1. Introduction
Nucleoside analogues (NAs) are highly significant pharmaceutical agents. Their relevance is based on the biological functions of their natural counterparts (Scheme 1a) as building blocks of nucleic acids or as regulators of gene expression, mRNA translation, and cell signaling. The main fields of application of nucleoside drugs are the treatment of cancer and viral infections [1, 2]. The desired activity is achieved by alteration of the canonical nucleoside scaffold and its functional groups (Scheme 1b). The resulting β-NAs inhibit key enzymes such as methyltransferases, deaminases, and polymerases, thereby preventing infection or curing diseases. Additionally, the application of NAs has been approved for the treatment of the autoimmune disease multiple sclerosis [3], and some NAs even exhibit antibiotic properties [4, 5].
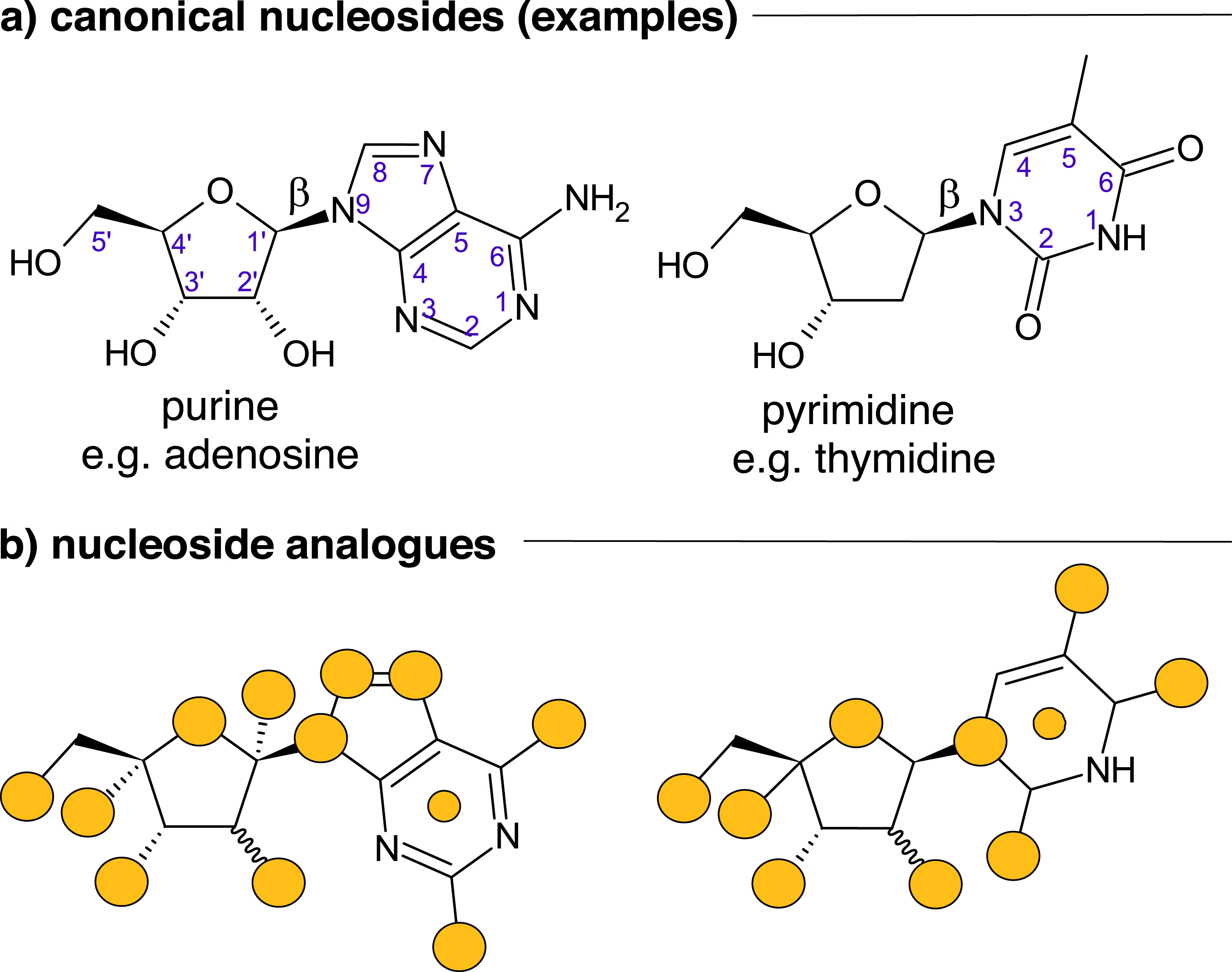
Nucleoside scaffolds: (a) canonical nucleosides and (b) nucleoside analogues. Numbering convention (blue numbers) and reported modification sites (yellow circles). Yellow circles within nucleobases signify modifications of the core structure such as heteroatom position or incomplete rings.
