1. Introduction
Each of us continuously emits more than a thousand volatile organic compounds (VOCs) that are responsible for our odor fingerprint. With the emergence of volatolomics, i.e., the discipline that investigates VOCs produced by living systems, the human volatolome has provided new insights into biological processes in real-time [1] (Figure 1).
Volatolomics and induced-volatolomics paradigms. Volatolomics is the study of endogenous molecules naturally produced and released by living systems [1, 2]. The concept of induced-volatolomics specifically monitors exogenous volatiles that come from the metabolisation of a probe [3].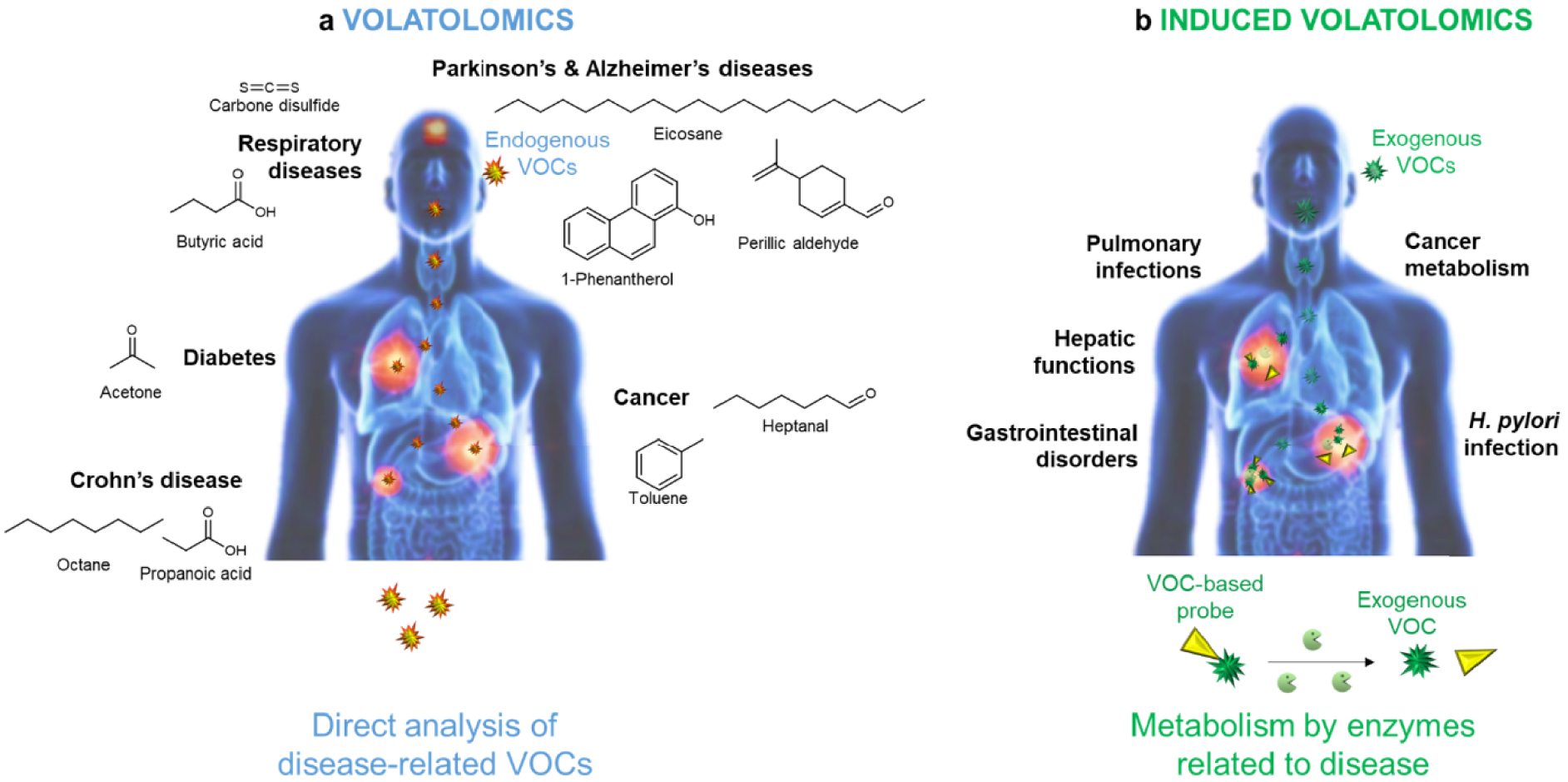
Indeed, as evidenced by canine olfaction-based studies, volatile molecules can serve as chemical tracers for assessing health status in real time in a non-invasive way [2, 4]. Along with the progress in analytical technologies, many research groups have attempted to find the perfect volatile marker for a given disease (Figure 1). Hence, viral (Influenza viruses, adenovirus, rhinovirus [5, 6]), bacterial, parasitic, and fungal infections (tuberculosis [7], malaria [8], pneumonia [9, 10]) were shown to be associated with the release of specific VOCs. Similarly, respiratory disorders, such as asthma [11], or acute respiratory distress syndrome [12] as well as metabolic disorders such as diabetes [13] could be diagnosed by the detection of alkanes and ketones in patients’ breath. Similarly, a strong correlation was reported between cancers, Parkinson’s and Alzheimer’s diseases and the airborne release of hydrocarbons, alcohols, aldehydes, ketones, esters, nitriles, and aromatic compounds [14, 15, 16, 17, 18, 19, 20].
Despite these extensive studies, no endogenous volatile compound has been proven to be of sufficient diagnostic value so far. Such an issue is mainly explained by both interindividual variability [21, 22] and individual daily variation [23]. Indeed, variables such as overall health status, diet, medication intake, exercise, and smoking habits induce qualitative and quantitative changes in patient volatolome that are unrelated to pathologies [24, 25]. Moreover, although several studies have sought to demonstrate the biosynthesis patterns of endogenous VOCs, their metabolic pathways remain enigmatic, which adds to confusion [26, 27, 28]. Finally, given the very low concentration of VOCs in body fluids and gases (e.g., 1 × 10−11 M in blood and 0.02 ppb in breath [24]), more efficient sampling