.31–11.55) versus 1.68 (95% CI: 0.7–4.03) [48]. Chaouch et al. [49] have compared the variants of the UGT1A1 gene in sickle cell and thalassemia patients and they have shown a significant association between heterogeneous TA(6)/TA(7) or homogenous TA(7)/TA(7) mutations and the risk of cholelithiasis among these patients. The Gilbert syndrome-associated UGT1A1 mutation is a common finding affecting about 10% of the general population, and can be involved in hyperbilirubinemia states, intermittent episodes of jaundice and black pigment gallstones [50, 51, 52, 53]. In cystic fibrosis patients, it was reported that those with UGT1A1 allele mutations were susceptible to gallstones (OR = 7.3 versus normal UGT1A1 gene) and higher serum levels of unconjugated bilirubin [54]. In a recent meta-analysis of 34 studies, it was reported that the overall prevalence of cholelithiasis among sickle cell patients was 25.3% and that the frequencies of (TA)7 and (TA)8 were significantly higher in patients with cholelithiasis [23].
Note that other types of gallbladder stones may be related to a defect in the UGT1A1 gene. In our series of stones we found three cases of calculi that contained atazanavir, a protease inhibitor extensively used in the past decade in therapy of HIV+ patients. One stone was almost pure atazanavir and the two others were pigment stones with a high content of both calcium bilirubinates and atazanavir. Indeed, it has been reported that serum bilirubin level was correlated to the plasma concentration of atazanavir and that atazanavir is able to inhibit the hepatic UGT1A1 metabolic pathway [55]. Moreover, it has been shown that atazanavir-treated patients carrying a variant of the UGT1A1 gene had a higher baseline bilirubin level and a slower atazanavir hepatic clearance [56], favouring the drug crystallization.
Several studies have reported that crystallization of calcium salts could be reduced by proteins such as mucin [57, 58, 59], and that imbalance of bile composition with respect to calcium, carbonate, and macromolecules, could favour calcium carbonate crystallization. Gleeson et al. [60] and more recently Yu et al. [61] provided convincing arguments that bile pH, calcium concentration and
Other predisposing factors such as chronic inflammation and reactive oxygen species production may be involved in gallstones as well as in other clinical complications of sickle cell disease [58, 61, 62, 63].
It has been known for some time that bile infection is another possible cause of gallstones [64, 65, 66]. Bilirubin deconjugation by bacterial glucuronidases could explain why brown pigment stones are more frequent in Asia than in western countries [67, 68]. These are often located in the bile ducts and associated with obstruction [68, 69]; calcium palmitate is often identified within such infected stones. Its presence could be linked to increased activity of bacterial phospholipases increasing the generation of free fatty acids which can precipitate as calcium salts [70]. In sickle cell patients, such a mechanism cannot be excluded but seems of limited importance in stone formation. We note that in our series of 75 stones, only five (6.7%) contained calcium palmitate and SEM images of four of them suggested the presence of bacterial imprints.
An unexpected finding from stone analysis was the high proportion of gallstones from sickle cell patients that were nucleated from calcium phosphate (23.5% versus 5.5% in other conditions, p < 0.0001). It has been reported that serum phosphate is increased in sickle cell disease [71, 72, 73]. However, phosphate content was determined in bile of patients who formed pigment stones and no difference was found in comparison with patients producing cholesterol stones. We have shown in Table 4 that calcium phosphate is not frequent in pigment stones from patients without sickle cell disease. In addition, the frequency of phosphate nuclei was very high in homozygous patients (31.4%), but was not different from other bile stone contexts in the heterozygous subjects (5.9%, p < 0.01). A possible explanation could be a more frequent and severe haemolysis in homozygous than heterozygous sickle cell patients. However, biochemical data to demonstrate this link with stone formation are lacking. It would be of interest to determine the phosphate content in bile of sickle cell patients with stones nucleated from calcium phosphate, and to correlate the data with their homozygous or heterozygous status, and also compare with the bile phosphate of sickle cell patients without stones.
All these observations suggest that several factors may contribute to the formation of stones in patients with sickle cell disease. Given the diversity of aetiologies and the number of identifiable components in gallstones, it would certainly be interesting to refine the composition studies of these stones by improving the phenotyping of patients, as we have shown over several years with respect to urinary tract stones [74, 75, 76, 77, 78]. This would improve correlation between clinical history, etiopathogenic factors, and the composition of calculi. Admittedly, unlike renal lithiasis, cholelithiasis leads to clinically symptomatic recurrences of stones in only a small proportion of cases, which limits the clinical interest in these stones and their cause(s). However, the chronic metabolic context that characterizes sickle cell disease predisposes to recurrence of bile duct lithiasis more than other pathological conditions [22]. This justifies improving understanding of the individual lithogenic risk factors in order to mitigate them with more selective protective measures.
5. Conclusion
Gallstones of sickle cell patients appear significantly different from those from the general population. As expected, chronic haemolysis favours the formation of pigment stones. However other factors may be involved and this should prompt more studies. For instance, an accurate examination of stone composition, especially of the stone nucleus, has revealed that a high proportion of stones are initiated from carbapatite. An increase in serum phosphate could be indicated, but, as shown for calcium carbonate crystallization in bile, the phosphate content of bile in sickle cell patients remains to be assessed in the future.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0