Given the significance of NAs as therapeutic agents, various chemical, biocatalytic, and chemoenzymatic methods have been developed (Schemes 2 and 3). In this mini-review, chemical synthesis refers to processes that do not involve biocatalysts (enzymes) in contrast to biocatalytic synthesis. Chemoenzymatic synthesis, on the other hand, denotes methods that combine both chemical and enzymatic strategies. These terms specifically refer to the individual reaction steps or corresponding cascade reactions under consideration. If the synthesis of starting materials were included in this discussion, the enzymatic production of NAs would always be classified as a chemoenzymatic approach since the precursor building blocks are typically derived from synthetic sources.
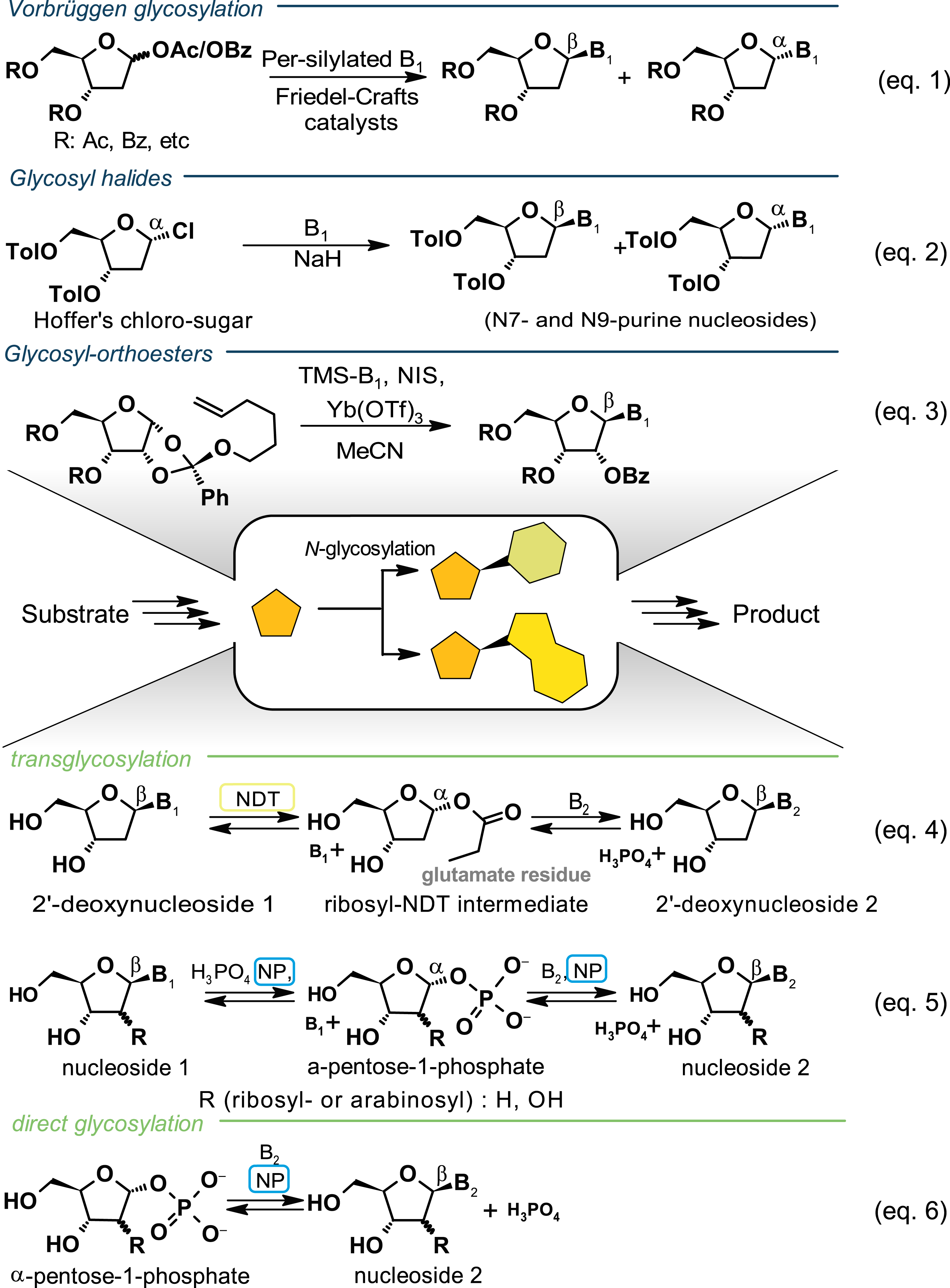
N-glycosylation as a key step in nucleoside analogue synthesis. Chemical glycosylation reactions are adapted from [6]. B1—nucleobase 1 and B2—nucleobase 2. Nucleobases typically have either a purine or a pyrimidine scaffold. NP, nucleoside phosphorylase; NDT, nucleoside 2′-deoxyribosyltransferase.