techniques and analysers are necessary to obtain reliable quantification [29, 30, 31, 32, 33]. In this respect, identification of rotational fingerprints with high accuracy, sensitivity, and selectivity is a challenge that can be addressed and automated using artificial intelligence and supervised or unsupervised machine learning algorithms. In very recent works, deep learning models were developed to provide breath volatolomics-based classifiers for the presence or stage different diseases [34, 35, 36, 37, 38]. These cutting-edge approaches demonstrate that machine learning methods could be the answer to make volatolomics a non-invasive clinical diagnostic tool for point-of-care applications [39].
Although volatolomics was booming in the early 2000s, another strategy, which relies on the monitoring of exogenous volatiles, was emerging. This novel paradigm, very recently called “induced-volatolomics” (Figure 1), is part of the chemical biology toolbox. It implies the use of off/on probes that can be converted into a volatile compound in response to a pathogen or metabolic-specific enzymatic stimulus [40, 41, 42]. In the absence of the activating process, the probe remains inactive. In the presence of the biochemical stimulus, the probe is turned on, thus releasing an exogenous volatile molecule. This targeted action mechanism significantly reduces variability issues that are met with the standard screening of endogenous VOCs. By correlating molecular processes with phenotypic states, these probes can provide valuable information (e.g. kinetics, localization, progress …) on the biochemical events that lead to the progression or regression of a pathology.
2. Contribution of induced-volatolomics to chemical biology
Chemical biology has generated many innovative tools for monitoring metabolic processes in cells [43]. Researchers in this field have developed numerous stimuli-responsive imaging probes with imaging tracers—from radioisotopes to small-molecule dyes, proteins, and carbon or metal nanomaterials—to drive and localize with high spatial resolution disease outcomes [44, 45, 46, 47, 48, 49, 50, 51, 52, 53]. Such activity-based probes can now detect and localize a wide range of biomolecules with high resolution in cell culture, liquid or solid biopsies, and animal models [54, 55, 56, 57]. However, the use of these stimuli-sensitive probes in mammalian models remains limited because of toxicity, stability, pharmacokinetics, and tissue distribution hurdles [58, 59].
