1. Introduction
For a while, transformation of various petroleum hydrocarbons to more valuable products and their valorization was considered as a topic that frequently has been addressed in the literature. This topic is still relevant today, as petroleum remains essential for the maintenance of industrial civilization. In the transformation of petroleum hydrocarbons, one of the main processes that valorize saturated hydrocarbons refers to the aromatization of light alkanes in the presence of various catalysts. Over time, the catalytic activity of different materials, especially of those exhibiting zeolite structures, for this purpose was investigated [1, 2]. Among them, one of the most investigated was MFI-type zeolite framework, due to its high surface area, well-defined porosity and selectivity [3, 4, 5, 6, 7, 8].
After being discovered at the end of the last century, the MFI-type zeolites have drawn a great deal of interest due to their unique 2D channel system and a relatively slow deactivation [9]. One of the well-known zeolites with MFI structure is ZSM-5, found to be a highly shape-selective catalyst for a variety of catalytic reactions. Findings reported in the literature elsewhere indicate a feature synergy that enables a mechanism of specific selectivity and a very slow coking process. This is the result of the action of strong acid sites on the catalyst surface, besides the hydrophobic character of the zeolite pore walls and the channel system of specific size. As is well known, MFI-type zeolite in general, and ZSM-5 in particular, belongs to the pentasil family of high-silica zeolites (Si/Al > 10) whose 3D porous structure is formed by 2D types of intersecting channels containing 10-membered rings, one of which is straight along the b direction, with almost circular opening (0.53 × 0.56 nm) and another sinusoidal (zig-zag) along the a crystallographic direction, with an elliptical opening (0.51 × 0.55 nm) [10]. Both channels meet at a relatively open intersection creating an approximately spherical super cage cavity with a diameter of about 0.85–0.9 nm, where the strong acid sites responsible for hosting C6–C8 aromatic hydrocarbons are located. The normal aliphatic hydrocarbons can diffuse freely in both channels but aromatics and iso-alkanes prefer the sinusoidal elliptical channels [11, 12].
One of the catalytic processes in which the modified ZSM-5-like catalysts have proved their superiority refers to the conversion of light alkanes and alkenes (C2–C4) into more useful aromatic compounds, such as BTX (benzene, toluene and xylenes). Thus, due to their important role in various petrochemical and chemical processes, the production of BTX aromatics by valorization of hydrocarbon fraction resulting in the petroleum refining processes, has caught the attention of researchers all over the world [13, 14].
Over time, numerous researches have been reported in literature focusing on the ability of HZSM-5 and HZSM-5 modified with gallium and zinc, that were used to enhance the conversion of alkanes, as well as the selectivity toward the formation of aromatics [15]. However, other manuscripts reporting the use of HZSM-5 catalysts modified with Pt, Ag and Ni too could be found in the literature. But, although Pt showed an improved catalytic activity, as well as an increased dehydrogenation rate of light alkanes, its preparation remains a high-cost procedure. More than that, its use as catalyst has a major disadvantage because of its active participation in the hydrogenolysis reaction leading to the formation of unreactive alkanes (C1, C2) and cyclic compounds, that are responsible for the decrease of aromatic selectivity [16, 17]. On the other hand, Ni- and Ag-modified HZM-5 zeolite shows good catalytic activity, being highly selective toward BTX aromatics which have been involved in the conversion process of light alkanes and alkenes [15, 18, 19]. Moreover, MFI-type zeolites modified with various metals such as Sn, Zr, Cu, and Ge could exhibit as well remarkable catalytic activity considering the same conversion processes [20].
Generally, H-form of ZSM-5 zeolite used as catalyst in the conversion reaction of light alkanes and/or alkenes to aromatic hydrocarbons, has a low selectivity due to a very fast β-scission side reactions [21, 22]. However, by hosting metal species like Ga, Zn, Pt through zeolite framework the rate of aromatization reactions and selectivity toward aromatics increases, while the β-scission reactions are inhibited, thus avoiding the formation of unwanted products. Basically, the main role of the metal in Me-ZSM-5 catalysts is the catalytic dehydrogenation of alkanes and naphthenic intermediates, while the acid sites (Brønsted and Lewis) from the catalyst surface are responsible for the oligomerization reaction of alkenes to dienes and trienes, as well their cyclization to naphthenes [23]. Moreover, side reactions such as catalytic cracking of C–C bond over strong Brønsted acid sites, hydroisomerization in the presence of excess hydrogen, alkylation, isomerization, disproportionation and hydrogenolysis also occur with various yields over monofunctional and bifunctional catalysts [24].
Nowadays, aromatization procedure for converting light hydrocarbons to BTX fraction over HZSM-5-based catalysts is already available at the industrial level. Thus, ZSM-5 zeolite is currently used in various industrial processes as M2-Forming, M-Forming, BP/UOP Cyclar, Z-Forming, RZ-Platforming, Alpha, AromaxTM, and Aroforming processes [25, 26]. But, to the best of our knowledge, no reports focusing on the evaluation of catalytic performance of Zn–Cu modified ZSM-5 bimetallic catalyst in the conversion reaction of butane (nC4 + i‐C4) technical fraction, especially at a pilot-scale reactor are available in the literature. Hence, this paper deals with the evaluation of catalytic properties of Zn–Cu/ZSM-5 bifunctional catalyst in the conversion process of gaseous butane technical mixtures to BTX-rich liquid fractions, and its performance validation in a pilot-scale reactor.
