1. Introduction
High-valent oxoiron species play important roles in both heme and non-heme monooxygenase enzymes and their biomimetic reactions. These enzymes catalyze many oxidation reactions including oxygen atom transfer (hydroxylation, epoxidation) and dealkylation, but much less activation was observed when iron was replaced by manganese. However, in biomimetic systems oxomanganese complexes mediate most of the oxidations brought by the iron oxygenases leading to a wide range of manganese catalysts for the epoxidation of alkenes, sulfoxidation of thioethers and hydroxylation of unactivated C–H bonds [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11]. The advantage of these systems is that they are inexpensive and less toxic than other transition metal complexes. We have shown earlier that manganese 1,3-bis(2′-Ar-imino)isoindoline complexes can mediate many types of biomimetic redox reactions [12]; including the oxidation of catechols to quinones (catechol oxidase) [13], the oxidative cyclization of 2-aminophenol to phenoxazinone (phenoxazinone synthase) [13], the catalytic disproportionation of hydrogen peroxide (catalase) [14, 15, 16], and the dismutation of superoxide radicals (superoxide dismutase, SOD) [17]. Furthermore they can be used as hydrogen peroxide-based bleach catalysts [18]. In this ligand the introduction of various aryl groups on the bis-iminoisoindoline moiety provided a new method for tuning the reactivity and electronic structure of the catalyst. This work focuses on our recent efforts to tune the reactivity of our manganese system and investigate how the catalytic activity can be optimized compared to our iron system in the oxidation of organic substrates including thioanisols and benzyl alcohols. Organic sulfoxides and carbonyl compounds produced by the oxidation of alcohols are used as intermediates for the synthesis of various agrochemicals, plastic additives, pharmaceuticals and drugs [19, 20, 21, 22, 23, 24]. Therefore, there has been considerable interest in the development of non-toxic, cheap and highly efficient oxidation catalysts for selective oxidation of organic sulfides and benzyl alcohols [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53], including Mn-based systems with various oxidants such as PhIO, mCPBA and H2O2 [43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53].
Structure of the 1,3-bis(2′-Ar-imino)isoindoline ligands used for the synthesis of [MnII(HL1-6)Cl2] (1–6) catalysts involved in this study.
2. Experimental section
2.1. Materials and methods
The ligands 1,3-bis(2′-Ar-imino)isoindolines (HLn, n = 1–6, Ar = 4′-methyl-pyridyl, pyridyl, imidazolyl, thiazolyl, benzimidazolyl and N-methylbenzimidazolyl, respectively) and their complexes [MnII(HL1-6)Cl2] (1–6) were prepared according to published procedures (Scheme 1) [12, 18]. GC analyses were performed on an Agilent 6850 gas chromatograph equipped with a flame ionization detector and 30 m SUPELCO BETA DEX225 columns. GC–MS analyses were carried out on Shimadzu QP2010SE equipped with a secondary electron multiplier detector with conversion dynode and a 30 m HP5MS column.
2.2. Description of the catalytic oxidation reactions
For the oxidation of thioanisoles and benzylalcohols, the reactions were carried out under argon atmosphere at room temperature (25 °C). In a typical experiment, 1 mL of H2O2 (diluted from 38–40% solution), mCPBA (77%), or tBuOOH (diluted from 70% solution) in acetonitrile was delivered by syringe pump to a stirred solution (2 mL) of catalyst and p-substituted thioanisole or benzylalcohol derivatives. The final concentrations were 10 mM catalyst, 250 mM co-oxidants, and 500 mM substrate. The PhIO was added as a solid into the water (100 μL) containing CH3CN solution. The manganese complexes were removed by passing the internal standard containing reaction mixture through a silica column followed by elution with ethyl acetate. The products were identified by GC–MS based on authentic samples, and quantified by GC relative to bromobenzene as an internal standard.
PhS(O)Me: m/z (%) = 140 (100), 125 (87), 97 (78), 77 (71), 65 (34), 51 (94); 4Cl-PhS(O)Me: m/z (%) = 174 (46), 159 (100), 131 (38), 111 (25), 75 (64), 50 (51.5); 4NO2-PhS(O)Me: m/z (%) = 185 (100), 170 (67), 139 (38.6), 76 (47.7), 63 (34), 50 (48); 4Me-PhS(O)Me: m/z (%) = 154 (67), 139 (100), 111 (21.2), 91 (44.6), 77 (55), 65 (43); 4MeO-PhS(O)Me: m/z (%) = 171 (100), 107 (80), 77 (51.2), 64 (17.6), 50 (12.4).
PhCHO: m/z (%) = 106 (95), 105 (90), 77 (100), 74 (11.2), 51 (57.6), 50 (35); 4Me-PhCHO: m/z (%) = 120 (85), 119 (90), 91 (100), 65 (51.3), 62 (17.6), 51 (25); 4Cl-PhCHO: m/z (%) = 141 (47), 140 (86), 139 (100), 713 (23), 111 (83.2), 75 (47.6), 50 (45); 4MeO-PhCHO: m/z (%) = 137 (87), 135 (100), 92 (37), 77 (84), 63 (19.2), 50 (17.7); 4NO2-PhCHO: m/z (%) = 151 (100), 150 (95), 105 (31), 77 (81.4), 51 (77.6), 50 (34).