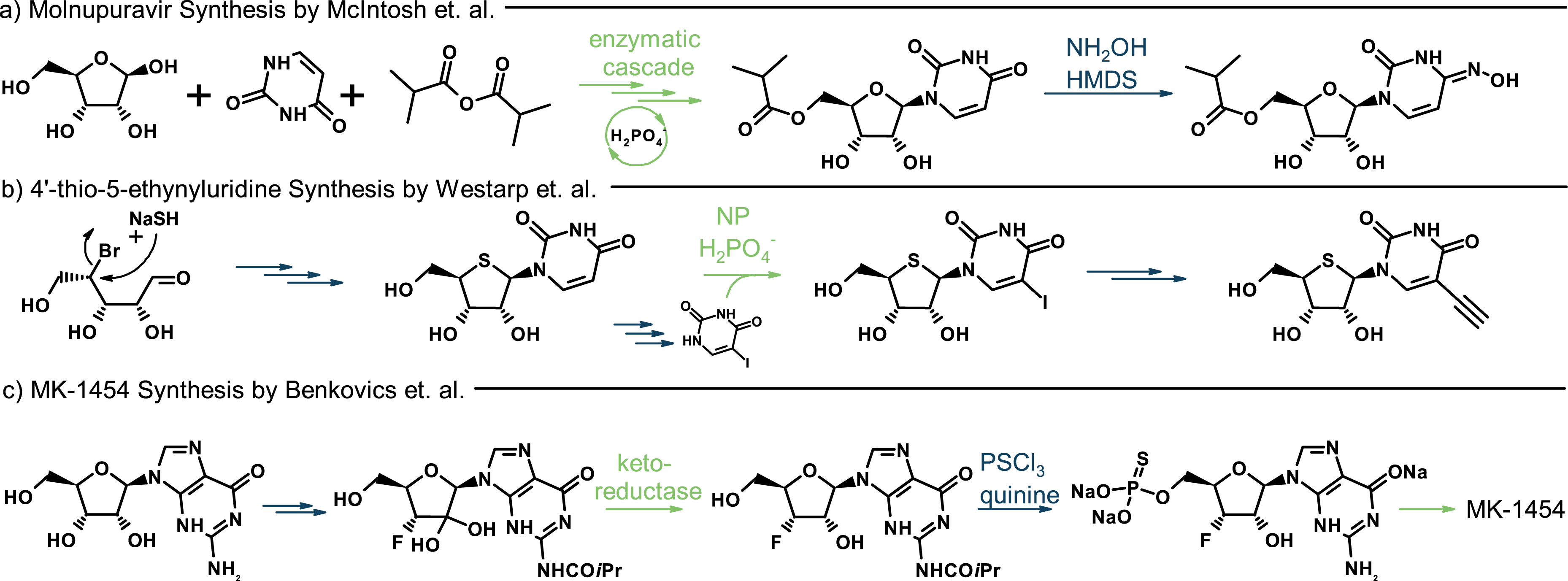
Chemoenzymatic workflows. (a) Chemoenzymatic cascade reaction towards molnupuravir [7]. (b) Biocatalytic base diversification of synthetic 4′-thiouridine and subsequent ethynylation via a Sonogashira cross-coupling reaction [8]. (c) Section of the synthesis route to the cyclic dinucleotide MK-1454 [9]. NP, nucleoside phosphorylase.
