Dimetallic centres 〚1, 2〛 containing metal–metal bonds present a range of interesting structural 〚3〛, electronic 〚4〛 and photochemical 〚5〛 properties. In particular, diruthenium cores have been used as connectors in nanoscale organometallo-wires 〚6〛. 1,8-Naphthyridine may serve as a well-adapted binding site for such dimetallic units 〚7, 8〛. On the other hand, heterocyclic bridging units such as pyrazine 〚9〛, pyrazolyl 〚10〛 or bipyrimidine 〚11〛 provide electronic communication between redox-active or photoactive moieties. Both units may be combined to serve as components for molecular electronic devices 〚12〛.
In the course of the design of polyheterocyclic molecular strands capable of self-organisation into specific molecular and supramolecular architectures 〚14, 15〛, we synthesised a set of molecules combining naphthyridine-binding sites and pyrimidine-bridging units that wrap into helical shape and form extended stacks 〚16〛. We also explored their potential to act as ligands for generating multiple dimetallic arrays and we report here the synthesis and characterisation of the crescent-shaped bis-dirhodium complex {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2 formed by the ligand 2-aryl-4,6-bis(2-(7-pyridyl)-1,8-naphthyridyl)-pyrimidine (L1) (Fig. 1).
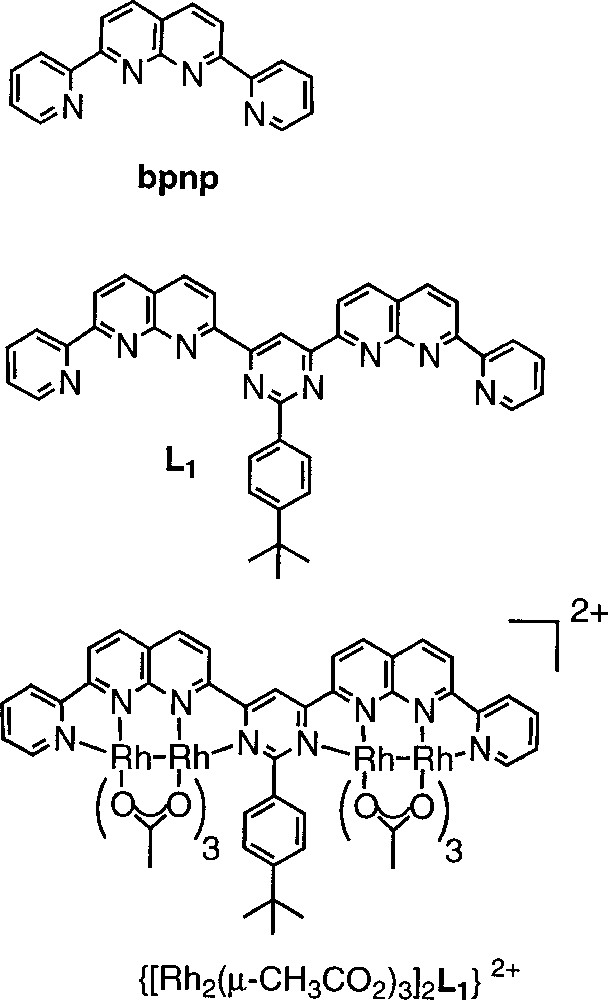
Structures L1, bpnp, {〚Rh2(μ-CH3CO2)3〛2L1}2+.
Although the 〚Rh2(μ-CH3CO2)3bpnp〛PF6 complex had already been described in the literature 〚7, 8〛, the preparation of the ligand and complex were repeated for comparison purposes. Both ligands were synthesised according to standard Friëdlander methodology 〚17, 18〛, and were characterised by 1H, 13C NMR and elemental analysis. The experimental procedures will be described elsewhere.
Heating a 1:2:2 mixture of ligand L1 (resp. bpnp), Rh2(OAc)4 and 1.0 M aqueous HCl in de-aerated methanol under argon for 20 h gave a deep green (respectively purple) solution. Addition of aqueous ammonium hexafluorophosphate to the methanolic solution of {〚Rh2(μ-CH3CO2)3〛2L1}(CH3CO2)2, followed by slow diffusion of diethylether into a solution of the formed precipitate in acetonitrile, yielded green crystals {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2 (>80%).