In this context, a novel set of off/on probes has been designed to explore and understand, at the molecular level, the pathophysiological mechanisms arising in biological systems. These probes can be converted into a small exogenous volatile molecule by a disease- or pathogen-associated enzyme with optimal affinity. Once produced, the volatile tracer diffuses in the biological system and can be released either in animal breath [40, 42, 60] or in sample headspace (cell culture, liquid or tissue biopsy) [42, 61, 62]. Although this action mechanism is common to all volatile-based probes, the nature of the exogenous volatile compounds released from this biochemical process has significantly evolved. The first probes were natural carbohydrates that could be metabolized into inorganic and very volatile compounds (e.g. H2, CH4 and CO2) [63, 64, 65]. They were then succeeded by synthetic probes, among which a myriad of isotopic CO2-based probes were designed for diagnosis purposes [66].
Among these probes, the 13C-urea breath test (UBT) is the best standardized and most widely used test worldwide. UBT is prescribed when Helicobacter pylori (H. pylori) infection is suspected [40]. This gram-negative micro-aerophilic bacterium can be found on the luminal surface of the gastric epithelium. It is responsible for various upper gastrointestinal disorders, sometimes resulting in ulcers and cancers. In patients with H. pylori infection, the 13C-urea probe is hydrolyzed by bacterial urease to NH3 and 13CO2. 