2. Experimental
2.1. Materials
Sodium silicate solution (Reagent Grade, Sigma Aldrich), H2SO4 (96%, Chemical Company), Al2(SO4)3 ⋅ 18H2O (Sigma Aldrich), ethylene glycol (ReagentPlus®, ⩾99%, Sigma Aldrich), NH4OH (p.a., 25%, Chemical Company), Cu(NO3)2 ⋅ 3H2O (puriss. p.a., Sigma Aldrich), Zn(NO3)2 ⋅ 6H2O (Reagent Grade, Sigma Aldrich), and deionized water. All chemicals were used as received.
2.2. Catalyst preparation
A few steps were followed in the synthesis procedure of ZSM-5-based catalyst, starting first with the preparation of Na-ZSM-5 zeolite form, as described by the authors in [27]. The Na-form of ZSM-5 zeolite was obtained by hydrothermal synthesis procedure (455 ± 5 K) in 24 h, using an alkaline sodium silicate solution, H2SO4, Al2(SO4)3 ⋅ 18H2O, ethylene glycol (EG), deionized water, NH4OH. The molar ratio of Na-ZSM-5 precursors in the synthesis procedure of the zeolite catalyst was SiO2:Al2O3:EG:H2O = 58.92:6.49:29.43:1832. Finally, the Si/Al ratio in the as-prepared Na-ZSM-5 was found to be of 36.02, while the content of Na2O was found to be 2.65 wt%.
Further, the H-form of ZSM-5 zeolite material was prepared by ion-exchange procedure. Therefore, the resultant Na-ZSM-5 sample was subjected to a chemical treatment with 1 M NH4NO3 solution, at 353 K for 6 h. The treatment has been repeated three times, afterwards the solid was recovered by filtration, dried overnight at 283 K and calcined at 823 K (at a heating rate of 1 degree⋅min−1) for 6 h, resulting in the H-ZSM-5 zeolite form. The third step continued with the synthesis of Zn–Cu/ZSM-5 bifunctional catalyst containing 1.73 ZnO wt% and 0.64 wt% CuO. The sample was prepared following the synthesis procedure reported in detail by the authors in [28]. First, the Zn-ZSM-5 intermediate was prepared by immersing H-ZSM-5 sample in 0.1 M Zn(NO3)2 aqueous solution. The recovered solid was filtered, washed, dried and calcined at 823 K, in order to get ZnO distributed in the zeolite matrix. Further, the resultant Zn-ZSM-5 sample was subjected to another ionic exchange reaction using 0.1 M Cu(NO3)2 solution. By applying the same thermal treatment as for ZnO, Zn–Cu/ZSM-5 bifunctional catalyst was obtained. Finally, the resultant Zn–Cu/ZSM-5 powder was extruded with 20 wt% γ-Al2O3 as binder, in short cylindrical forms (3 × 10 mm), that were dried at 383 K for 6 h, then calcined at 823 K in air for 6 h.
2.3. Characterization of the prepared catalyst
The Na-ZSM-5 sample prepared has been characterized from the structural point of view, confirming the MFI structure of high crystallinity, using a Philips PW 1830 instrument (Ni-filtered CuKα radiation) [29]. By using a Microspec WDX-2A Scanning Electron Microscope (SEM) with an accelerating voltage of 25 kV, the morphology could be investigated. Thus, the prepared catalyst was found to reveal a well-defined cubic morphology of crystals. Temperature Programmed Desorption (TPD) technique using ammonia was used to determine the strength and acidity distribution on Zn–Cu/ZSM-5 catalyst surface. Thus, a known amount of the sample was activated in a dry N2 flow at 773 K for 4 h, then cooled to 353 K to introduce ammonia. Another adsorption experiment has been performed, and after adsorption, the catalyst has been heated up to 1073 K (at a heating rate of 10 degree⋅min−1) and the desorbed ammonia was quantified by absorption in 1 M HCl. Further, the desorbed ammonia was correlated with the total acidity (weak and strong) of the catalyst, and it was found to be of 0.863 mmol NH3/g for the prepared bifunctional catalyst. As the TPD plot shows two peaks, the acidity at two different temperatures (LT453 K—low temperature, and HT723 K—high temperature) has been estimated. Thus, the low-temperature peak was determined to correspond to weak acid sites, while the high-temperature peak was assigned to strong acid sites. The calculated amounts of weak-acid sites and strong-acid sites were found to be of 0.737, and 0.126 mmol NH3/g, respectively. Further, the information about the strength and the number of the acid sites on the catalyst surface was obtained [28]. As textural properties of a material play an important role in catalytic processes, the specific surface area has been evaluated by applying the BET (Brunauer–Emmett–Teller) equation to the registered N2 adsorption–desorption isotherm on a Carlo–Erba Sorptomatic Series 1800 apparatus, at 77 K. The nonlocal density functional theory (NLDFT) approach has been considered as well to determine the pore size distribution [31].
2.4. Catalytic performance evaluation
The catalytic activity of Zn–Cu/ZSM-5 sample in the conversion reaction of C4 (n‐C4 + i‐C4) technical fraction at 793 K, under atmospheric pressure and 1 h−1 WHSV (Weight Hourly Space Velocity), to BTX, was studied. All tests occurred in a Twin Reactor System Naky Metrimpex-type fixed-bed continuous flow stainless-steel reactor. In advance, a thermal pre-treatment of the catalyst has been performed (at 773 K for 6 h, N2 gas flow) in order to eliminate all impurities and moisture adsorbed on its surface. The composition of C4 (n‐C4 + i‐C4) technical fraction included mostly of iso-butane, i-C4 (76%–82% (v/v)) and n-butane, n-C4 (15%–22% (v/v)), through which small amounts of butenes, ∑C4 = (0.3%–1.5% (v/v)), and C3-fraction (0.5%–1.5% (v/v)) were found.