3. Results and discussion
Iron and manganese containing porphyrin and phthalocyanine complexes as catalysts can be used for a wide range of oxidation reactions due to their distinguishing properties such as variable oxidation states and readily tunable Lewis acidic behavior and redox properties [54, 55, 56, 57, 58]. Based on the advantageous properties of the above systems the solubility, electronic structure and steric properties of the ligand as well as their complexes can be further developed by the synthesis of 1,3-bis(2′-Ar-imino)isoindolines, an open-chained phthalocyanine mimic, introducing various aryl groups on the bis-iminoisoindoline moiety [12]. In this study we have investigated the catalytic properties of manganese 1,3-bis(2′-Ar-imino)isoindolines for the oxidation of organic sulfides and benzyl alcohols with various co-oxidants compared to the recently published iron 1,3-bis(2′-Ar-imino)isoindoline system [59, 60].
Comparison of the product (PhS(O)Me) formation in the manganese (2)-catalyzed oxidation of PhSMe with various co-oxidants in CH3CN at 25 °C (entries 1, 6–8 in Table 1).
Various [MnII(HL1-6)Cl2] (1–6) complexes-catalyzed thioanisole (4R-PhSMe) oxidation by H2O2, tBuOOH, PhIO and mCPBA in MeCN at 25 °C
| Entry | Cat. | Timea (min) | Oxidant | R | Epc b (mV) | Epa b (mV) | Yield (%) | TONc | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | mCPBA | H | 880 | 1016 | 948 | 66 | 16.4 |
| 2 | 2 | 10 | mCPBA | H | 865 | 987 | 926 | 34 | 3.4 |
| 3 | 2 | 15 | mCPBA | H | 865 | 987 | 926 | 48.8 | 4.9 |
| 4 | 2 | 20 | mCPBA | H | 865 | 987 | 926 | 61 | 15.1 |
| 5 | 2 | 30 | mCPBA | H | 865 | 987 | 926 | 60.4 | 15.1 |
| 6 | 2 | 20 | PhIO | H | 865 | 987 | 926 | 26.4 | 6.6 |
| 7 | 2 | 20 | tBuOOH | H | 865 | 987 | 926 | 8 | 1.9 |
| 8 | 2 | 20 | tBuOOH/AcOHd | H | 865 | 987 | 926 | 0.5 | 0.1 |
| 9 | 2 | 20 | tBuOOH/AcOHe | H | 865 | 987 | 926 | 1.8 | 0.5 |
| 10 | 2 | 20 | H2O2 | H | 865 | 987 | 926 | 1 | 0.2 |
| 11 | 2 | 30 | mCPBA | OMe | 865 | 987 | 926 | 68.4 | 17.1 |
| 12 | 2 | 30 | mCPBA | Me | 865 | 987 | 926 | 65 | 16 |
| 13 | 2 | 30 | mCPBA | Cl | 865 | 987 | 926 | 52 | 13 |
| 14 | 2 | 30 | mCPBA | NO2 | 865 | 987 | 926 | 39 | 9.9 |
| 15 | 3 | 20 | mCPBA | H | 685 | 816 | 750 | 53 | 13.2 |
| 16 | 4 | 20 | mCPBA | H | 573 | 625 | 600 | 45 | 11.3 |
| 17 | 5 | 20 | mCPBA | H | 354 | 421 | 388 | 42 | 10.5 |
| 18 | 6 | 20 | mCPBA | H | 395 | 455 | 425 | 38 | 9.4 |
aReaction conditions: Thioanisole (1.5 mmol), catalyst (0.03 mmol), oxidant (0.75 mmol) in MeCN (3 ml) at 25 °C. bmV versus SCE [18]. cTON = mol S/mol Cat. dAcOH (0.75 mmol). eAcOH (0.075 mmol).