13CO2 is then excreted via the lungs and measured in the expired air. However, because 1.1% of the natural carbon atoms in the human body are present in the stable isotope 13C form, high 13CO2 background noise is observed in patients’ breath. Therefore, and despite its high sensitivity and specificity (both around 97%) [67, 68], false-negative and positive tests represent a clinical issue, especially for the 5% of samples near cut-off values. This issue has led researchers to design a new generation of volatile-based probes that can release a tracer absent from the pool of endogenous VOCs (Figure 2) [42, 61, 62, 69, 70]. These emergent probes have very recently broadened the scope of “induced-volatolomics” offering the opportunity to explore a wide range of fundamental biological processes.
Exogenous VOCs released from VOC-based probes activation.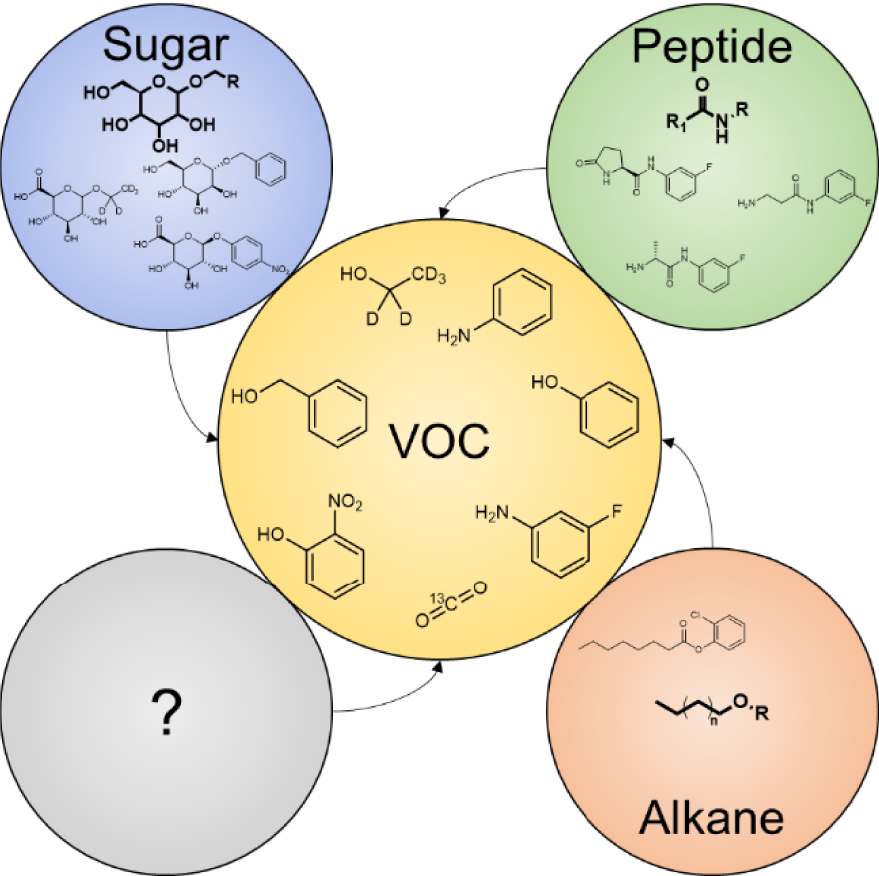
A set of VOC-based probes was designed to detect in vitro pathogenic strains such as Pseudomonas aeruginosa [62], Salmonella [69] and Listeria monocytogenes [70] (Figure 3). These probes are generally activated by hydrolytic enzymes such as peptidases or glycosidases to release an exogenous molecule (e.g. aniline, 3-fluoroaniline, 2-chlorophenol, phenol) in biological or food sample headspace. Very recently, Chan et al. [60] proposed bioorthogonal peptide-VOC reporters to probe the inflammatory response to respiratory lung infection (Figure 4). In their work, the authors designed a set of VOC-based probes consisting of chemically modified tetrapeptide substrates specific to both human and mouse serine protease neutrophil elastase. At the C-terminal end, an amine-containing VOC was attached via an amide bond. Hydrofluoroamines (HFAs; CF3(CF2) ×CH2NH2) were selected as exogenous VOC reporters because of their high volatility. VOC-based peptide probes were formulated into volatile-releasing Activity-Based Nanosensors (vABNs) by conjugation onto an eight-arm PEG nanocarrier in such a way that the VOCs were