1. Introduction
Fossil fuels are still the primary source of energy used commercially. The massive use of these fossil fuels will impact its unreliable role in the long run. Therefore, fossil fuels need to be replaced with eco-friendly renewable fuels. Bioethanol is one of the biofuels produced from the fermentation of biomass containing sugar, starch, and cellulose [1]. Bioethanol can be produced sustainably since the raw materials come from various biological resources and plant waste, such as lignocellulose plants [2, 3, 4, 5, 6, 7, 8, 9]. Low toxicity, fewer emissions, and biodegradability are bioethanol advantages compared to fossil fuels. Bioethanol production was initially produced by using starch such as cassava, corn, sugarcane and potato. However, these feedstocks were confronted to their function as food [10]. Due to this reason, the new raw materials for bioethanol production were identified such us lignocellulosic biomass. The latter contains the main components constituting its cell walls in aromatic polymers, namely lignin and carbohydrates (cellulose and hemicellulose). Coconut husk is one of the lignocellulosic biomass for bioethanol production. The constituent chemical components of coconut husk includes 45.84% lignin, 43.44% cellulose, 5.25% water, 3.00% pectin, and 0.25% hemicellulose [11]. Bioethanol production from lignocellulosic biomass involves several processes, including delignification, hydrolysis, fermentation, and purification. The delignification aims to degrade the lignin bonds in lignocellulose. The lignin content in coconut husk must be removed by pretreatment as delignification because lignin can be a barrier to bioethanol production. In fact, lignin is a hardness complex compound because it consists of composed of 3-dimensional polymers of phenolic or branched phenylpropanoids [12]. The lignin position in lignocellulosic is in the outermost layer that covers the cellulose and hemicellulose bonds. Therefore, cellulose can only react with the catalyst to produce glucose after the lignin bonds have been degraded. Different types of solvent systems have been implemented in lignin dissolving from coconut husk lignocellulose, such as ionic liquids hydrothermal [13], NaOH [14, 15, 16], H2SO2 [17, 18], alkaline and oxidative pretreatment [19, 20] or combination between two of them. The delignification by using those chemical agents will negatively impact the environment. Chemicals used as catalysts will be dissolved in the wash water during the delignification process and become waste that can damage the environment.
Due to this reason, a “green” solution was involved in the delignification process. Deep Eutectic Solvents (DES) and Natural Deep Eutectic Solvents (NADES) are two solvents currently being continuously developed for biomass delignification. Both solvents are environmentally friendly since they are reusable, biodegradable, non-toxic and simply prepared [21]. Deep eutectic solvents (DES) are inexpensive, easy to manufacture, and can be customized to be non-toxic, biocompatible, and biodegradable [22, 23]. If the components that produces DES are primary metabolites, DES is called NADES (meaning natural DES). However, when the component that constitutes a DES is a natural metabolite, DES is called a NADES [23]. DES and NADES solvents are produced by reacting a mixture of substances formed due to the bonding of Hydrogen Bonding Donors (HBD) and Hydrogen Bonding Acceptors (HBA) at a certain molar ratio [24].
The results of delignification of coconut husk using DES made from Choline Chloride (ChCl):Monoethanolamine (MEA) and NADES made from Betaine (Be):Lactic Acid (La) are studied in this investigation. The using of ChCl-MEA compared to other solvents can reduce costs through the use of inexpensive ingredients, an easier preparation method, moderate conditions, and reduced polysaccharide loss. In previous studies, the using of ChCl-MEA as solvent for pretreatment wheat straw effectively degraded lignin by 71.4% [25]. Meanwhile, the using of Betaine as NADES based solvent couple with lactic acid has been used for different feedstock [26, 27, 28]. Betaine as based solvent is characterized by essential element and widespread among organisms [29], inexpensive and eco-friendly [30]. The terms of variation of the HBA:HBD molar ratio in DES and NADES are 1:4, 1:6, and 1:8, respectively, with delignification times of 2, 4, 6, and 8 h. Delignification results from the two solvents were analyzed by the Chesson–Datta method. Furthermore, hydrolysis was carried out on a sample, followed by an analysis of glucose content using a Brix refractometer. Hydrolysis was then fermented and distilled until pure bioethanol was obtained and then analyzed with alcohol refractometers to determine the resulting bioethanol content.
2. Materials and method
The raw material used in this study was coconut husk obtained in Palembang, South Sumatra. The used chemicals include Choline Chloride 99%, Monoethanolamine 99%, Betaine, Lactic Acid 88.5%, H2SO4 98%, Cellulase Enzymes, Saccharomyces Cerevisiae yeast, NPK, Urea fertilizer, and aquadest. The materials and the process of bioethanol production was showed in Figure 1.