The 1H NMR spectrum in CD3CN of the complex derived from L1 is in accordance with a single symmetrical species, distinct from the ligand. Both species of composition {〚Rh2(μ-CH3CO2)3〛2L1}2+ and {{〚Rh2(μ-CH3CO2)3〛2L1}PF6}+ were identified by ESMS. These data agree with the formulation of the complex formed as the bis-dirhodium species. It was confirmed by determination of the crystal structure {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2. As shown in Fig. 2, it consists of two dirhodium cores bound to the naphthyridine units and bridged by the central pyrimidine. In each dimer centre, the bond lengths Rh(1)–Rh(2) (2.40 Å), Rh(1)–N(2) and Rh(2)–N(3) (2.00 and 1.98 Å resp.) are in agreement with those found for the ‘parent’ dirhodium complex of 2,7-bis(2-pyridyl)-1,8-naphthyridine (bpnp), 〚Rh2(μ-CH3CO2)3bpnp〛PF6 〚7〛. The axial Rh(1)–N(1) distance is slightly shorter (2.16 Å compared to 2.20 Å), whereas the Rh(2)–N(4) (pyrimidine) distance is longer (2.31 Å), as expected for these less basic sites. In order to cope with steric interactions between the aromatic protons on the naphthyridine and pyrimidine groups, the structure is slightly twisted (11° between the two naphthyridine planes), giving an overall propeller shape to the molecule. The two helicoidal enantiomers co-crystallize.

Crystal structure of the complex cation {〚Rh2(μ-CH3CO2)3〛2L1}2+, ball and stick representation (top), and space-filling representation (bottom).
Cyclic voltammetry was performed on the complexes and ligands in both dry acetonitrile (when possible) and dichloromethane using tetra-n-butylammonium perchlorate as supporting electrolyte, platinum as auxiliary electrode, silver as ‘reference’ and glassy carbon as working electrodes. The E1/2 potentials were measured using ferrocene as an internal standard, and are reported vs SCE in Table 1.
Electrochemical data (E1/2 vs SCE) for the dirhodium and bis-dirhodium complexes, and the corresponding ligands.
| Compound | Conditions | Reduction (a) | Oxidation (a) |
| bpnp | CH2Cl2, 0 °C | –1.64 (rev.) | none (< 1.8) |
| L1 | CH2Cl2, 0 °C | – 1.72 (rev.); –1.36 (quasi–rev.); –1.36 (rev.) | none (< 1.8) |
| 〚Rh2(μ–CH3CO2)3bpnp〛PF6 | CH2Cl2, 0 °C | –1.40 (rev.); –0.57 (rev.) | 1.40 (rev.) |
| {〚Rh2 (μ–CH3CO2)3〛2L1}(PF6)2 | CH2Cl2, 0 °C | –1.69 (rev.); –1.40 (rev.); –1.18 (rev.); –0.58 (rev.); –0.19 (rev.) | 1.49 (rev.) |
| bpnp | CH3CN, 25 °C | –2.00 (quasi–rev.); –1.56 (rev.) | none (< 1.8) |
| 〚Rh2(μ–CH3CO2)3bpnpPF6 | CH3CN, 25 °C | –1.34 (rev.), –0.64 (rev.) | 1.30 (rev.) |
| {〚Rh2 (μ–CH3CO2)3〛2L1} (PF6)2 | CH3CN, 25 °C | –0.49 (rev.); –0.21 (rev.) | 1.46 (rev.) |
Due to the limited solubility of the doubly reduced species starting from {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2 in acetonitrile, the electrochemical behaviour of both complexes and of both ligands L1 and bpnp were more thoroughly studied in dichloromethane.
In these conditions, the longer ligand L1 is easier to reduce, compared to bpnp, due to the presence of the pyrimidine unit and the ability for charge delocalisation.
The ligand L1 shows three reduction waves and no oxidation. As expected, the complex is more easily reduced (five reversible waves) and oxidised (one reversible wave).