3.1. Catalytic oxidation of thioanisole
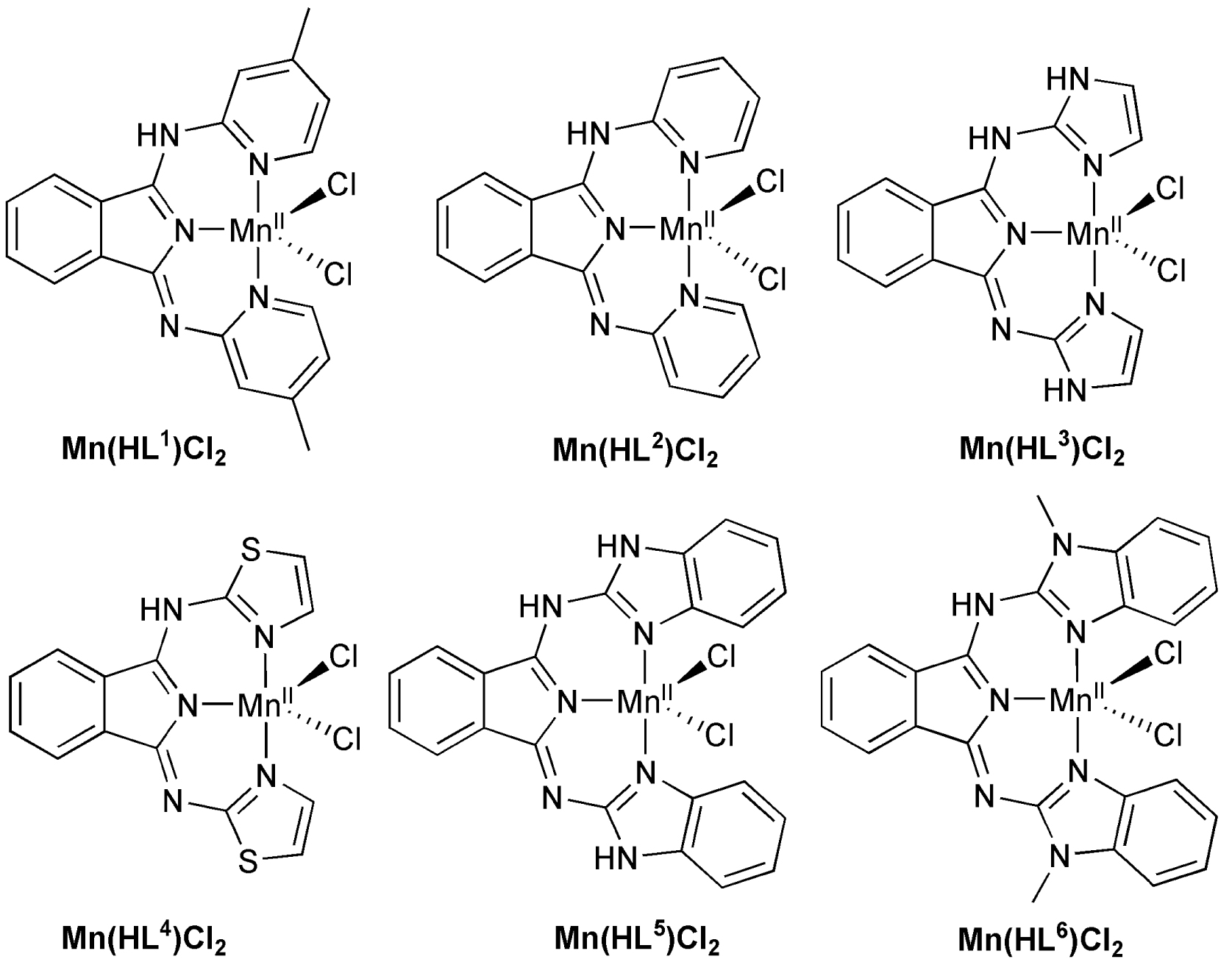
The catalytic activity of [Mn(HL2)Cl2] (2) was studied in the oxidation of thioanisole and benzyl alcohol utilizing tert-butyl hydroperoxide (tBuOOH), meta-chloroperoxybenzoic acid (mCPBA), H2O2 and PhIO as co-oxidants. Using an identical concentration of catalyst/substrate/co-oxidant (1/PhSMe/co-oxidant = 1/50/25), Table 1 and Figure 1 show that there is an increase of the yield when the co-oxidants employed are H2O2, tBuOOH, PhIO and mCPBA from 1 to 61% (entries 1, 6–8 in Table 1). The highest conversion is attained with mCPBA, where 15 turnovers were observed in the course of 20 min, after which time there was no further product formation (entries 1–5 in Table 1). Under this condition we were not able to catch the characteristic absorption band around 900 nm in case of our coordinatively unsaturated complexes, which can be assigned to the proposed metastable intermediate, MnIVO. It is not surprising that the best results were obtained with mCPBA compared to tBuOOH, because the reaction with Mn(II) complexes can be seen as following: MnII + HO–B → MnII–O–B + H+ → MnIV(O) + B−, thus the reactivity can be correlated with the pKa of the couple BH/B-. The pKa values for mCPBA/mCPBA- and tBuO-/tBuOH are 3.8 and 19.2, respectively. Based on previous literature, acetic acid (AcOH) as an additive may play a key role in promoting the rapid cleavage of O–O bonds [61]. In our case, unfortunately much worse or just slightly better results were obtained, depending on the amount of AcOH, which can presumably be explained by the formation of catalytically inactive complexes (Tables 1 and 2). Low yields were obtained for H2O2, probably because it competes with 2 for the catalase-like activity.
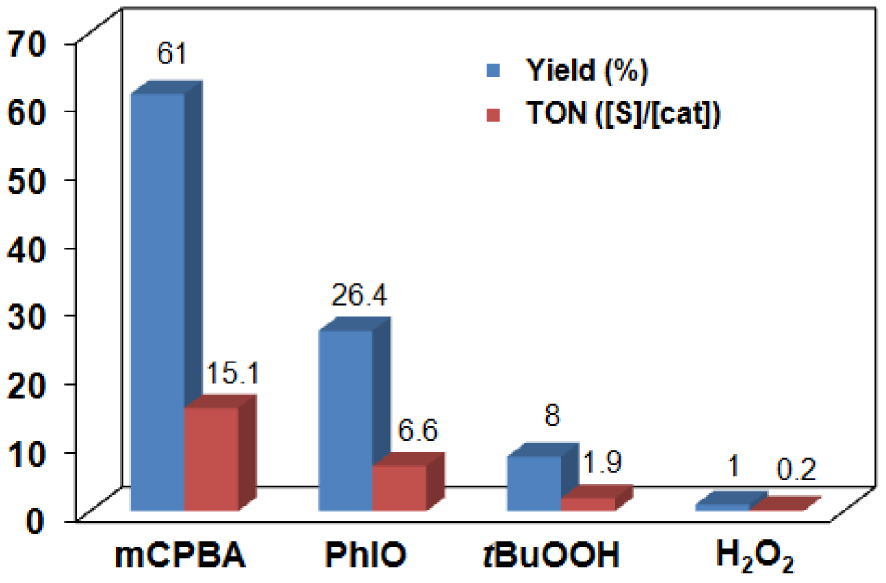
Catalytic oxidation of PhSMe with [MnII(HL2)Cl2] (2) in CH3CN at 25 °C (entries 1–5 in Table 1).