Process of producing bioethanol from coconut husk.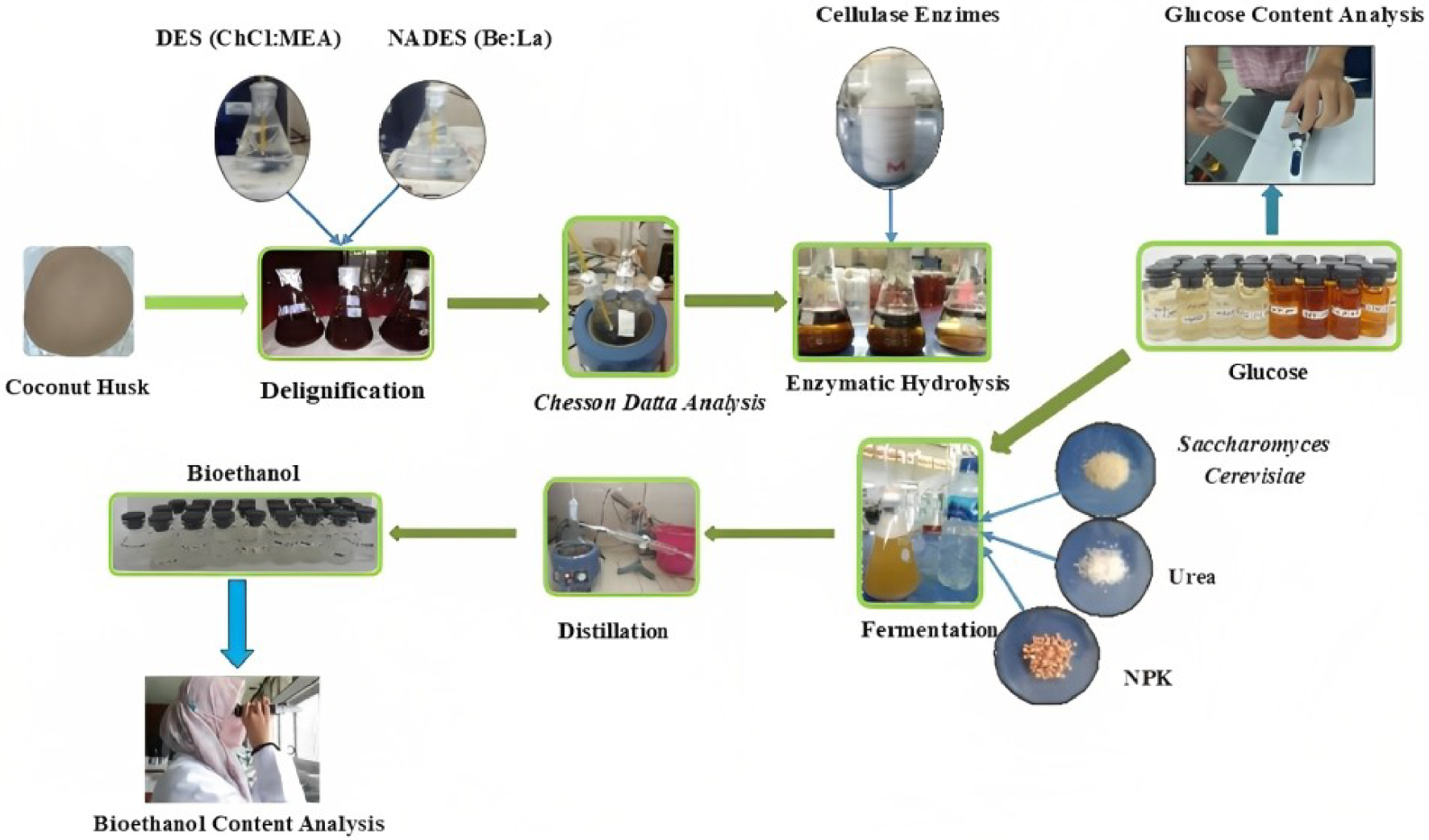
2.1. Preparation of raw materials and solvents
Coconut husks were washed with water until clean, rinsed using aquadest and oven-dried at 105 °C for 2–3 days. The dried coconut husk was mashed using a blender and sifted using a 35 mesh screen test sieve. One gram of coconut husk was taken for proximate analysis using the Chesson–Datta method to determine the initial composition of lignocellulose in the coconut husk. Furthermore, in a separate place, a DES solution was prepared to consist of Choline chloride-monoethanolamine (ChCl-MEA) and NADES consisting of betaine-lactic acid (Be-La).
Both solvents were reacted with molar ratios of 1:4, 1:6, and 1:8. Reagent was carried out using a 500 mL erlenmeyer and closed with a cork at a temperature of 60 °C then homogenized using a hot plate equipped with a magnetic stirrer at a speed of 100 rpm for 10–15 min in samples with NADES treatment [31] as well as at 80 °C and 100 rpm for 1 h for samples with DES treatment [25].
2.2. DES and NADES pretreatment
DES and NADES solutions were added into separate 1000 ml Erlenmeyer filled with 50 g of coconut husk. The solution was homogenized by maintaining a total volume of 500 mL. Then the sample was heated using an autoclave at a temperature of 121 °C with time variations of 2, 4, 6, and 8 h. After the delignification, the samples were removed from the autoclave and cooled until reaching the room temperature (28–30 °C). Sample residue was separated from lignin extract and washed using distilled water until the pH of the washing water became neutral (pH-7). Washed samples were then dried in an oven at a temperature of 105 °C for about 24 h. Dry samples were already separated by as much as one g to determine the lignocellulose content after the delignification process using the Chesson–Datta method.
One g (a) of clean and dry coconut husks was boiled and added with 150 mL of distilled water which was heated using a heating mantle for 1 h. The sample residue, after heating, was washed with 300 mL of distilled water and dried using an oven for 8 h or until the weight was constant (b). Then 150 mL of 1N H2SO4 was refluxed using a heating mantle for 1 h at 100 °C. The residue from the reflux process was filtered and rewashed with distilled water to remove the H2SO4 content until neutral pH (pH 7). Residual pH measurement was carried out using a pH meter. After reaching a neutral pH, the residue was dried in an oven at 100 °C for 8 h or until the weight was constant (c).
A total of 10 mL of 72% H2SO4 solution was added to the dry residue and left for 4 h at room temperature, then 150 mL of 1N H2SO4 was added and refluxed with a heating mantle at 100 °C for 1 h. The residue after reflux was then filtered and washed using distilled water until pH 7. After that, the residue was dried using an oven at 105 °C until the weight was constant and weighed (d). The dried residue was then oxidized in a furnace at a temperature of 575 °C for 5 h and the residual ash was collected. The weight of the ash obtained after the furnace was then weighed to get the final weight (e).