Compared to 〚Rh2(μ-CH3CO2)3bpnp〛PF6, the bis-dirhodium complex is markedly easier to reduce (δE1/2 1rst red = E1/21rst red 〚(Rh2)2〛 – E1/21rst red (Rh2) = 0.4 V) and more difficult to oxidise (δE1/2 1rst ox = 0.1 V), which is expected when a pyridine is replaced by a pyrimidine bound to two metallic centres. The presence of the bridging unit may also provide communication between the two dirhodium sites.
The electrochemical behaviour is mirrored by the electronic absorption spectrum in acetonitrile (Table 2). Most remarkably, the lowest energy electronic transition shifts from 578 nm in the purple 〚Rh2(μ-CH3CO2)3bpnp〛PF6 〚7〛 to 622 nm in the green {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2, which is consistent with a charge transfer (MLCT) assignment of the electronic transition with a shift to longer wavelength for {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2, in agreement with the presence of a lower lying LUMO (Fig. 3).
Electronic absorption spectral data: λmax (nm), log10ϵ (in acetonitrile).
| 〚Rh2(μ-CH3CO2)3bpnp〛, PF6 | 248 | 283 | 346 | 363 | 408sh | 440sh | 540sh | 578 |
| (4.69) | (4.33) | (4.46) | (4.63) | (3.34) | (3.08) | (3.40) | (3.52) | |
| {〚Rh2(μ-CH3CO2)3〛2L1} (PF6)2 | 250 | 292 | 347sh | 371 | 412sh | 468 | 543 | 622 |
| (4.9) | (4.6) | (4.5) | (4.6) | (4.2) | (4.02) | (3.71) | (3.71) |

Comparative electronic energy levels, HOMO and LUMO, for the complexes 〚Rh2(μ-CH3CO2)3bpnp〛PF6 (left) and {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2 1 (right), and corresponding long-wavelength electronic transition.
The tetrarhodium complex {〚Rh2(μ-CH3CO2)3〛2L1}(PF6)2 hence shows novel electronic features that are currently being further studied. A deep blue green (λmax = 595 and 690 nm in acetonitrile) bis-diruthenium complex was also obtained. Longer strands containing several naphthyridine and pyrimidine subunits have been synthesised and their conformational 〚16〛 as well as their metal complexation features are being explored. Such entities that contain communicating multiple dimetallic cores are promising components for extended molecular electronic devices 〚12, 13〛.
Appendix
Crystal structure data for 1
Single crystals of compound 〚C57H48N8O12Rh4·2 PF6·8 CH3CN〛 were grown by slow diffusion of diethylether into a solution in acetonitrile. The crystals were placed in oil and a single dark green crystal of dimensions 0.22 × 0.12 × 0.12 mm was selected, mounted on a glass fibre, and placed in low-temperature N2 stream. X-ray diffraction data for the complex were collected on a Nonius-Kappa CCD diffractometer with a graphite monochromatic Mo Kα radiation (λ = 0.710 71 Å), phi scans, at 173 K. The unit cell was triclinic with a space group of . Cell dimensions: a = 15.674(2), b = 17.183(2), c = 17.833(4) Å, α = 97.75°, β = 105.35°, γ = 112.37°, V = 4128(4) Å3 and Z = 2 (FW = 2091.24, ρ= 1.682 g cm–3). Reflections were collected from 1.23 ≤ θ ≥ 27.43 for a total of 18 079, of which 10 822 were unique (Rint = 0.074); number of parameters = 872. Final R factors were R1 = 0.091 (based on observed data), wR2 = 0.256 (based on all data), GOF = 1.366, maximal residual electron density = 0.226 e Å–3. The structure solution of the complex was solved using direct methods and refined (based on F2 using all independent data) by full matrix least squares methods (SHELXTL 96 V. 5.02). It contains disordered anion and acetonitrile species. Hydrogen atoms were included at calculated positions by using a riding model. CCDC 165256. See http://www.rsc.org/suppdata/cc/ for crystallographic files in .cif format.
Supplementary material
Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, as supplementary material No. SUP 165256 and can be obtained by contacting the CCDC.
Acknowledgements
We thank Dr M. Mayor for help in the electrochemical measurements.