Various [MnII(HL1-6)Cl2] (1–6) complexes-catalyzed benzyl alcohol (4R-BzOH) oxidation by H2O2, tBuOOH, PhIO and mCPBA in MeCN at 25 °C
| Entry | Cat. | Timea (min) | Oxidant | R | Epc b (mV) | Epa b (mV) | Yield (%) | TONc | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | mCPBA | H | 880 | 1016 | 948 | 34 | 8.5 |
| 2 | 2 | 20 | mCPBA | H | 865 | 987 | 926 | 30 | 7.5 |
| 3 | 2 | 20 | PhIO | H | 865 | 987 | 926 | 8.5 | 2.1 |
| 4 | 2 | 20 | tBuOOH | H | 865 | 987 | 926 | 11.4 | 2.8 |
| 5 | 2 | 20 | tBuOOH/AcOHd | H | 865 | 987 | 926 | 4.5 | 0.5 |
| 6 | 2 | 20 | tBuOOH/AcOHe | H | 865 | 987 | 926 | 15.2 | 3.8 |
| 7 | 2 | 20 | H2O2 | H | 865 | 987 | 926 | 2 | 0.5 |
| 8 | 2 | 30 | mCPBA | OMe | 865 | 987 | 926 | 40 | 10 |
| 9 | 2 | 30 | mCPBA | Me | 865 | 987 | 926 | 33 | 8.2 |
| 10 | 2 | 30 | mCPBA | Cl | 865 | 987 | 926 | 26 | 6.5 |
| 11 | 2 | 30 | mCPBA | NO2 | 865 | 987 | 926 | 21 | 5.2 |
| 12 | 3 | 20 | mCPBA | H | 685 | 816 | 750 | 26 | 6.5 |
| 13 | 4 | 20 | mCPBA | H | 573 | 625 | 600 | 22.8 | 5.7 |
| 14 | 5 | 20 | mCPBA | H | 354 | 421 | 388 | 19.2 | 4.8 |
| 15 | 6 | 20 | mCPBA | H | 395 | 455 | 425 | 16.8 | 4.2 |
aReaction conditions: BzOH (1.5 mmol), catalyst (0.03 mmol), oxidant (0.75 mmol) in MeCN (3 ml) at 25 °C. bmV versus SCE [18]. cTON = mol S/mol Cat. dAcOH (0.75 mmol). eAcOH (0.075 mmol).
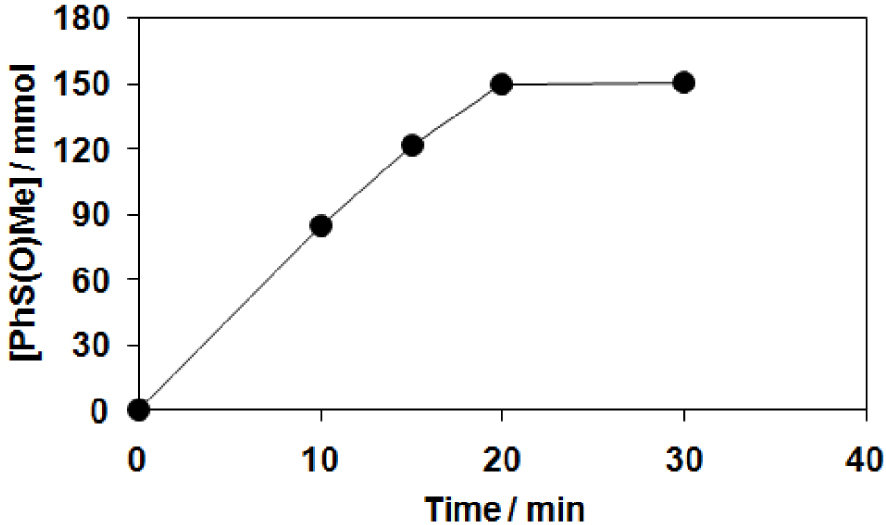
The reactivities of para-substituted thioanisols relative to that of thioanisole were also investigated (Figure 3). Hammett treatments of relative reactivities (krel = log(Xf∕Xi)∕log(Yf∕Yi), where Xi and Xf are the initial and final concentration of para-substituted thioanisols, and Yi and Yf are the initial and final concentration of thioanisole) of various substituents against 𝜎 gave 𝜌 value of − 0.26 (Figure 4A), which suggests that the behavior of the oxidant generated from [Mn(HL1)Cl2] with mCPBA is mildly electrophilic. Furthermore, this value is comparable to that of the closely related [Fe(HL2)]2+/H2O2 system (𝜌 = −0.40) [59]. When the logkrel values were plotted against the
Comparison of the product formation in the manganese-catalyzed (2) oxidation of substituted thioanisols (entries 4, 9–11 in Table 1).