Calculation of lignocellulose content (lignin, cellulose, and hemicellulose) can be done based on Equations (1)–(3):
| (1) |
| (2) |
| (3) |
2.3. Enzymatic hydrolysis
The hydrolysis process was carried out using residues that have been delegated and weighed by weight. The sample was placed into a 1000 mL Erlenmeyer mixed with 750 mL distilled water and homogenized using a glass stirring rod. The H2SO4 10% compound was gradually added into the sample solution to adjust the pH sample solution about 4–5. A cellulase enzyme of 5% (w/w) was added to the solution, which was then homogenized for 30 s with a glass stirring rod. The homogeneous solution was then hydrolyzed at 121 °C for 1 h using an autoclave. The hydrolysis solution was separated with coconut husk residues as hydrolysate. Then as much as 10 mL of hydrolysate was taken to analyze glucose content formed using a Brix refractometer. This refractometer has an accuracy of 0.5% and a scale of 0.5% Brix, which can be used at room temperature.
2.4. Fermentation and distillation
The hydrolysate from the hydrolysis process was transferred to a 1000 mL Erlenmeyer flask, and 10% H2SO4 was added dropwise to the solution, bringing the pH to around 4–5. 5% (w/v) of yeast containing Saccharomyces cerevisiae was added to the solution. 4% NPK fertilizer and 3% urea were added as nutrients and homogenized for 30 s with a glass stirrer. The Erlenmeyer, which holds the fermentation solution, was sealed with a cork with a plastic tube inserted in the middle. The hose was then connected to a plastic bottle filled with water, sealed with a cork, and stored under anaerobic conditions. Fermentation was carried out for seven days at room temperature (28–30 °C). Nutrients in the fermented solution were separated first using a filter, and the solution was transferred to a distillation flask. Distillation was carried out at a temperature of 78 °C according to the boiling point of ethanol until there were no bioethanol condensation drops (5–6 h). The distillate was then stored and cooled in a refrigerator at <5 °C for analysis using an alcohol refractometer to determine the bioethanol content produced. Bioethanol content was analyzed using a portable handheld alcohol refractometer 0–80% (v/v) with a scale of 1% (v/v) and an accuracy of 1%. The working principle of these two tools is by the light refraction, so their use must be performed in the sun or with sufficient light.
3. Results and discussion
3.1. Effect of DES and NADES on the delignification process
The lignin content can inhibit acid penetration and microbial growth in bioethanol fermentation [32]. Delignification can open the lignocellulosic structure by breaking bonds and dissolving lignin, making cellulose easier to access [33]. Delignification can increase enzymatic accessibility and helps the enzymatic hydrolysis process to convert cellulose and hemicellulose into monosaccharides [34]. Breaking bonds in LCC can be done using a eutectic solvent composed of ionic liquid and organic solvent (Figure 2) due to the strong hydrogen bonds used effectively for biomass pretreatment [35]. The compounds contained in coconut husk before and after delignification were analyzed using the Chesson–Datta method [36]. The results of the analysis of the initial content of lignocellulose in coconut fiber are attached in Table 1.
DES Pretreatment of Lignocellulose Scheme [25].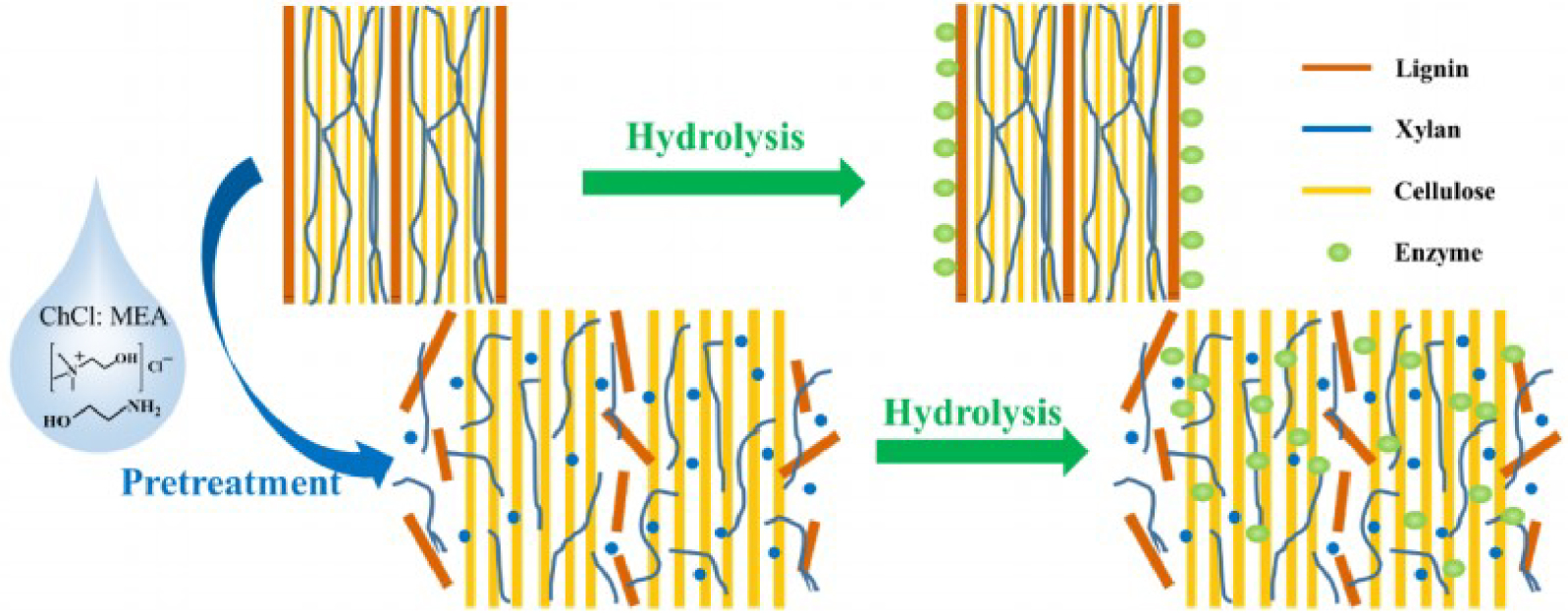
The initial composition of lignocellulosic content in coconut husk
| Composition | Content (%) |
|---|---|
| Cellulose | 22.2 |
| Lignin | 43.0 |
| Hemicellulose | 28.4 |
Delignification of coconut husk was carried out using two types of solvents, DES composed of choline chloride as the Hydrogen Bond Acceptor (HBA) and monoethanolamine as the Hydrogen Bond Donor (HBD) and NADES composed of betaine as HBA and lactic acid as HBD (Figure 3). The pH of each solvent was determined after HBA and HBD mixed. DES has an alkaline pH of around 14 and NADES has an acidic pH of around 1–3. The pH impact is essential for the use of DES as catalyst. The pH value varies significantly based on the used HBD[37]. This pH value seems to alter based on the relative acidity of anionic and cationic species that are combined [38]. However, the pH DES solvent content choline chloride-monoethanolamine is optimally ranged between 12 and 14 [39]. The hydrogen-bond donor has a significant impact on the resulting pH. Nature of hydrogen bond donor determines the pH of NADES. NADES pH impacts in range of 1.2 until 2.74 at ambient temperature (23 ± 2 °C). The lignocellulosic content in coconut husk changed where the lignin content decreased by 4.51–16.97% after the delignification process, while the cellulose content increased by 3.26–14.82%. The changes in lignocellulosic content in coconut fiber were influenced by the chemical properties of DES and the delignification time. DES, classified as a strong base solution, provides a strong lignin removal capacity for ethanolamine-based DES [25].