Chemical synthesis is the gold standard in NA production. Recently, this has been impressively demonstrated by Kothapalli and colleagues in their comprehensive review on synthetic routes for NAs with antiviral activity [10]. Chemical synthesis routes were described for all the 14 compounds investigated while a biocatalytic route was only outlined for one compound, namely islatravir [11]. This highlights the significant potential of synthetic chemistry and explains the number of approaches available for NA synthesis.
Since the discovery of nucleoside phosphorylases [12], biochemists have focused on enzymatic processes for NA production to overcome the limitations of chemical methods. The identification of additional suitable biocatalysts for NA synthesis has broadened the scope of enzymatic methods, and the production of a wide range of NAs has been reported. The field has experienced a revival driven by the demand for sustainable manufacturing processes and the challenges posed by the COVID-19 pandemic [7, 11].
N-Glycosylation is a key step in nucleoside (bio)chemistry, facilitating the formation of β-nucleosides from a sugar synthon and a nucleobase. In synthetic chemistry, over time, three core strategies for the N-glycosylation step have been developed. The Vorbrüggen glycosylation is frequently used but is known to produce nucleosides with poor α/β anomeric purity, particularly for 2′-deoxynucleosides, and exhibits low regioselectivity for purine nucleosides (Scheme 2, Equation (1)). To address these challenges, several alternative synthesis methods have been introduced as reviewed by Wang and colleagues [6]. Some of these approaches, such as gold-catalyzed N-glycosylation with ester or ortho ester ribosyl donors or the use of halide leaving groups, offer good regio- and stereoselectivity, even for 2′-deoxyribosides [6, 13] (Scheme 2, Equations (2) and (3)). To meet the ongoing demand for novel NAs to be synthesized and evaluated as potential drugs, numerous specialized synthesis routes have been developed over time [14, 15]. For example, intramolecular ring formation represents an alternative strategy and was first described by Hager and Liotta in 1991 [16]. It involves the formation of the N-glycosidic bond prior to ring closure. This method provides greater control over the stereochemical configuration of the N-glycosidic bond and offers more versatility compared to the aforementioned N-glycosylation reactions. More recent efforts have focused on developing protecting-group-free synthesis routes [17].
All reported biocatalytic N-glycosylation reactions are either transglycosylation or direct glycosylation reactions (Scheme 2, Equations (4)–(6)). These reactions utilize enzymes from nucleotide salvage pathways and are anomer-specific. Two enzyme classes have been identified as efficient biocatalysts for the synthesis of β-nucleosides: nucleoside phosphorylases and nucleoside 2′-deoxyribosyltransferases [18, 19, 20]. Recent advancements include one-pot nucleobase diversification of unconventional sugar modifications, such as 4′-methylated nucleosides [21] and 4′-thionucleosides [8], using nucleoside phosphorylases as well as a comprehensive characterization of the substrate scope of LlNDT-2 [22]. To enhance the competitiveness of biocatalysis compared to synthetic chemistry, several strategies have been explored to (i) improve solubility, (ii) address thermodynamic limitations, and (iii) broaden the range of substrates. For instance, employing thermostable enzyme variants enables reactions at temperatures up to 90 °C and the use of solvents, which can significantly increase substrate concentration [23, 24, 25]. Thermodynamic limitations can be overcome through direct glycosylation [26, 27] and the removal of released phosphate using enzymes like sucrose phosphorylase and pyruvate oxidase [7, 11]. Additionally, enzyme engineering presents a promising approach to broaden the substrate range of biocatalysts [7, 11, 28].
This review provides an overview of advantages and drawbacks of purely chemical and biocatalytic synthesis routes. Regarding chemoenzymatic approaches, solutions are presented that overcome still existing challenges of both synthetic chemistry and biocatalysis, showcasing the advantages of combining both methods. By presenting the key strategies of NA synthesis, the authors aim to inspire researchers from both disciplines to collaborate to harness the full potential of this integrated approach. Hopefully, this will help address the existing challenges in NA synthesis and pave the way for more efficient production methods.
2. Strengths and weaknesses of synthetic chemistry and biocatalysis
The success of synthetic chemistry in NA synthesis can be attributed to several key advantages: the wide availability of sugar synthons, the ability to work with high substrate loads, and the tolerance for diverse and often extensive modifications (Figure 1). Disregarding factors such as yield, efficiency, and sustainability, synthetic chemistry offers the flexibility to develop a process for virtually any NA. However, despite its established dominance, chemical synthesis routes for NAs still show several drawbacks, including the requirement for harsh reaction conditions, multiple protection and deprotection steps, and challenges with regard to regioselectivity and stereoselectivity. These factors often result in complex multistep procedures, which in sum decrease overall process efficiency [29].