During the catalytic tests, the resultant products were separated through an ice-trap, to a liquid and a gas fraction. Two different chromatographs were used to determine the composition of the resultant phase products. The composition of the liquid phase was determined by using a GC Carlo Erba (model C) apparatus equipped with FID detector (flame ionization) and a fused silica capillary column (of 25 m length and 0.32 mm i.d.) with SE-52 stationary phase. While, the gaseous phase composition was determined on a GC Carlo Erba (model Vega), equipped with a TCD detector (thermal conductivity) and a column, of 6 m length, containing squalane and dimethylsulpholane.
In order to validate the catalytic performance of the synthesized Zn–Cu/ZSM-5 catalyst, ten further catalytic tests, at 793 K, using butane technical fraction (with variable compositions) were performed. After each experiment, the catalyst was regenerated by a thermal treatment at 823 K for 6 h, under N2-flow containing 2 wt% oxygen. In order to achieve a high yield in the liquid product, the temperature, WHSV and pressure were optimized in advance and optimal values of 593 K, 1 h−1, and atmospheric pressure, respectively, were considered.
3. Results and discussion
3.1. Catalyst characterization
The structural characterization of the synthesized Zn–Cu/ZSM-5 catalyst was done by registering the XRD pattern (Figure 1a). As observed, the catalyst exhibits characteristic peaks corresponding to ZSM-5 zeolite MFI-type structure (JCPDS: 44-0003), being indicative of the retention of zeolite crystal structure when copper and zinc were added [29]. Specific regions assigned to copper oxide (JCPDS: 80-1268) and zinc oxide (JCPDS 36-1451) phases in bimetallic catalyst were identified, as well. Moreover, XRD pattern allowed to calculate the interplanar distances of 0.25 nm and 0.19 nm, which were assigned to CuO and ZnO, respectively. Further, according to the Scherrer’s equation, the average crystallite sizes of 24.5 nm and 22.8 nm for CuO and ZnO, respectively, were estimated. Besides, the catalyst was characterized from the textural point of view and the N2-sorption isotherms registered are presented in Figure 1b. Thus, according to IUPAC classification [30], the isotherm was of the type I–IV combined model, characterized by a high sorption at very low relative pressures (p∕p0 < 0.05) due to the microporosity (pores of d < 2 nm), as well as at higher relative pressures (0.2 < p∕p0 < 1) due to the mesoporosity of the prepared catalyst (2–50 nm). The adsorption–desorption isotherm is accompanied by a type H4 hysteresis loop, with a pronounced uptake at low relative pressures that is associated mainly with the filling of micropores. In the inset of Figure 1b, the corresponding NLDFT pore size distribution could be found, yielding a bimodal distribution that proves the presence of micro and mesopores of 0.85 nm and 3.49 nm through the Zn–Cu/ZSM-5 catalyst structure. The catalyst was found to be characterized by an excellent pore structure of large specific surface area (271 m2⋅g−1), and a large total pore volume (0.262 cc⋅g−1), recommending the synthesized Zn–Cu/ZSM-5 bifunctional catalyst as a good candidate for catalytic processes. Due to the peak intensity of micropores given by NDLFT pore size distribution, the micropore surface area was found to be of 150 m2⋅g−1, while the external surface area is 121 m2⋅g−1, meaning that the microporosity of the prepared bifunctional catalyst is 55% of the total porosity.
Structural and textural characterization of the synthesized Zn–Cu/ZSM-5 catalyst: (a) XRD pattern, and (b) N2-sorption isotherm and in the inset the NLDFT pore size distribution isotherm graph is shown.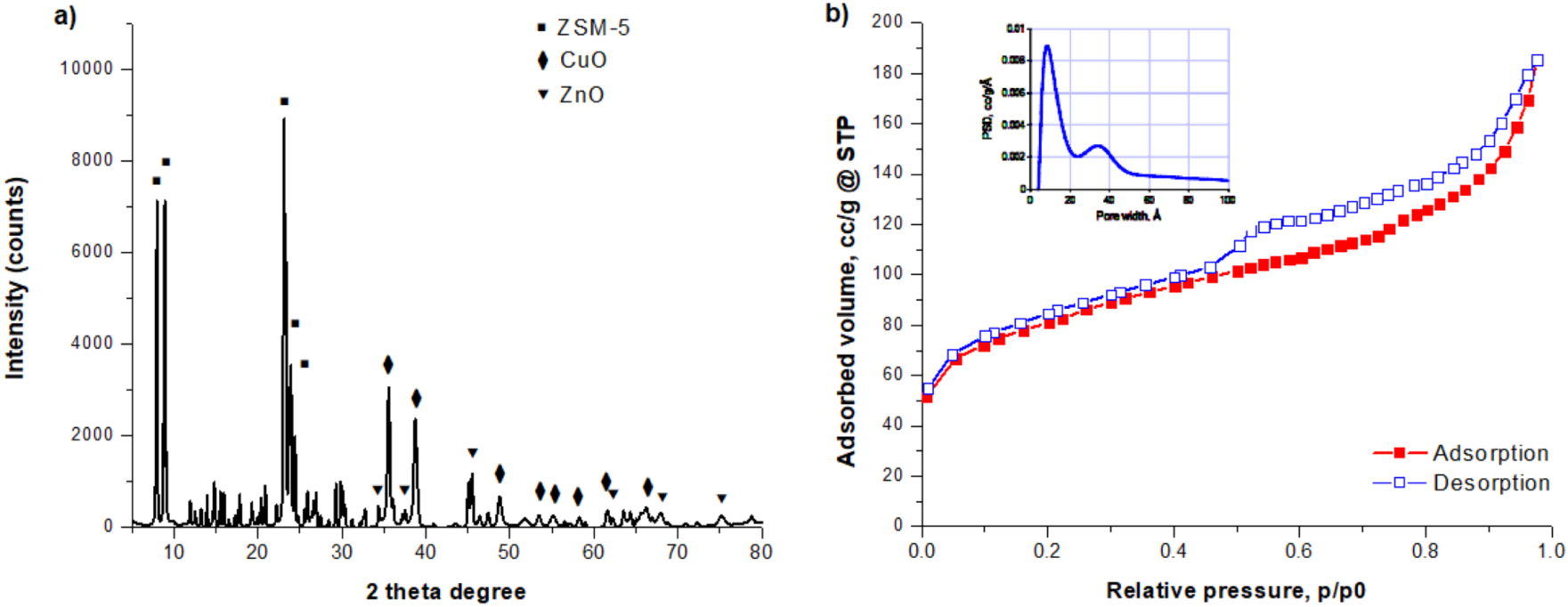
3.2. Catalytic performance evaluation at pilot-scale reactor
As a result of the conversion experiments of C4 (n‐C4 + i‐C4) technical fraction over the extruded Zn–Cu-ZSM-5 catalyst, the average yields in both liquid and gaseous phase products have been obtained. Thus, changes in the gaseous product composition over Zn–Cu/ZSM-5 sample on stream (monitoring every four hours) have been observed, and the results registered for the catalytic test No. 1 were drawn and are shown in Figure 2.