Solvent reaction with ratio 1:4 (a) DES, (b) NADES.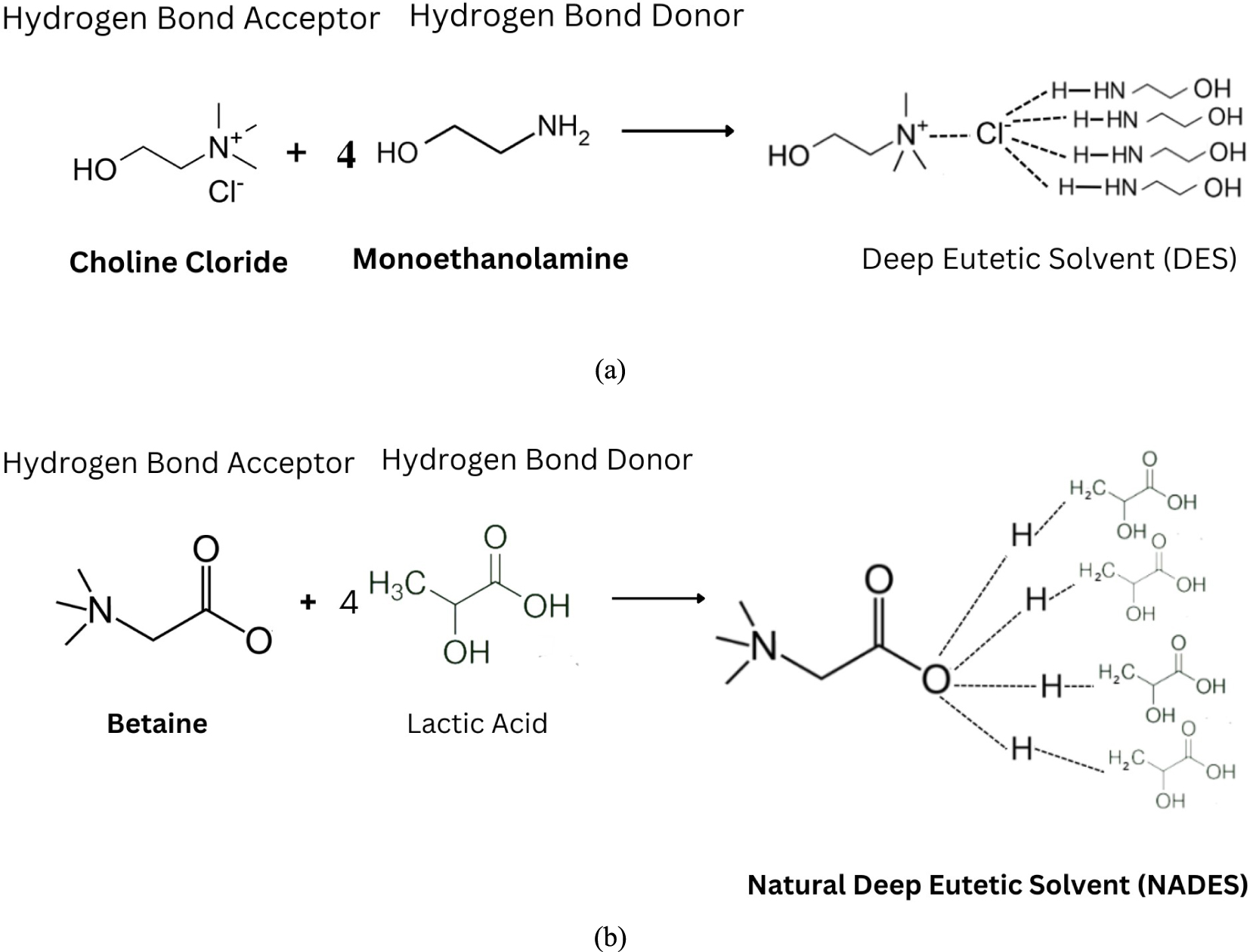
Figure 4 shows a decrease in lignin when coconut husk was delignified using DES for each time and ratio variation. The higher the solvent ratio, the more significant the delignification results, but it can be constant or even decrease as it passes the optimal point. Time also affects the delignification performance of DES at all ratios. In particuler the lignin decay decreased with increasing delignification time. The lignin dissolution content decreased at 2, 4, and 6 h and only marginal variations were observed for a further increase up to 8 h. Delignification for 2 h was the most optimal duration for delignification. It occurred due to the increase of cavitation by increasing pretreatment time. Cavitation produces high energy and turbulence, which helps the breakdown of lignin, increases alkaline activity to remove lignin, and increases the mass transfer rate [40].