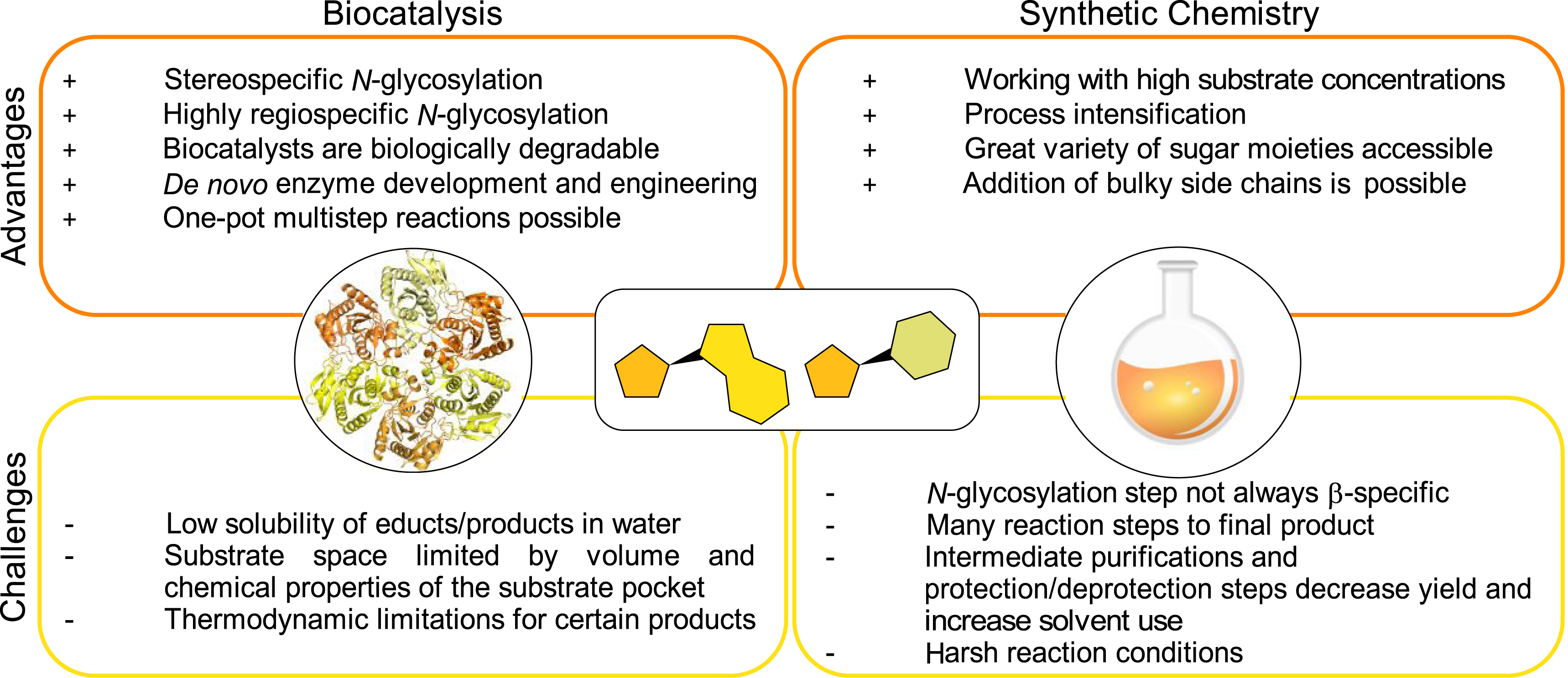
Strengths and drawbacks of chemical and biocatalytic synthesis routes.
In contrast, biocatalytic synthesis routes are highly regio- and stereoselective, enabling efficient one-pot cascade reactions under mild reaction conditions. De novo enzyme development and enzyme engineering now also allow the synthesis of highly modified compounds. However, despite significant advancements, biocatalytic production still faces challenges, such as the limited tolerance for large substituents and certain thermodynamic constraints (Figure 1).