Variation of the gaseous product composition vs. time on stream over prepared Zn–Cu/HZSM-5 bifunctional catalyst; data obtained as the result of catalytic test No. 1 in aromatization process of n‐C4 + i‐C4 technical fraction.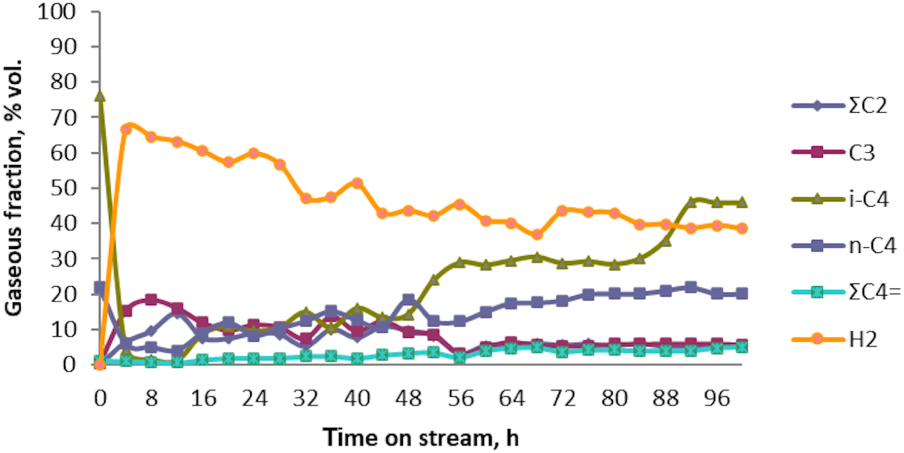
Starting from 76.21% (v/v) in the raw material, it was observed that in the first 20 h of conversion reaction, a large quantity of i-C4 hydrocarbons is consumed, thus decreasing its concentration to 2.5% (v/v). Thereafter, as observed from Figure 2, until the end of the experiment, an increase in concentration of i-C4 is achieved (up to 50%), reaching approximately more than half the value of the initial concentration. Even though the n-C4 fraction has an initial concentration three times smaller (22.03% (v/v)) than i-C4, in the raw material, the same trend could be observed. Thereafter, it consumes appreciably in the first 20 h of conversion reaction, after which the concentration is slightly increased, as could be observed in the Figure 2. At the same time, as the reaction progresses, C2 and C3 hydrocarbon fractions, which are virtually absent in the raw material, forms in small quantities (up to a concentration not exceeding 15% (v/v)). Whilst, ∑C4 = fraction, especially butenes, during the catalytic test No. 1, show a slow increase in its concentration from 1.25% (v/v) to 5% (v/v). Most likely, once formed, they are consumed due to the difference in reactivity with respect to butane. As hydrogen is the major gaseous product throughout the catalytic test, its concentration in the first 20 h of the conversion reaction exceeds 60% (v/v), then a decrease to 45% (v/v) could be observed, when 100 h of conversion reaction have passed.
Regarding the liquid fraction, the resultant of the conversion reaction of butane’s technical fraction, it was found that all catalytic tests lead to an average yield of the liquid phase product exceeding 40 wt%, with a content of aromatic hydrocarbons exceeding 80 wt%. Thus, the variation in the product concentration of the liquid fraction, as the result of the catalytic test No. 1 of aromatization of the technical fraction n‐C4 + i‐C4, on the Zn–Cu/ZSM-5 bimetallic catalyst surface is shown in Figure 3.