Effect of DES (a) and NADES (b) ratio along with delignification time on lignin content in coconut husk.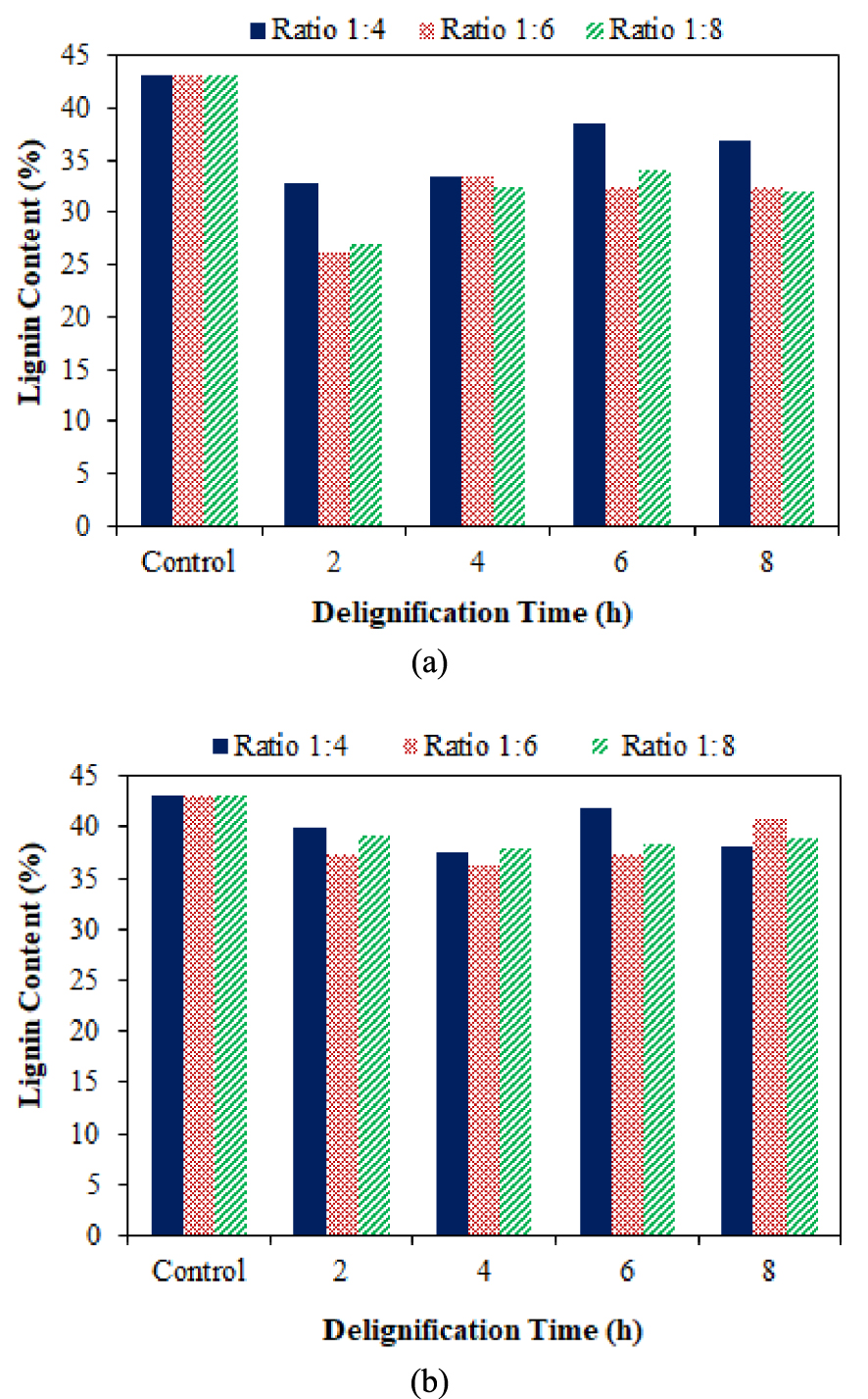
Delignification with NADES can reduce lignin content to around 36.19–41.73% with a maximum degradation of 6.81%. NADES was effective for dissolving lignin by not dissolving cellulose, making it suitable as a solvent in the delignification process [41]. Based on Figure 3, the lignin content decreased along with the increase in delignification time (2 and 4 h). The increase in delignification time caused the decrease in lignin content because the intensity of the solvent to extract lignin also increased [42].
The efficiency of delignification with NADES at 6 and 8 h decreased compared to delignification at 2 and 4 h. Similar behavior was obtained in the literature [42]. The effectiveness of NADES delignification increased as the NADES ratio increased (1:4 and 1:6 ratio). The strong hydrogen bonding between the betaine carboxylate atom and the hydroxyl group in lactic acid and the interaction of the methyl group on betaine and oxygen in lactic acid contributes to the strength of their interaction [30]. The effectiveness of coconut husk delignification with NADES at a 1:8 ratio decreased compared to NADES at 1:4 and 1:6 ratios. Delignification of biomass with betaine-lactic acid solvent was typically performed at a ratio of 1:2, with a maximum ratio of 1:5 [31]. Therefore, the best lignin degradation for coconut husk delignification with NADES was found at a ratio of 1:6 with 4 h of delignification.
The decrease in lignin content during delignification using DES and NADES at a ratio of 1:4 for 6 h resulted in the lowest degradation compared to other ratios and times. Maximum lignin degradation for DES solvent was 16.97%, and for NADES, only 6.81%, where DES had a higher average delignification than NADES. In the literature, it was shown that delignification using NADES made from betaine: lactic acid can only degrade lignin with a concentration of not more than 10% [43].
Delignification using DES ratios 1:6 and 1:8 at each variation of the delignification time had higher cellulose content with an increase of 9.00–14.31% and 9.73–14.82%, respectively. DES at a ratio of 1:4 was only able to increase cellulose up to 3.26–11.28%, while DES at a ratio of 1:6 and 1:8 was able to degrade more lignin. The amount of lignin degradation in the ratio 1:6 and 1:8 was linear with the cellulose content in that ratio. The increased cellulose content was also influenced by the pH of the solvent used.