undetectable in a non-volatile state. vABNs were further delivered into the lungs via intratracheal instillation, and extracellular proteases produced during respiratory disease cleaved the surface-conjugated peptide substrates, thereby releasing the VOCs. VOCs were finally exhaled and breath samples were collected into a glass vial. VOCs concentrations were quantified using an MS detector (Figure 4).
Induced-volatolomics for the in vitro detection of pathogens [61, 62, 69, 70].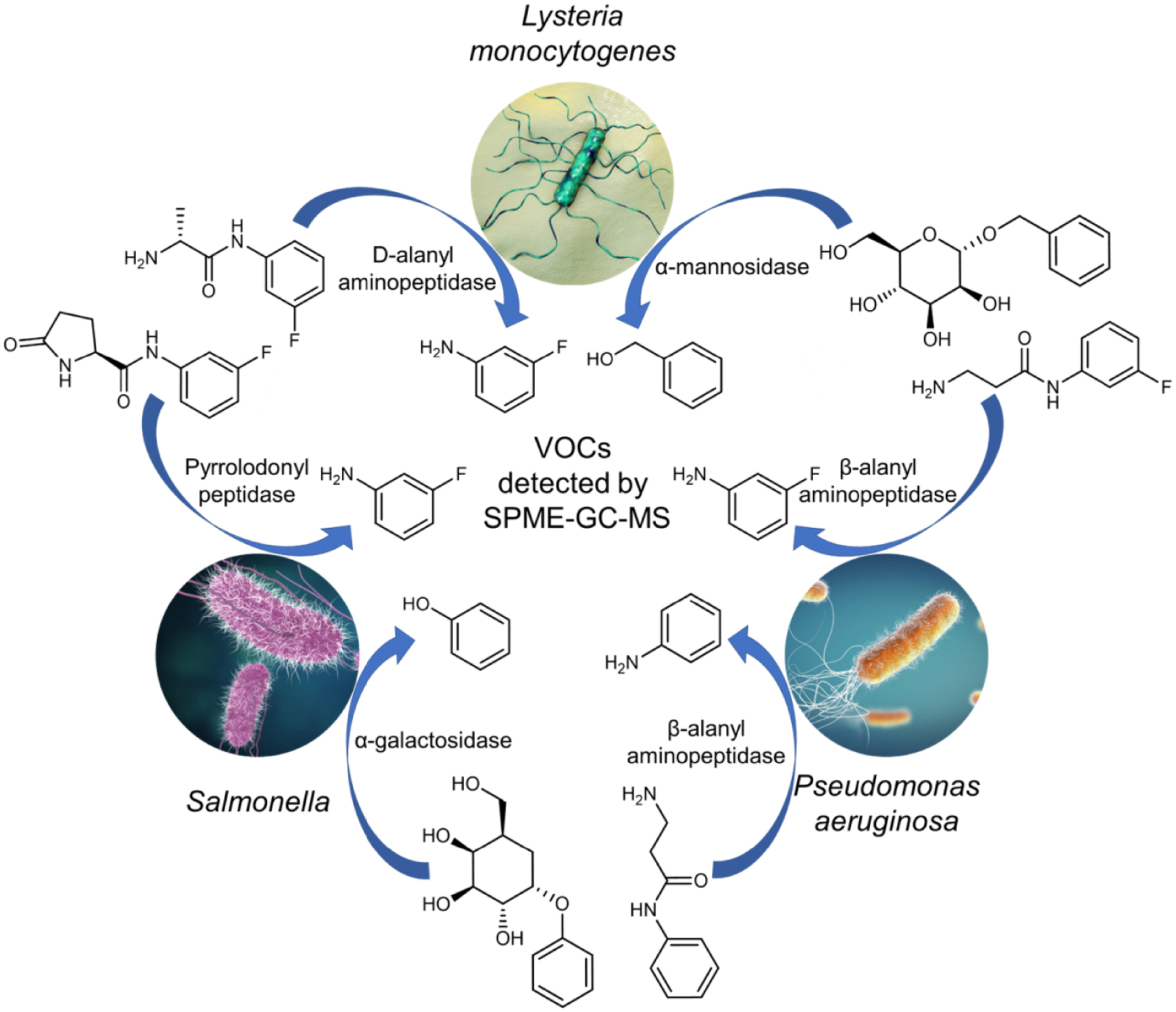
Volatile-releasing Activity-Based Nanosensors (vABNs) for the diagnosis of respiratory infections. (a) VOC-modified peptide substrates are formulated into vABNs by conjugation onto an eight-arm PEG nanocarrier and delivered into the lungs via intratracheal instillation. When attached to the vABN, VOC reporters are in an undetectable, non-volatile state (grey). Extracellular serine protease neutrophil elastase (NE) produced during respiratory disease cleaves the surface-conjugated peptide substrates, thereby releasing VOC reporters. Upon release from vABNs, reporters recover their characteristic mass and volatility (red) and are then exhaled. (b) Enzymatic cleavage by serine protease neutrophil elastase. (c) Breath signal after intrapulmonary delivery of HFA-releasing vABNs in healthy controls and mouse models of lung infection. Figures are reproduced from Chan et al. [60].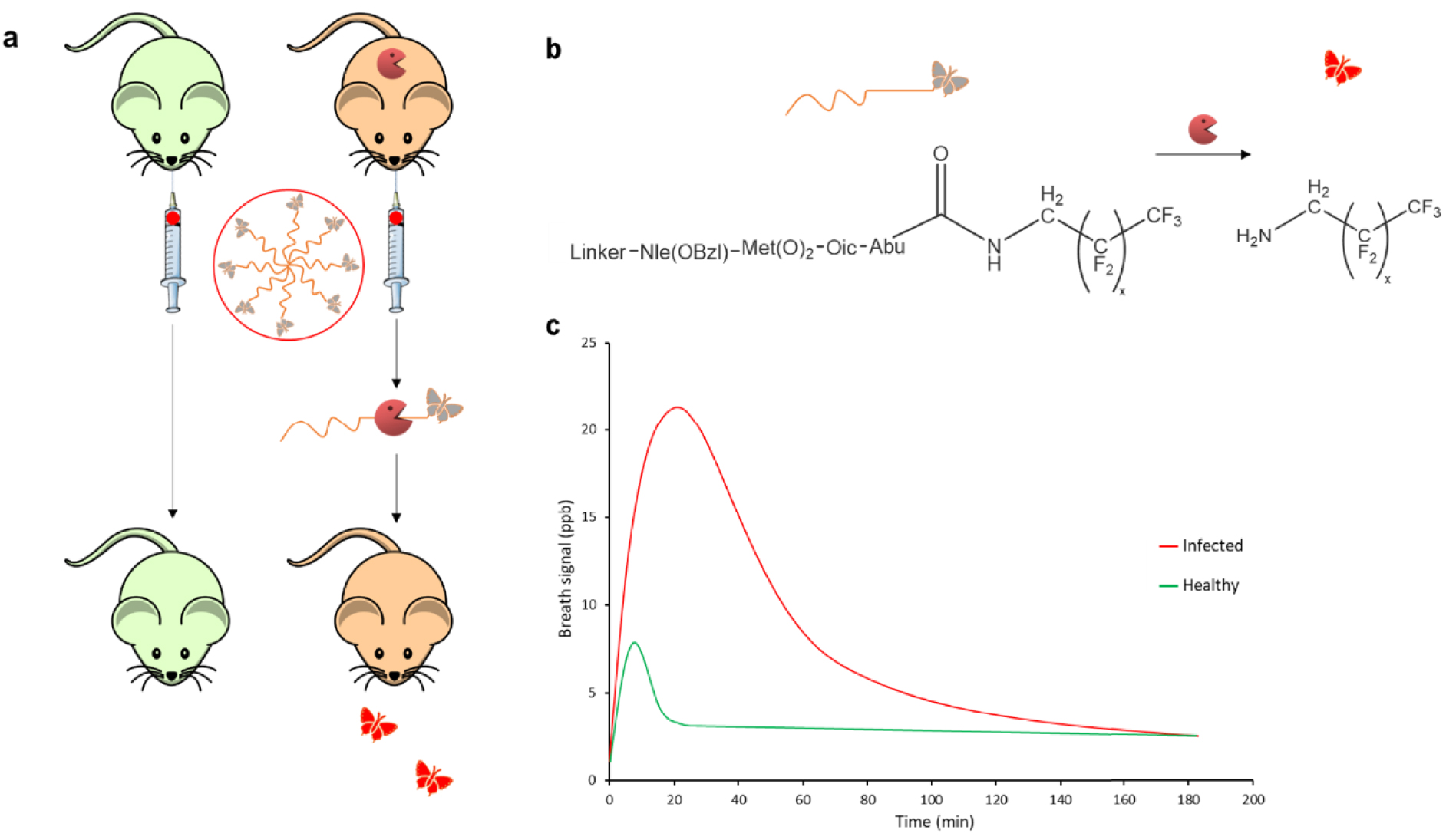
In parallel, our group demonstrated the efficacy of a VOC-based probe to diagnose cancers and monitor their dynamics during chemotherapy [42]. Our labelled probe, D5-ethyl-𝛽-d-glucuronide, was designed to target, with high spatial resolution, extracellular 𝛽-glucuronidase accumulated selectively in the microenvironment