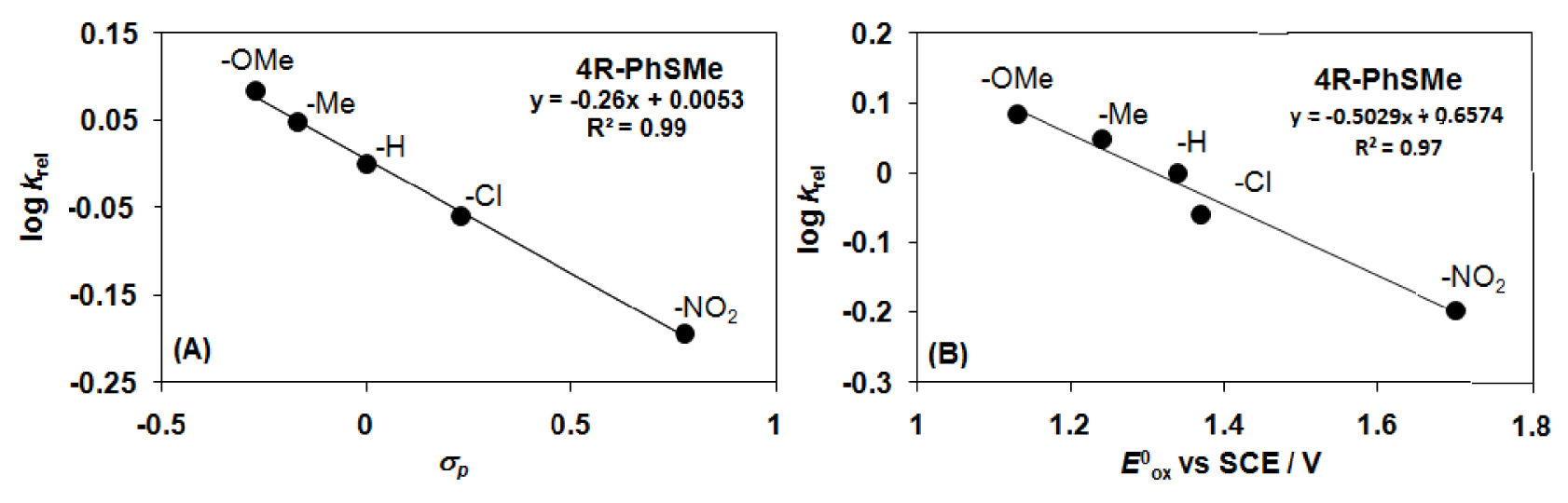
Catalytic oxidation of substituted thioanisols with [MnII(HL2)Cl2] (2) in CH3CN at 25 °C (Table 2). (A) Plot of logkrel against the 𝜎p of para-substituted thioanisols. (B) Plot of logkrel against the Eox of para-substituted thioanisols (entries 4, 9–11 in Table 1).
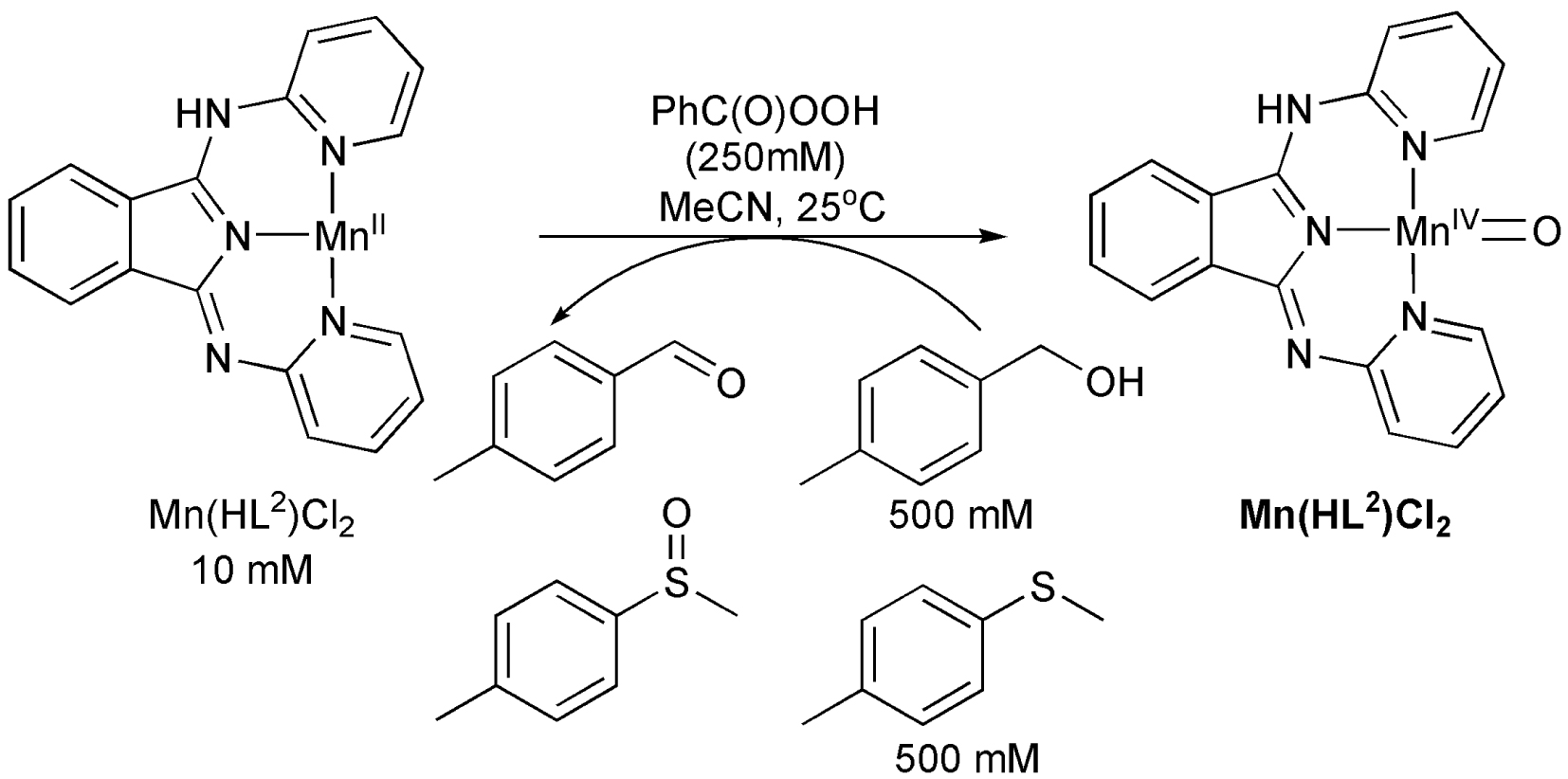
O-atom and H-atom transfer reactions: catalytic oxidation of sulfides and benzyl alcohols by manganese(II) complexes.
3.2. Catalytic oxidation of benzyl alcohols
The oxidation of benzyl alcohol by [Mn(HL2)Cl2] with mCPBA, PhIO, tBuOOH and H2O2 at 25 °C resulted in the formation of benzaldehyde in 30, 8.5, 11.4 and 2% yield, respectively (entries 2–5 in Table 2 and Figure 5). The highest