According to Figure 5, the increase in cellulose content occurred in all samples for each ratio and was optimum at 4 h but decreased at 6 and 8 h. This was consistent with the findings of Chamidy et al. [44], who found that the highest cellulose content was obtained after 1 h of delignification and decreased after 2 h due to a large amount of cellulose degraded by alkali treatment. Delignification with NADES increased the cellulose content to 23.47–36.97%, with a maximum increase of 14.77% obtained at a delignification ratio of 1:6 for 6 h. Delignification with NADES ratios of 1:4, 1:6, and 1:8 for 2 and 4 h increased cellulose content but decreased at 6 h for the ratios of 1:4 and 1:8 and again at 8 h for each ratio. Delignification at 6 and 8 h decreased due to a decrease in delignification effectiveness [45].
Effect of DES (a) and NADES (b) ratio along with delignification time on cellulose content in coconut husk.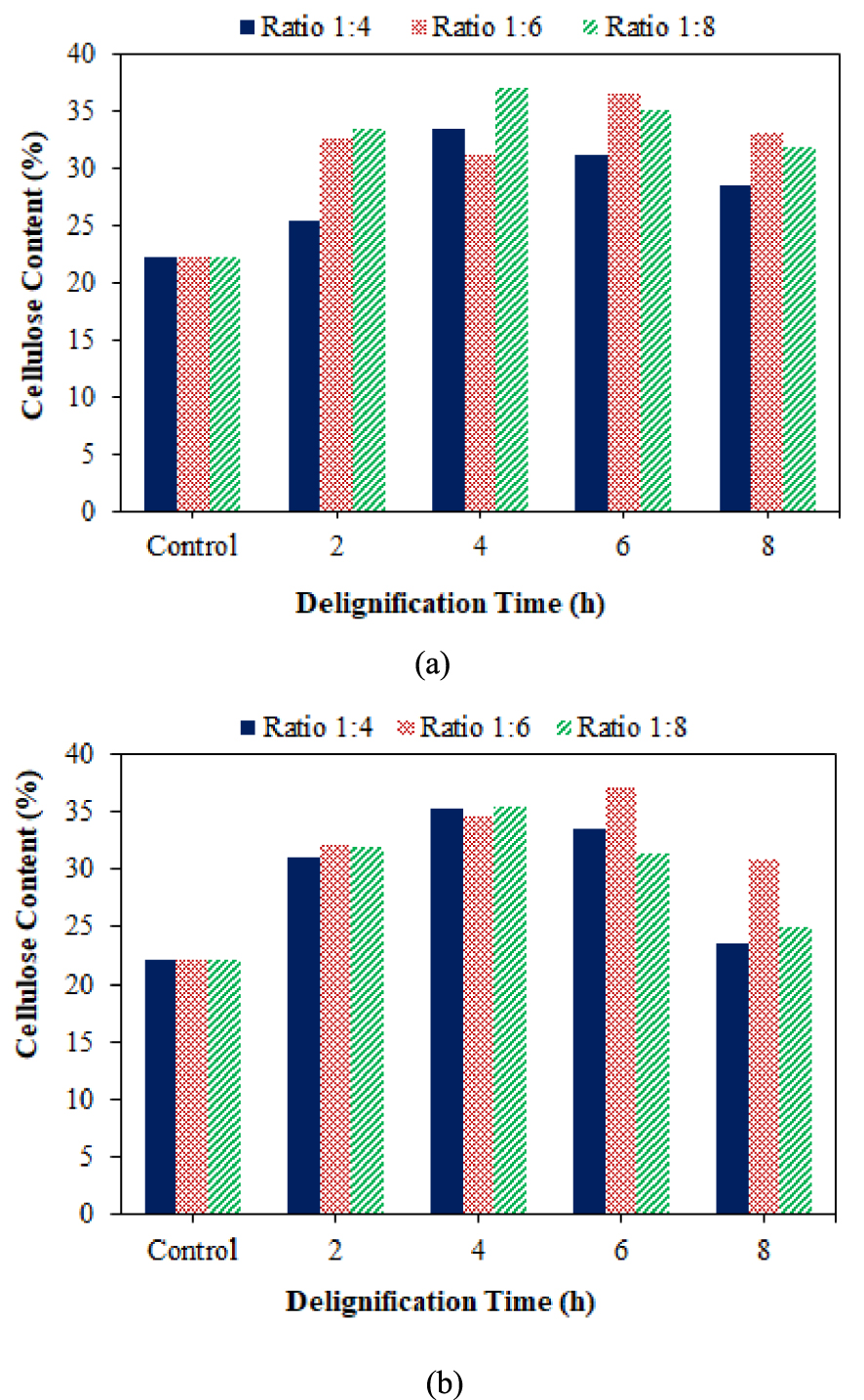
3.2. Effect of the delignification process on glucose contents
The purpose of hydrolysis was to break down the crystal structure of cellulose to facilitate the decomposition of cellulose into glucose. Hydrolysis can also break down the hemicellulose structure into simple sugars such as glucose [46]. The hydrolysis was carried out enzymatically using the cellulase enzyme with a concentration of 5% (w/w). The enzyme works selectively, where the enzyme that can hydrolyze cellulose is the cellulase enzyme [47]. Enzymatic hydrolysis was performed at a pH of about 4.5–5 [2] and isolated for 1 h [47]. Glucose conversion will increase with increasing hydrolysis time, but if the hydrolysis time exceeds the optimum time, then glucose conversion will decrease. A long time has an impact on the death of the enzyme because the amount of nutrients is not sufficient for enzyme activation in batch hydrolysis and contaminates glucose [48].
Figure 6 shows the DES solvent ratio and delignification time effect on hydrolysis glucose content. Glucose content increased after delignification with DES at 4 h, except in a 1:6 ratio, which decreased at 6 and 8 h. The 1:4 ratio sample had the lowest glucose content in each delignification time variation. This behavior was due to the sample’s low cellulose content compared to other samples. Several DES samples contain the same amount of glucose but differ in cellulose content.