of numerous solid tumors [71, 72, 73] (Figure 5). After intravenous administration at a dose as low as 50 μg⋅kg−1, D5-ethyl-𝛽-d-glucuronide was converted into D5-ethanol and glucuronic acid after 𝛽-glucuronidase selective activation within the tumor. D5-ethanol was then passed to the bloodstream, exhaled in the breath where it was trapped and finally analyzed. This strategy enabled unambiguous discrimination between healthy mice and mice bearing different solid tumors (cervix, mammary, and pulmonary; Figure 5b–d). Furthermore, this approach allowed for the first time to monitor either tumor growth or regression during cancer chemotherapy [74] (Figure 5e,f). Indeed, we showed that tumor regression observed in animals under chemotherapy was accompanied by a decrease in the D5-ethanol amount in their breath. In contrast, in untreated mice, tumor growth was correlated with an increase in the D5-ethanol concentration breathed out by the animals. Therefore, these results highlight that our 𝛽-glucuronidase-responsive VOC-based probe is a useful tool to follow tumor progression as well as tumor response to chemotherapy.
(a) Enzymatic reaction. (b–d) Efficacy of D5-ethyl-𝛽-D-glucuronide probe to diagnose solid tumors. KB: mouth epidermal carcinoma (n = 13 per group); MDA-MB-231: breast adenocarcinoma (n = 8 per group); LLC: Lewis Lung Carcinoma (n = 6 per group). ∗, ∗∗, ∗∗∗: Significant differences for p-value < 5%; NS: Non-significant. (e) Average tumor volume of mice with KB xenografts (n = 8 per group). (f) Spearman’s correlation test (p-value = 0.005) relating the evolution of tumor volumes (orange dot line) and the evolution of D5-ethanol exhaled (purple line) by KB mice treated with chemotherapy. Figure reproduced from Lange et al. [74].