Liquid composition vs. time on stream over Zn–Cu/ZSM-5 catalyst; catalytic test No. 1.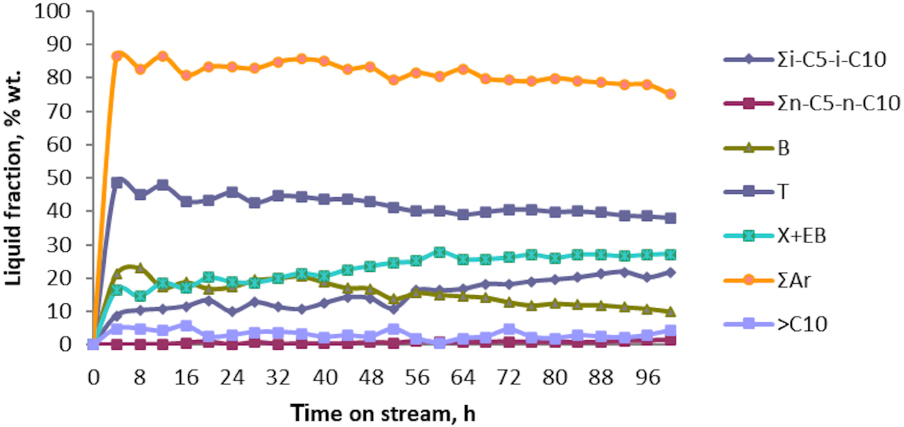
As observed, BTX aromatic hydrocarbons (∑Ar) represent the majority products of the liquid fraction that resulted throughout the catalytic test. Their concentration exceeds 80 wt% in the first 60 h of reaction and reaches 75 wt% after 100 h of reaction. Through BTX aromatics, toluene (T) is the major aromatic hydrocarbon whose concentration exceeds 40 wt%, after 84 h of the conversion reaction. On the other hand, benzene (B) concentration exceeds 20 wt% in the first 20 h, after which its concentration decreases monotonously, while the aromatic hydrocarbons C8, as xylenes and ethyl benzene (X+EB), form in quantities lower than 20 wt%, in the first 36 h of the catalytic test. Therefore, the concentration of aromatic fraction C8 reaches about 28 wt% after 100 h. This can be explained by the strong acidic centers formed during the first hours of aromatization reaction over Zn–Cu/ZSM-5 catalyst, that most likely favors C8 aromatic hydrocarbon dealkylation reactions. Furthermore, the “oligo” fraction ( >C10) seems to be formed in small quantities throughout the catalytic test, their concentration being below 6 wt%. Further, C5–C10 fractions (∑i‐C5 − iC10), namely iso-alkanes, in amounts corresponding to concentrations ranging between 8.73 wt% after 4 h and 21.75 wt% after 100 h of conversion reaction, were formed. The concentration of n-alkanes from C5–C10 fraction (∑n-C5–n-C10) do not exceed 2% in the liquid fraction of the resultant products. It was as well observed that iso-propylbenzene (IPB) is formed in quantities lower than 2%, while naphthalene is present in quantities lower than 1.5%.
Figures 4 and 5 show the variation in the concentration of i-alkanes and n-alkanes of the resultant liquid fraction of the catalytic test No. 1 of aromatization reaction of butane technical fraction over the prepared Zn–Cu/ZSM-5 catalyst.
Variation of the i-alkanes i-C5 − i-C10 concentration in liquid fraction vs. time on stream as the result of catalytic test No. 1 of technical fraction (n‐C4 + i‐C4) aromatization over Zn–Cu/ZSM-5 sample.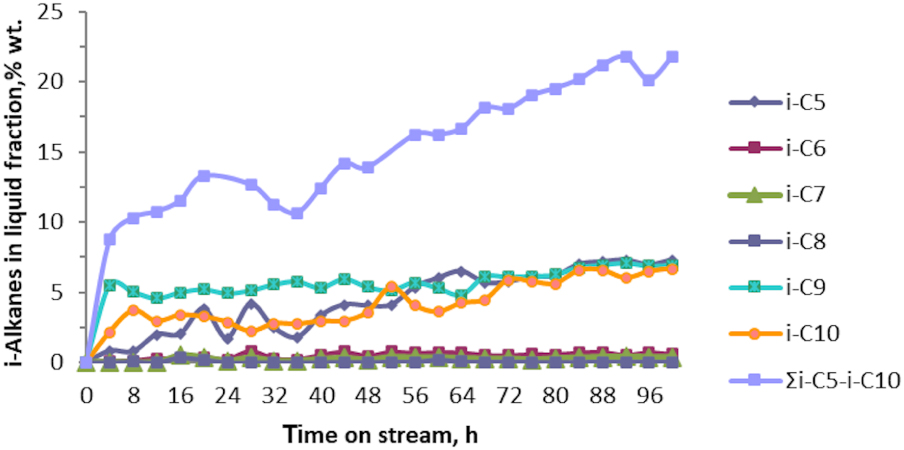
Variation of the n-alkanes n-C5 − n-C10 concentration in liquid fraction vs. time on stream in catalytic test No. 1 of technical fraction (n‐C4 + i‐C4) aromatization over Zn–Cu/ZSM-5 sample.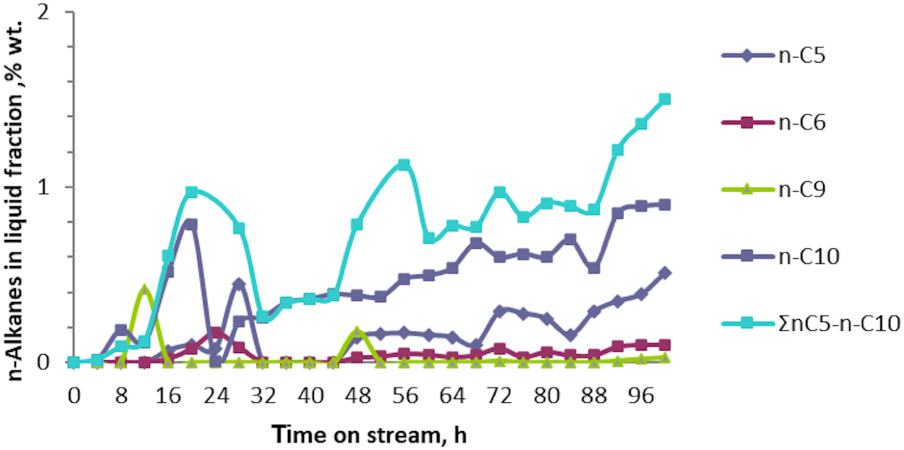
From the Figure 4 it is observed that i-alkanes (i-C5, i-C9, and i-C10) were obtained in more significant quantities. Thus, their concentrations were found to exceed 5 wt% in the resultant liquid fraction as 50 h of reaction passed. Moreover, altogether, the sum of i-alkanes, ∑i-C5 − i-C10, does not exceed 23 wt% in the liquid fraction after 100 h of the reaction. In contrast, n-alkanes, ∑n-C5 − n-C10, were obtained in very small quantities. Their concentration exceeds 1 wt% in the liquid fraction only when ∼90 h of the reaction time passed. When the concentration of BTX hydrocarbons in the liquid fraction dropped below 75 wt%, the regeneration procedure to the Zn–Cu/ZSM-5 catalyst was applied.