reactivity with a 7.5 turnover was observed for a mCPBA co-oxidant in the course of 20 min, after which time there was no further product formation.
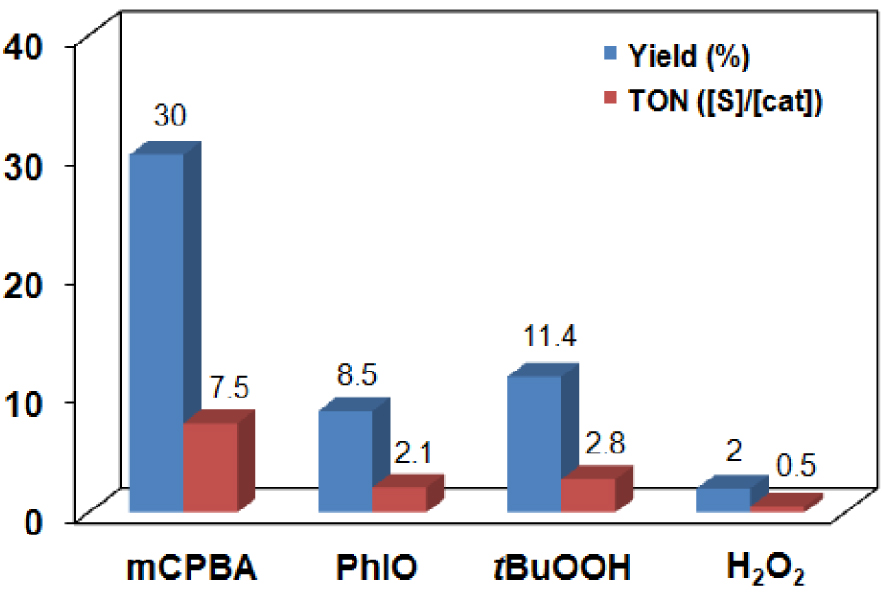
Comparison of the product (PhCHO) formation in the manganese (2)-catalyzed oxidation of BzOH with various co-oxidants in CH3CN at 25 °C (entries 2–5 in Table 1).
A Hammett plot of krel values gave a 𝜌 value of − 0.27, suggesting an electrophilic oxidant formation during the oxidation process (Figure 6, Scheme 2). This value is a little bit higher than those obtained for bona fide complexes, [FeIV(O)(N4Py/TPA)]2+ (𝜌 = 0.07) [63], but three times smaller than that was observed for the closely related [Fe(HL2)]2+/H2O2 system (𝜌 = −0.85) [59].