On the basis of our study conducted in mice, Owlstone Medical (Cambridge, UK), an industry leader in breath research and technical innovation, recently started testing the D5-ethyl-𝛽-d-glucuronide probe in clinic [75]. Their objective was to provide an early diagnosis test for lung cancer detection. After preclinical tests on lung tumor biopsy and mice bearing lung tumors, Owlstone initiated a phase Ia clinical trial. In this study, they administered D5-ethyl-𝛽-d-glucuronide to healthy individuals in single ascending dose assays to verify safety and background D5-ethanol levels. They reported no side effects in these patients. In addition, they did not detect a D5-ethanol signal in their breath, thereby proving that D5-ethyl-𝛽-d-glucuronide was not hydrolyzed by intracellular 𝛽-glucuronidase.
Despite these successes, induced-volatolomics approaches have challenges related to the selection of the best enzymatic marker. Hence, we recently proposed to combine several VOC-based probes into a cocktail to simultaneously monitor the dysregulation of several tumor-associated enzymes in living mice or biopsies (Figure 6) [76]. Such an outcome is a great advantage in comparison with fluorescent probes, which cannot be used in cocktails because of imaging interferences. The cocktail was composed of four enzyme-responsive VOC-based probes, each targeting a particular glycosidase (𝛽-galactosidase, 𝛼-L-fucosidase, 𝛽-glucuronidase, N-acetyl-𝛽-D-glucosaminidase). Furthermore, in the presence of the corresponding enzyme, each probe led to the release of a specific ethanol isotope (D2-, D4-, D5- and 13CD5-ethanol), allowing the identification of the activating glycosidase. When the cocktail was injected into mice bearing tumors, two ethanol isotopes were detected in the animal breath (Figure 6a), highlighting the upregulation of 𝛽-glucuronidase and N-acetyl-𝛽-D-glucosaminidase in the tumor microenvironment (Figure 6b). Applied ex vivo on tumor biopsies, our cocktail again indicated an increased catalytic activity of N-acetyl-𝛽-D-glucosaminidase in tumors compared with that in healthy tissues (Figure 6c). Having identified this glycosidase as a potential target for cancer therapy, we designed an enzyme-responsive albumin-binding prodrug [77, 78, 79, 80] of the potent monomethyl auristatin E programmed for the selective release of the drug into the tumor microenvironment. This tumor-activated therapy produced remarkable therapeutic efficacy on orthotopic triple-negative mammary xenografts implanted in mice, leading to the disappearance of tumors in 66% of treated animals (Figure 6d). Thus, this study demonstrated the potential of induced-volatolomics for the exploration of biological processes as well as the discovery of novel therapeutic strategies.
Cocktail of VOC-based probes for the design of tumor activated therapy. To highlight the dysregulation of glycosidases activity in tumors, a cocktail of VOC-based probes (D2-EtGal, D4-EtFuc, D5-EtGlu and 13CD5-EtGlcNAc) has been designed. (a) After administration to tumor-bearing mice, the probes can be selectively hydrolyzed in the tumor microenvironment by the corresponding glycosidases to release D2, D4, D5 or 13CD5-ethanol isotopes. When released into tumors, labelled VOCs pass through the bloodstream and are exhaled in the breath. (b) Analysis of VOCs signals allows the identification of the most active enzymes, 𝛽-glucuronidase and N-acetyl-𝛽-D-glucosaminidase, in the microenvironment of human cervical tumors. Amount of D5-ethanol and 13CD5-ethanol exhaled by athymic nude healthy mice (green bars; n = 6); amount of D5-ethanol and 13CD5-ethanol exhaled by mice with KB subcutaneous xenograft 12 days after tumor implantation (orange bars, n = 6); amount of D5-ethanol and 13CD5-ethanol exhaled by mice with KB subcutaneous xenograft 15 days after tumor implantation (red bars, n = 6). For each isotope, significant differences in terms of ethanol isotope amounts were tested by ANOVA (confidence interval of 95%). Significant differences are indicated by the different letters. (c) Ex vivo protocol for the detection of exoglycosidases on solid biopsies (healthy kidneys and MDA-MB-231 grafts). The four ethanol isotopes were trapped in the sample headspace and analyzed. VOCs signals tended to show high catalytic activity of N-acetyl-𝛽-D-glucosaminidase in tumor tissues. (d) Structure of the 𝛽-GlcNAc-responsive albumin-binding prodrug 1. MDA-MB-231 tumor growth inhibition under therapy with vehicle and 1. Each point shows mean ± s.e.m. from 6 tumor volumes. Figure reproduced from Châtre et al. [76].