Further, Figure 6 illustrates the variation in the composition of the gaseous fraction produced as a result of the catalytic test No. 10 of the aromatization of a technical fraction of n‐ + i‐C4 over Zn–Cu/ZSM-5 catalyst surface.
Aromatization of n‐ + i‐C4 technical fraction: catalytic test No. 10. Evolution of the gaseous products composition vs. time on stream over Zn–Cu/ZSM-5 catalyst.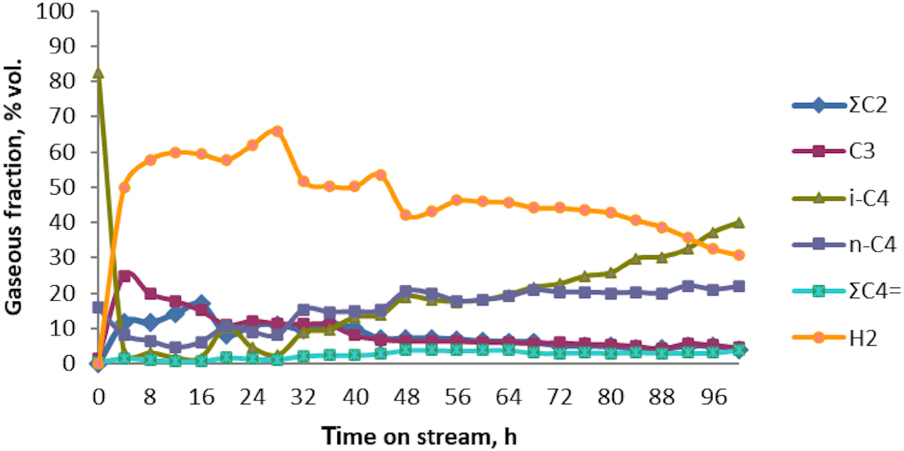
Regarding i-butane, i-C4, its concentration in the raw material was found to be of 82% (v/v), being consumed appreciably during the catalytic process. Therefore, its concentration was found to decrease to 40% (v/v) when 100 h of the reaction time passed. Concerning n-butane fraction, in the first 40 h of the reaction time, n-C4 present at 15.77% (v/v) in the feed gas, was consumed appreciably, after which its concentration slowly rose and exceeded 20% (v/v) when 100 h of the reaction time were achieved. At the same time, C2–C3 hydrocarbons (∑C2 and C3), virtually absent in the raw material, were formed in quantities exceeding 10% (v/v) when about 35 h of the reaction time have passed, but did not exceed 25% when 100 h were passed. Further, butenes, , absent in the raw material were obtained in quantities not exceeding 4% (v/v) in gaseous fraction. As observed, hydrogen, H2 is the predominant resultant gaseous product, its concentration exceeding 40% (v/v) in the first 88 h of the conversion reaction.
Figure 7 shows the variation of the resultant liquid fraction composition in the catalytic test No. 10 of aromatization reaction of butane technical fraction on the Zn–Cu/ZSM-5 catalyst.
Variation of liquid composition vs. time on stream of aromatization of butane technical fraction in the presence of Zn–Cu/ZSM-5 sample; catalytic test No. 10.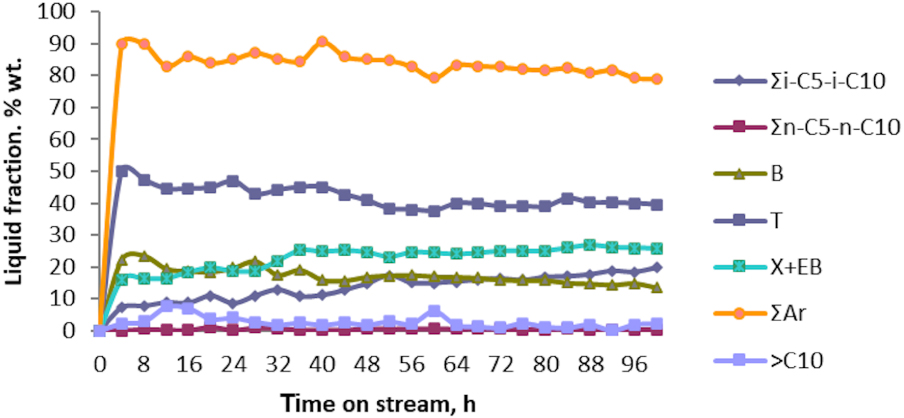
Thus, BTX aromatics, ∑Ar, were found to dominate the liquid fraction, ranging from 91.0 wt% in the first 8 h to 79 wt% after 100 h of the reaction. Toluene (T) is the aromatic hydrocarbon which is obtained as major component, its concentration exceeding 40 wt% within 100 h of the reaction. Benzene (B) concentration in the aromatic hydrocarbon fraction was found to achieve 20 wt% in the first 32 h, then monotonously decreases to 14 wt% when 100 h of the catalytic reaction has passed. Regarding C8 aromatic hydrocarbons, (X+EB—xylenes + ethyl benzene), their concentrations did not exceed 20 wt% in the first 30 h, but later their concentration increased up to 26% when 100 h of the conversion reaction passed. The variations in B concentration and C8 aromatic hydrocarbons are correlated with the presence of strongly acidic centers in the first hours of catalytic reaction. The concentration of i-alkanes C5–C10, ∑i-C5 − i-C10, averages between 7.3 wt% after the first 4 h and 19.7 wt% after 100 h, while the n-alkanes C5–C10, ∑n-C5 − n-C10, are formed in quantities lower than 1 wt%. The concentration of “oligo” ( >C10) fraction varies between 2 wt% and 7 wt% throughout the catalytic test.