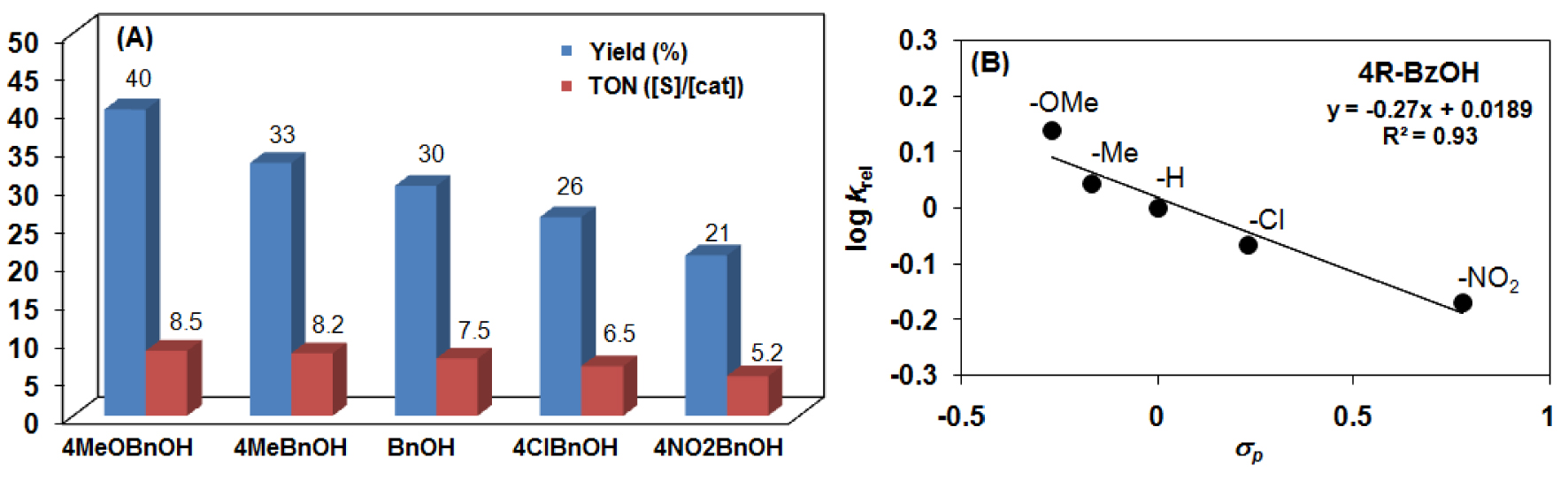
Catalytic oxidation of substituted benzyl alcohols with [MnII(HL2)Cl2] (2) in CH3CN at 25 °C (Table 2). (A) Comparison of the product formation in the manganese-catalyzed (2) oxidation of substituted benzyl alcohols. (B) Plot of logkrel against the 𝜎p of para-substituted benzyl alcohols (entries 2, 6–9 in Table 2).
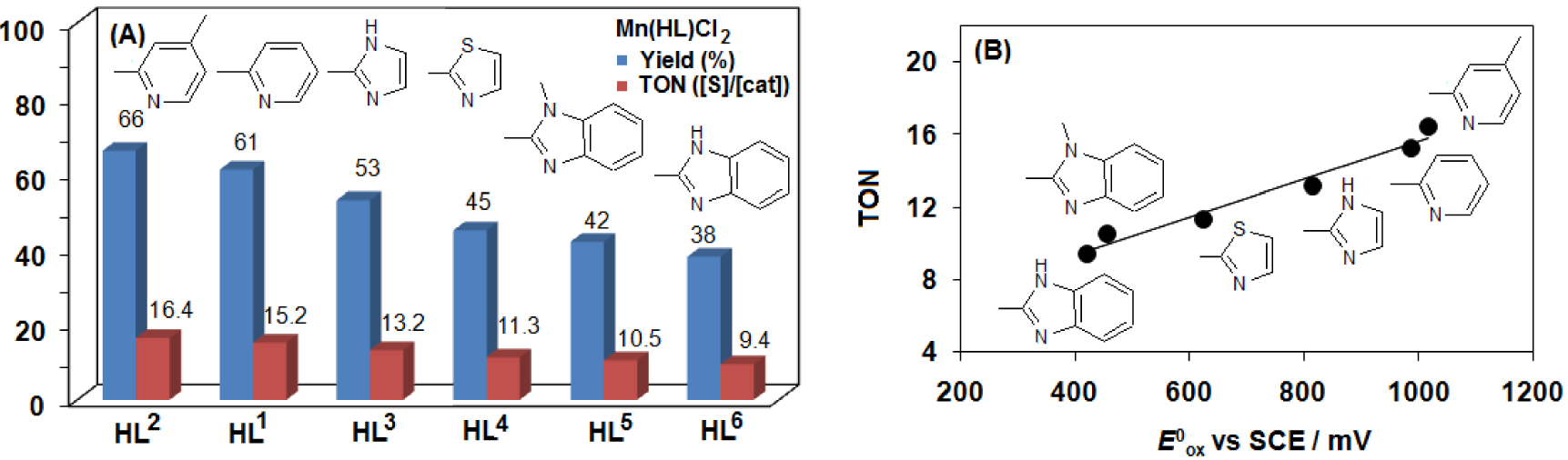
(A) Comparison of the oxidative reactivity of [MnII(HL1-6)Cl2] (1–6) complexes for OAT reactions with thioanisole. (B) Dependence of the TON on the oxidation potential (Epa) of the [MnII(HL1-6)Cl2] (1–6) complexes (entries 1, 4, 13–16 in Table 1).