The effect of DES ratio (a) and NADES (b), along with delignification time on glucose content in coconut husk.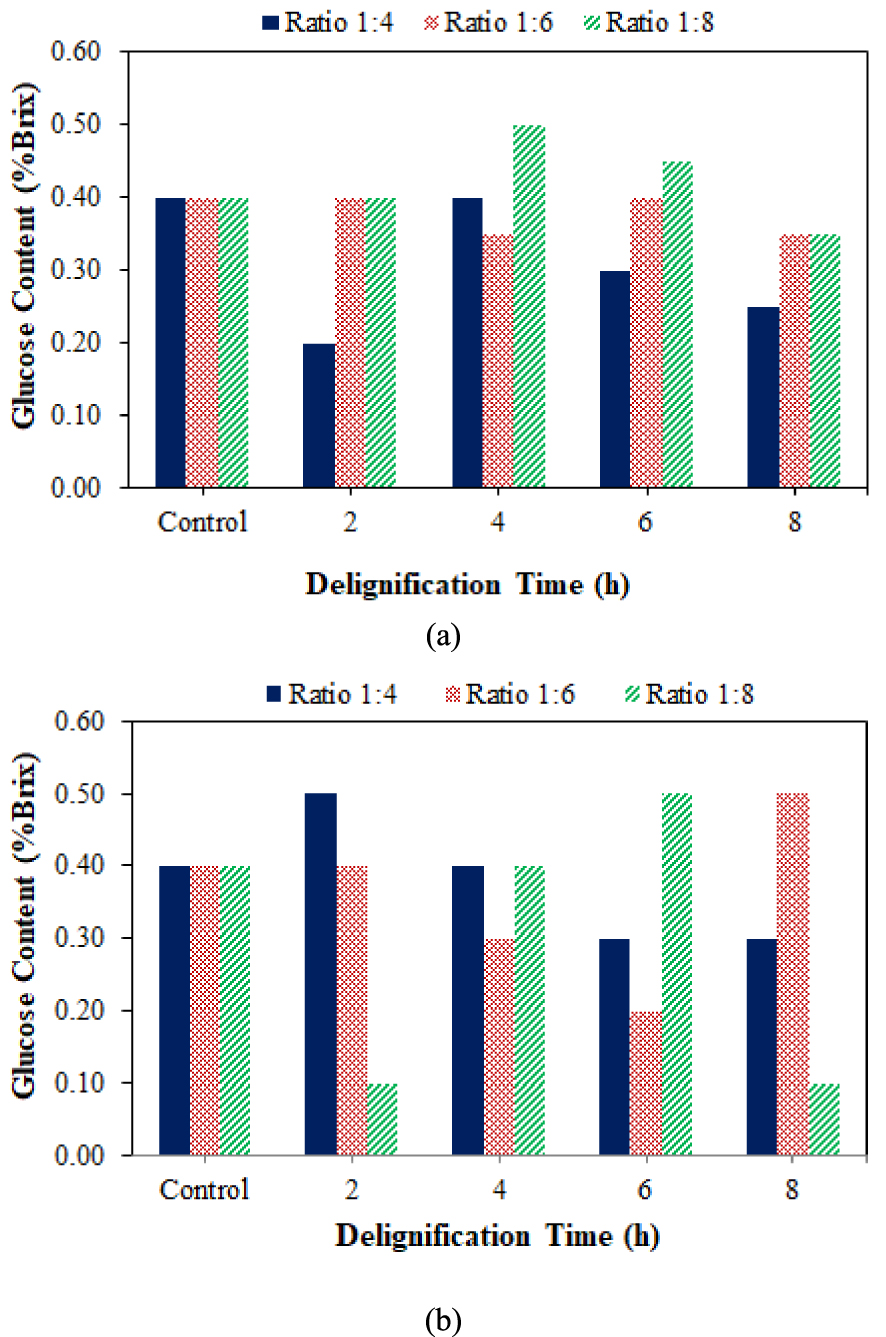
Glucose content at the delignification ratio of 1:4 and 1:6 for 2 h increased compared to the initial coconut husk samples. According to [49], delignification can increase glucose content due to an increase in cellulose accessibility due to the removal of hemicellulose and lignin content. DES and NADES solvents broke down the lignin bond from lignocellulose and diluted it to wash water after the delignification process. After delignification, the sample was washed using distilled water with the purpose of neutralizing the sample as well as dissolving the degraded lignin and existing solvents. Furthermore, pure cellulose becomes easier to be hydrolyzed by enzymes into glucose. Based on the glucose data obtained from the two solvents, it was known that delignification also gives different results to glucose content in general.
3.3. The effect of the delignification process on bioethanol contents
The hydrolysate was fermented using Saccharomyces cerevisiae at a concentration of 5% (w/v) for seven days with the addition of urea and NPK (Nitrogen Phosphor Potassium) fertilizer as much as 3% and 4% as nutrients to support the growth and activity of microorganisms [50]. According to Yuniarti et al. [51], the highest bioethanol content was found in adding 5% (w/v) yeast, with the most effective fermentation time of 7 days. Saccharomyces cerevisiae was used since it can produce a high ethanol yield, has a fast fermentation rate and has a high tolerance for glucose and ethanol [52]. The hydrolysate was added with H2SO4 10% until the pH of the solution became around 4–5 because the optimum pH for the growth of Saccharomyces cerevisiae was in the range of 4–5 [53]. The fermented solution was purified by distillation at 78–80 °C according to the boiling point of ethanol and kept from reaching the boiling temperature of water (100 °C).