Figures 8 and 9 show the variations in the composition of the i- and n-C5–C10 of the liquid fraction at the end of the catalytic test No. 10. The main i-alkanes resulting in quantities exceeding 5 wt% in the liquid fractions are i-pentanes, i-nonans and i-decanes. As observed, the amount of i-alkanes (∑i-C5 − i-C10) monotonously increased up to 20 wt%, but did not exceed this value even when 100 h of the catalytic reaction were passed. On the contrary, n-alkanes (∑n-C5 − n-C10) were obtained in small quantities, their concentration being up to 1 wt% in the liquid fraction throughout the tenth catalytic test.
Variation of the i-alkanes i-C5 − i-C10 in the liquid fraction obtained in catalytic test No. 10 of aromatization of the technical fraction i‐C4 + n‐C4 over Zn–Cu/ZSM-5.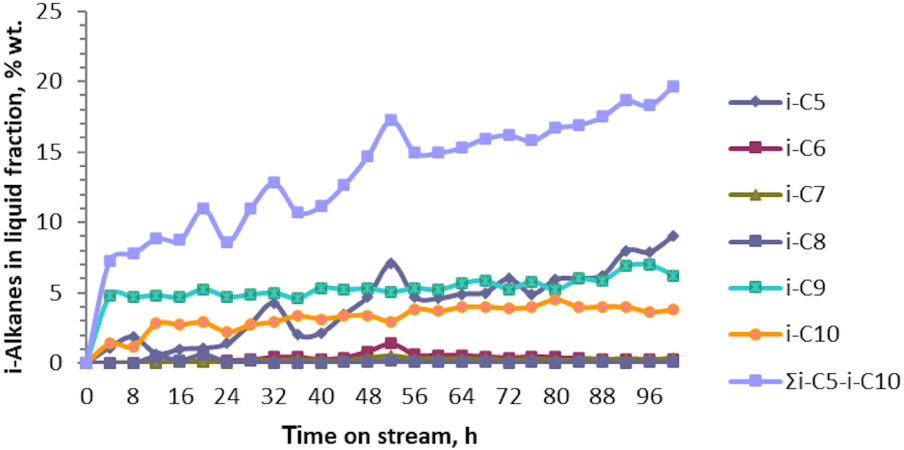
Variation of the n-alkanes n-C5 − n-C10 in the liquid fraction obtained in catalytic test No. 10 of aromatization of the technical fraction i‐C4 + n‐C4 over Zn–Cu/ZSM-5.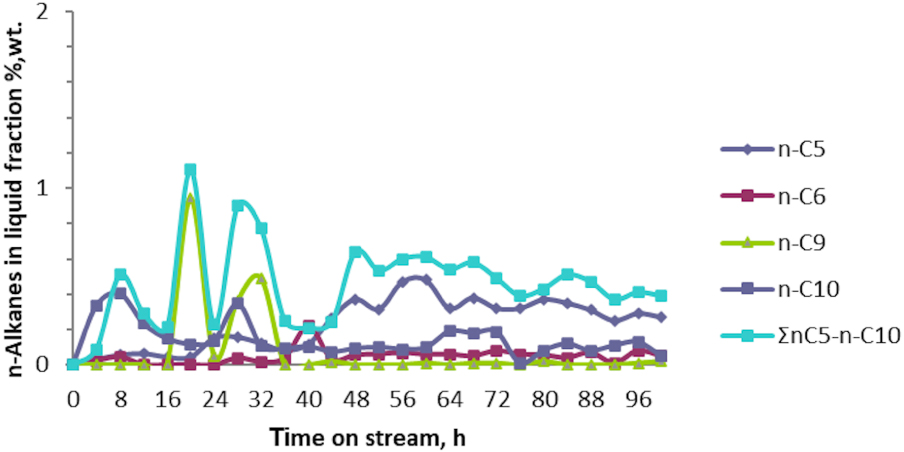
Further, the evolution of average BTX aromatics in ten cycles of the catalytic tests in Figure 10 was drawn. In essence, the aromatization reaction of technical fraction of butane (n‐C4 + i‐C4) over Zn–Cu/ZSM-5 bifunctional catalyst, at pilot scale, was found to show more or less the same yield, at the end of each catalytic cycle. As a matter of fact, these results proved that the prepared bifunctional catalyst is stable and could be reused in a pilot-scale reactor, at least ten times, without losing its catalytic activity in the aromatization process of butane technical fraction (n‐C4 + i‐C4).