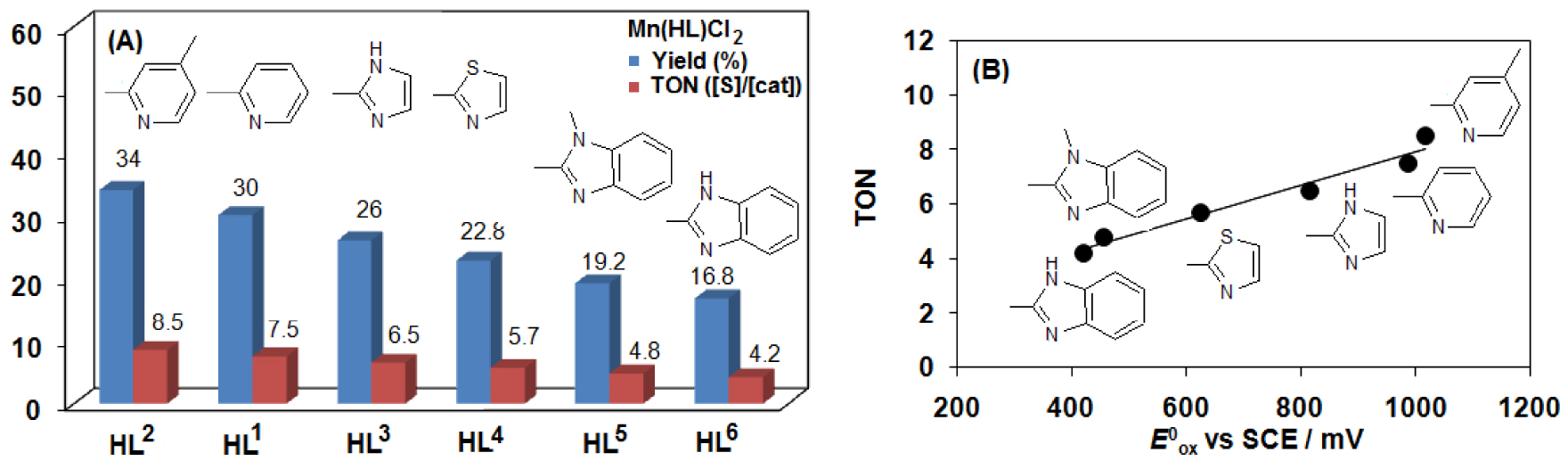
(A) Comparison of the oxidative reactivity of [MnII(HL1-6)Cl2] (1–6) complexes for HAT reactions with benzyl alcohol. (B) Dependence of the TON on the oxidation potential (Epa) of the [MnII(HL1-6)Cl2] (1–6) complexes (entries 1–2, 10–13 in Table 2).
Based on our previous experience we expected that varying the aryl group on the bis-iminoisoindoline moiety would directly tune electron density in the metal center and hence allow us to evaluate the influence of these electronic changes on reactivity. Cyclic voltammetry measurements on complexes 1–6 reveal that the Mn(III/II) redox potentials can vary by up to 560 mV between the most electron-withdrawing (948 mV for 1) and the most electron-donating (388 mV for 5) [18]. With a systematic series of [MnII(HL1-6)Cl2] (1–6) complexes, we investigated their oxidative reactivity for HAT (hydrogen atom transfer) and OAT (oxygen atom transfer) reactions with benzyl alcohol and thioanisole. Among these catalysts, we observed that the complex 5 and 6 containing the most electron-donating benzimidazole arms, show the smallest rates of oxidation for both OAT (Figure 7) and HAT (Figure 8) reactions. In other words, the electron-deficient, Lewis acidic catalyst (1 and 2) is much more active than the electron-rich ones (5 and 6). Similar trend was observed for the disproportionation of H2O2 by [MnII(HL1-6)Cl2] (1–6) complexes [18]. The reactivity enhancement with the appropriate aryl arm is similar to that observed for the axial ligand effect in the oxoiron(IV)-containing model complex, FeIV(O)(PY5Me2) (PY5Me2 = 2,6-bis(1,1-bis(2-pyridyl)ethyl)pyridine) [64] and FeIV(O)(TPA) (TPA = tris(2-pyridylmethyl)amine) [65], but it is moderate compared to the axial effect of the anionic ligand in case of FeIV(O)(TMC) (TMC = tetramethylcyclam) [66, 67], FeIV(O)(TMP+∙) (TMP = Tetramesitylporphyrin) [68] and MnV(O)(TBP8Cz) (TBP8Cz = octakis(p-tert-butylphenyl)corrolazinato3-) [69, 70, 71] models. In our case the change in the ligand set varies the reactivity by less than 3-fold based on TONs.
4. Conclusion
We have investigated the effect of systematic changes on the 1,3-bis(2′-Ar-imino)isoindoline by introducing various heteroaromatic arms on the bis-iminoisoindoline moiety toward the reactivity of their manganese(II) complexes for HAT and OAT reactions. These transformations resulted in a predictable and significant 560 mV shift in the MnIII∕MnII redox potentials with concomitant changes in the reactivity toward thioanisols and benzyl alcohols. The effect is small in both cases, but based on these results, including the linear correlations between the oxidation reactivity of the catalysts and MnIII∕MnII redox potentials and the Hammett correlations (𝜌 = −0.27 for 4R-PhSMe and 𝜌 = −0.27 for 4R-PhCH2OH) electrophilic oxomanganese(IV) intermediate has been suggested as key oxidant.




 CC-BY 4.0
CC-BY 4.0