3. Discussion
The induced-volatolomics is an emerging area of science that could revolutionize our vision of analytical chemistry and strengthen the toolbox of chemical biology. Similar to enzyme-responsive probes, VOC-based probes provide critical information on enzymatic activity changes associated with disease appearance and progression both in cellulo and in vivo [42, 60, 62, 75].
Whereas off/on optical probes deliver visual images allowing the localization of biochemical events in tissues or body, VOC-based probes can provide additional precision on their spatial distribution by discriminating catalytic events occurring inside versus outside cells [42, 60]. Moreover, by using different isotopes of the same volatile tracer (i.e. labelled ethanol), VOC-based probes can be employed in cocktail to measure in real time and simultaneously several enzymatic activities in biological systems (organism or biopsy) [76]. This outcome is of particular interest compared with the usual colorimetric or fluorescent tracers. For instance, simultaneously probing several glycosidase activities through the conversion of the corresponding 4-methylumbelliferyl glycosides is not possible because all these probes release the same fluorescent tracer. While activatable dual and/or multimodal fluorescent probes [81, 82, 83, 84] can be envisioned to highlight at least two catalytic activities, their development remains limited due to imaging interferences and/or high background noise. Moreover, differences in the sensitivity between certain imaging modalities (e.g. MRI and PET) remain an issue, which requires balancing the probe concentration necessary for each imaging technique. On the other hand, tracking the same volatile tracer, as we did with labelled ethanol-based probes [76], prevents analytical variability issues linked to volatile compound detection and quantification. Therefore, VOC-based probe cocktails could soon become very useful tools for the multimodal exploration of global enzymatic dysregulation related to various diseases.
Moreover, the usefulness of VOC-based tracers could cross the boundaries of chemical biology because they are complementary to conventional “omics” approaches. In these fields of research, continuous efforts are being made to design strategies that could provide fundamental insights into enzymatic markers. Within this framework, proteomics has held a central position with the development of multiplex workflows involving antibody-based strategies, electrophoresis strategies, mass-spectrometry analysis, or in vitro enzyme assays [85, 86, 87]. Although these methodologies showed high specificity, they do not cleanly notify on the fundamental understanding of the roles of these enzymes in their physiological environment. Indeed, several key physical and chemical features of cells are ignored when separating protein markers from their actual biological contexts. Hence, they may sometimes fail because of the complexity of biological systems that could hamper the purification, detection, and sequencing of low-concentration markers. Thus, induced-volatolomics could provide major advances in terms of sensitivity and normalisation because the biological matrix should not interfere with the detection of exogenous volatile tracers. Furthermore, novel probes targeting other enzyme classes, such as esterases and oxidases, could be developed. By combining these probes with those targeting proteases and glycosidases, several enzymatic processes occurring in different cell compartments (e.g. membrane, cytosol, nucleus, bacteria periplasm and wall) could be integrated to better understand complex biochemical networks. This would certainly enhance our knowledge of enzyme interactions when a biological system is evolving towards diseases. Overall, induced-volatolomics could address fundamental and unsolved questions in biology.
In summary, the induced-volatolomics paradigm has great potential for fundamental understanding of how living cells function [58]. VOC-based probes represent a new generation of tools in the field of chemical biology, which would probably initiate scientific bridges between various disciplines and promote translational science and culture.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors thank the local committees Vienne and Deux-Sèvres of the “Ligue Contre le Cancer” and “La Ligue nationale contre le Cancer”, and the “Région Nouvelle Aquitaine” for financial supports. This work was also funded by the ANR through the Young Researcher grant “Volatolomix” (ANR-21-CE18-0009). We also thank the CNRS, which supplied the project through the “AAP Emergence” and FEDER.




 CC-BY 4.0
CC-BY 4.0