BTX concentrations (average values per test) in liquid fraction obtained in ten catalytic cycles in the aromatization process of butane technical fraction (n‐C4 + i‐C4) over extruded Zn–Cu/ZSM-5 catalyst (793 K, atmospheric pressure, WHSV = 1 h−1).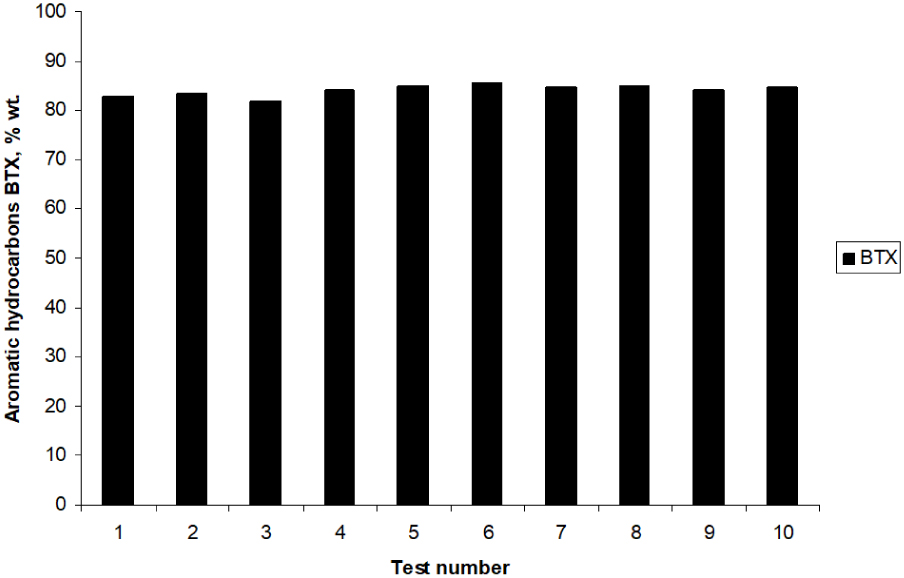
In conclusion, during all ten catalytic aromatization experiments of butane technical fraction (n‐C4 + i‐C4) carried out on Zn–Cu/ZSM-5 sample, at optimized experimental conditions (793 K, WHSV = 1 h−1 and atmospheric pressure) the concentration of BTX aromatic hydrocarbons in liquid fraction (average values per test) was found to exceed 81 wt%. Mention could be made that after each cycle, the catalyst has been regenerated by thermal treatment at 823 K in a N2 flow with 2 vol.% O2, without its removal outside the test reactor.
In short, the conversion of butane mixture over prepared Zn–Cu/ZSM-5 bifunctional catalyst has been found to take place via a complex suite of reactions that include cracking and cyclization, further dehydrogenation, oligomerization and isomerization, β-scission and H transfer [32]. Actually, the aromatization process has been proved to be complex, through which the low molecular weight alkanes are transformed to BTX aromatics following a three-stage process [33]: alkanes transformation to alkenes (), cracking and oligomerization of alkenes, and alkenes cyclisation to aromatic hydrocarbons C6–C10. Among these reactions, the most relevant are: activation of C–H bonds of alkanes; transformation of carbonium to carbenium ions, and finally to small alkenes, ; oligomerization of alkenes to ; isomerization and β-scission; dehydrogenation to dienes; cyclization to cyclic alkenes; and finally, dehydrogenation to cyclic dialkenes and aromatic hydrocarbons.
4. Conclusions
In conclusion, the prepared bifunctional catalyst, Zn–Cu/ZSM-5, showed a high selectivity to BTX aromatics, being involved in the aromatization process of butane technical mixture. In the investigated process BTX were formed as a result of dehydrogenation reaction of alkanes to alkenes, dehydrocyclization step of alkenic oligomers to intermediates (naphthenes), and, finally, to aromatics, all reactions occurring on the surface of the synthesized Zn–Cu/ZSM-5 bifunctional catalyst. In consequence, the average content values of the resultant BTX aromatics in the liquid phase exceeded 81 wt%, which is considered a significant value. Their formation on the prepared Zn–Cu/ZSM-5 catalyst surface is explained in terms of increased production of alkenes as the result of dehydrogenation reaction for which the zinc and copper species are responsible.
Moreover, the distribution of various fractions in gaseous and liquid phases during the conversion process of butane technical fraction at 793 K on the surface of the prepared catalyst appears time variable. Thus, the synthesized bifunctional Zn–Cu/ZSM-5 catalyst was shown to be very active, still exhibiting a good selectivity towards the production of BTX aromatics for a longer period, in a pilot-scale reactor. In this case the role of the introduced zinc and copper oxides was associated with the reverse spillover of hydrogen from the zeolite surface where Zn and Cu acts as active sites, where the molecular hydrogen is produced.
During this investigation, almost no loss of the catalytic activity of Zn–Cu/ZSM-5 sample at the pilot-scale reactor was observed even after ten catalytic cycles. This finding is most probably due to the synergistic effect that occurs between zinc and copper species and Brønsted and Lewis acid sites found on the zeolite surface. Accordingly, this synergism is responsible for the suite of dehydrogenation reactions (alkanes to alkenes (n + i) ), alkenes oligomers to dienes (), and cyclonaphthenes to aromatics (C6–C10→C6–C10), isomerisation of , oligomerization of , and cyclization of dienes to cyclic naphthenes () that lead to the formation of BTX fraction.
In conclusion, even after ten experimental tests of aromatization process of technical fraction of butane (n‐ + i‐C4), in a pilot-scale reactor, with a corresponding regeneration of the catalyst (considering about 1000 h of continuous catalytic activity), the prepared Zn–Cu/ZSM-5 bifunctional catalyst did not show reduced activity and selectivity towards BTX aromatic hydrocarbons. Thus, the obtained results validated the performance of the Zn–Cu/ZSM-5 bifunctional catalyst at pilot scale, in the aromatization reactions of butane technical fraction. These findings recommend the prepared catalyst as suitable for successful industrial use to convert the low-value light hydrocarbon byproducts in refineries to high value-added BTX aromatics, with hydrogen as co-product.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0