3. Chemoenzymatic approaches for NA synthesis
In the literature on nucleoside chemistry, only a few chemoenzymatic synthesis routes have been described so far. Most of the reported methods primarily focus on biocatalytic endgame reactions, utilizing either nucleoside phosphorylases or nucleoside 2′-deoxyribosyltransferases. This section aims to highlight three recent cascade synthesis strategies for NAs, each designed to leverage the complementary strengths of both chemical and enzymatic methods. Two examples illustrate biocatalytic reactions that have been coupled with a chemical reaction step, and one example demonstrates a chemical synthesis that incorporates a biocatalytic step.
One impressive chemoenzymatic cascade reaction is the synthesis of molnupuravir, which was reported by McIntosh and colleagues. After the C5-acylation of ribose by Candida antarctica lipase B, ribosyl-1-phosphate was formed using engineered 5-(S)-methylthioribose kinase and N-glycosylation was realized by engineered uridine phosphorylase (Scheme 3a) [7]. The reaction was driven by a sophisticated ATP regeneration system based on pyruvate oxidase and acetate kinase. The last reaction step required the interconversion of the amidic carbonyl in the uracil ring to the corresponding oxime and was performed using synthetic chemistry. It was necessary to follow this regime as uridine phosphorylase does not accept cytidine derivatives as substrates. Hence, this approach duly shows how to overcome substrate limitations of the biocatalyst by implementing a chemical step.
The second example is work from our own group: starting from commercially available ribose, where 1′-OAc-4′-thioribose was initially obtained in seven synthetic steps. From there, Pummerer-type glycosylation yielded β-4′-thiouridine with good selectivity [30, 31]. We employed thermostable nucleoside phosphorylase to convert 4′-thiouridine, for example, to 4′-thio-5-iodouridine with 82% yield. For this, 4 equiv of base was applied in the transglycosylation reaction. After purification by semi-preparative HPLC, a Pd-catalyzed Sonogashira cross-coupling reaction gave 4′-thio-5-ethynyluridine in quantitative yields (Scheme 3b). A final synthetic step was necessary since 5-ethynyluridine is thermodynamically unfavored with respect to most other nucleosides and therefore hard to access by nucleoside phosphorylase catalyzed transglycosylation. In the same publication, we demonstrated the versatility of this enzymatic platform by synthesizing a range of other halogenated purine and pyrimidine nucleosides [8].
The third example is the synthesis of nucleotide analogue MK-1454 reported by Benkovic and colleagues. In this process, engineered α-ketoreductase is used in between chemical transformations to ensure the correct anomeric configuration of the 2′-hydroxygroup from a 2′-ketonucleoside intermediate. This approach exhibited superior diastereoselectivity compared to several traditional catalysts explored by the authors [9] (Scheme 3c).
The limited number of examples of chemoenzymatic synthesis routes can likely be attributed to the highly specialized environments in which chemists and biochemists typically work. A 2023 survey by Gallou et al. examined how decision-makers in the chemical industry perceive biocatalysis. The findings revealed that they tend to rely more on the strengths of their own discipline to solve problems rather than seeking solutions from other fields. Interestingly, however, most participants in the survey recognized the potential of biocatalysis [32]. This raises the question of how to facilitate the development of new chemoenzymatic synthesis methods. If approached effectively, nucleoside chemistry could greatly benefit from the complementary strengths of both fields (Figure 1).
4. Conclusion
The most recent reviews on nucleoside chemistry and sugar synthesis primarily focus on the achievements and challenges within synthetic chemistry [14, 33]. Even though Wang et al. and Cosgrove and Miller acknowledge the potential of biocatalysts and highlight their respective strengths, they still predominantly emphasize chemical approaches [6, 34]. We believe that integrating both strategies is key to overcoming the most pressing challenges since biocatalytic synthesis can address some of the limitations inherent in chemical synthesis and vice versa.
CRediT author contributions
SW, AK: Conceptualization. SW: Methodology, Formal Analysis, Investigation, Resources, Data Curation, Writing—Original Draft. SW, AK, PN: Writing—Review & Editing. SW: Visualization. AK: Supervision. PN: Project Administration, Funding Acquisition.
Declaration of interest
Anke Kurreck is CEO of BioNukleo GmbH, Peter Neubauer is a member of the advisory board. The authors declare no conflict of interest.
Acknowledgment
The authors thank Laura Hillebrand for valuable feedback on the chemical section of the manuscript.





 CC-BY 4.0
CC-BY 4.0