Based on Figure 7, the variation of the delignification time has an increasing and decreasing trend in the same range. The lowest bioethanol content was obtained in the delignification sample of DES for 4 h, and the highest bioethanol content was obtained at the delignification time of 2 and 6 h. Glucose content at 8 h was low, so the content of bioethanol produced decreased since the amount of sugar converted into bioethanol was less. The fermentation that passed the optimum time decreased yeast activity and even reached the death phase due to decreased substrate availability, so bioethanol conversion also decreased [54]. The presence of dead yeast cells in the sample and the decomposition of bioethanol due to the length of fermentation resulted in a decrease in the purity of the bioethanol produced [55].
The effect of DES Ratio (a) and NADES (b), along with delignification time on bioethanol content in coconut husk.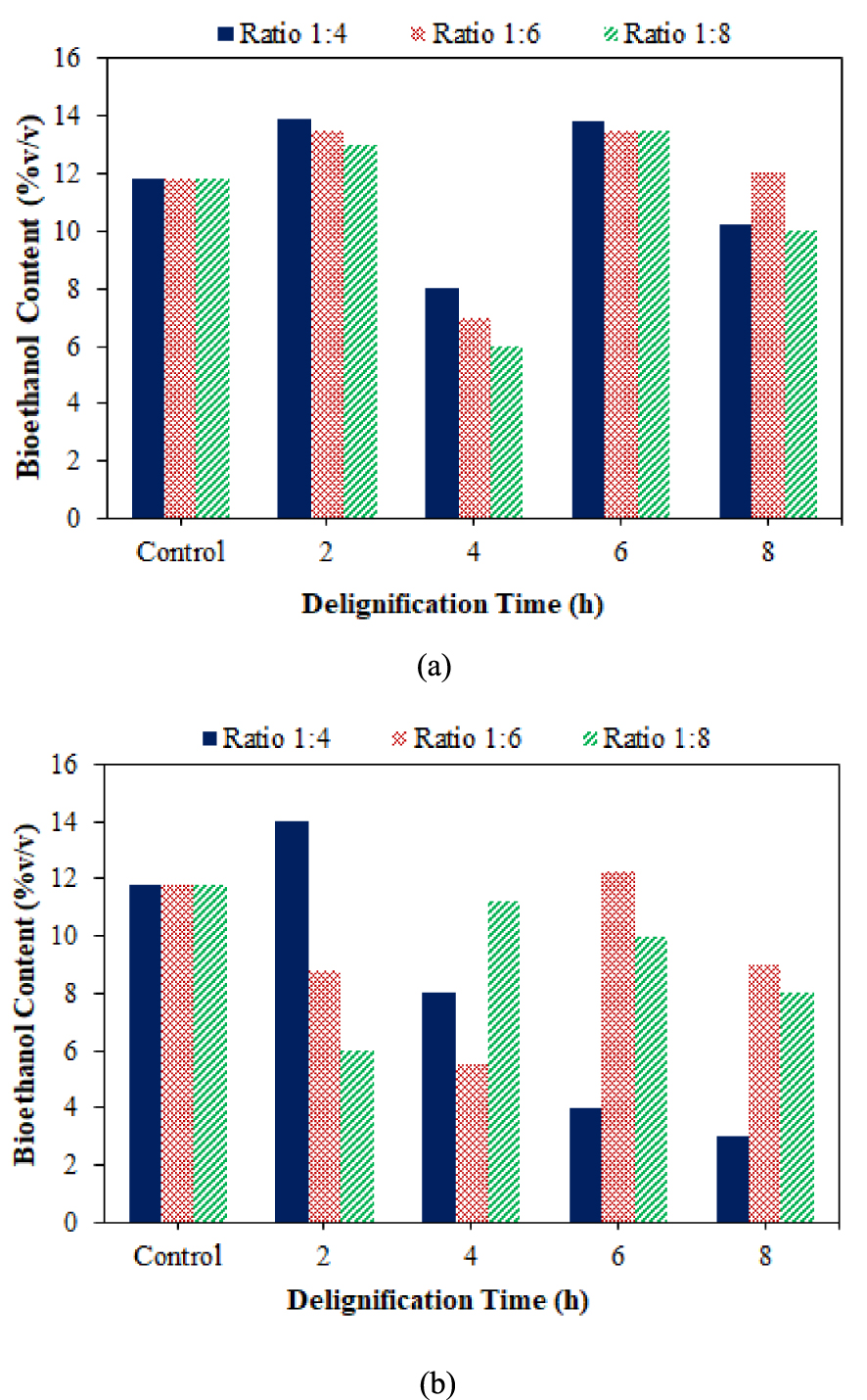
The increasing and decreasing bioethanol contents in the ratios 1:4, 1:6, and 1:8 and the delignification time for 2 and 4 h were the same as the statistics for increasing and decreasing glucose contents resulting from hydrolysis. The best bioethanol content was found at a ratio of 1:4 for a delignification time of 2 h, consistent with the high glucose content contained in that ratio and time. This was because Saccharomyces cerevisiae converted a large amount of glucose as a nutrient into bioethanol [56]. Based on the data from the analysis of bioethanol samples obtained from the two types of solvents (DES and NADES), coconut husk delignification using DES resulted in a higher average bioethanol content than NADES. This was directly proportional to the glucose content produced, where the average glucose concentration of the DES solvent was higher.
4. Conclusion
The increasing of DES and NADES molar ratios and the delignification time tended to increase delignification efficiency. The use of DES in the pretreatment process resulted in higher glucose contents and bioethanol than NADES. The best bioethanol contents for DES and NADES were discovered at a 1:4 (2 h) ratio of 13.9% (v/v) for DES and 14% (v/v) for NADES. Therefore, it was concluded that DES and NADES could affect the conversion of glucose to bioethanol in the delignification process. Therefore, both solvents are essential in bioethanol production.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgments
This research was financially supported by PNBP, Faculty of Engineering, Universitas Sriwijaya, in 2021 with Grant Number: SP DIPA-023.17.2.677515/2022, on November 17, 2021, and 0390/UN9.FT/TU.SK/2022, on May 13, 2022.




 CC-BY 4.0
CC-BY 4.0