1 Introduction
Numerous compounds containing the 1,2,4-triazine moieties are well known in natural materials and show biological, pharmacological and medicinal properties. An interesting characteristic of the derivatives of 1,2,4-triazine compounds is that they form colored complexes when they are coordinated to a metal ion. In particularly, the ligand 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine, noted dppt, has been widely used as an sensitive reagent for the determination of Fe(II) by spectrophotometric methods, in natural and waste waters [1]. Its role as transfer agent for transition metal ions has been showed by electrochemistry studies [2]. Some Cu(II) complexes have been isolated and studied as potential catalytically active redox agents [3]. More recently, it has been revealed that a zinc-dppt complex associated to a biocide could have significant biocidal effects on living cells including those of microorganisms (bacteria and fungi), cell culture system, plants and animals [4]. Because of our interest in the field of the metal ion and drug interactions, we have focused our work on the synthesis and properties of Zn-dppt complexes. This paper reports the preliminary work on the preparation and the characterization by X-ray diffraction, 2D-1H NMR, infra-red and UV–vis spectroscopies of two Zn(II) complexes Zn(dppt)Cl2·0.5 H2O (1) and Zn(dppt)2Cl2·2 H2O (2).
2 Experimental section
Reagents and solvents were obtained commercially and used without further purification. 5,6-Diphenyl-3-(2-pyridyl)-1,2,4-triazine was commercially available from Lancaster chemicals.
2.1 Physical measurements
NMR spectra were obtained on a Bruker Spectrospin 200 MHz. Infrared spectra were recorded as KBr pressed pellets on a Genesis Series FTIR Ati Mattson. UV–vis spectra were obtained from an HP8453 spectrophotometer. Measurements were made in 1 cm path length cells at 293 K. DCI and FAB mass spectra were obtained with a Nermag R10-10 spectrometer. CHN elemental analyses were performed in duplicate by Pierre Fabre Laboratories, Castres, France.
2.2 Synthesis of Zn(dppt)Cl2·0.5 H2O (1)
Dppt (209.6 mg; 0.674 mmol) and ZnCl2 (93.6 mg; 0.687 mmol) were added together in a mixture of 4 ml of CH2Cl2 and 2 ml of THF. The reaction solution was stirred at room temperature for 30 min. A white solid was isolated by filtration, washed with EtOH and dried with Et2O. C20H14N4Cl2Zn·0.5 H2O, yield 180 mg, 60% based on zinc chloride. Anal. Calc. for C20H14N4Cl2Zn·0.5H2O: C, 52.72; H, 3.10; N, 12.30. Found: C, 53.06, H, 3.34, N, 12.06%; UV–vis (EtOH) [λmax (nm) (ε (M–1 cm–1))]: 240 (14,162), 291 (19,428), 320 (sh); FT-IR (KBr) (cm–1): 538 (m), 600 (m), 696–884 (s, δC–H), 1013–1144 (s, Ar ring vibrations), 1375 (s), 1407 (s), 1447 (m), 1485 (m), 1518 (s), 1575 (m, νN=N), 1605 (s, νC=N), 3068 (m, μC–H); 1H NMR (acetone-d6, δ ppm) 9.13 (br, H6), 9.09 (br, H3), 8.64 (t, H4), 8.23 (t, H5), 7.91–7.57 (m, 10H, Ar–H), mass spectrum (DCI/NH3): 409 (M-Cl), 311 (dppt). M.p. > 300 °C.
2.3 Synthesis of Zn(dppt)2Cl2·2 H2O (2)
Dppt (209.5 mg; 0.676 mmol) and ZnCl2 (30.7 mg, 0.225 mmol) were stirred together at room temperature for 1 h in a mixture of 4 ml of CH2Cl2 and 2 ml of THF. The yellow precipitate was filtered, washed and dried with Et2O. C40H28N8Cl2Zn·2 H2O, yield 116 mg, 70% based on zinc chloride. Anal. Calc. for C40H28N8Cl2Zn·2 H2O: C, 60.65; H, 4.04; N, 14.13. Found: C, 60.69, H, 3.73, N, 14.10%; UV–vis (EtOH) [λmax (nm) (ε (M–1 cm–1))]: 240 (29,605), 292 (40,356), 324 (sh); FT-IR (KBr) (cm–1): 534 (m), 598 (m), 697–879 (s, δC–H), 1009–1113 (s, Ar ring vibrations), 1258 (w), 1372 (s), 1401 (s), 1445 (s), 1508 (s), 1600 (m, νN=N), 1651 (m, νC=N), 3060 (m, μC–H); 1H NMR (acetone-d6, δ ppm, (J Hz)) 9.06 (qd, 2H, H6, 3J6–5 = 4.90, 4J6–4 = 1.70, 5J6–3 = 1.00), 8.88 (dt, 2H, H3, 3J3–4 = 7.80, 4J3–5 = 1.05, 5J3–6 = 1.00), 8.37 (td, 2H, H4, 3J4–5 = 7.80, 3J4–3 = 7.80, 4J4–6 = 1.70), 7.93 (qd, 2H, H5, 3J5–4 = 7.80, 3J5–6 = 4.90, 4J5–3 = 1.05), 7.83–7.49 (m, 20H, Ar–H), mass spectrum (FAB/NBA): 721 (M-Cl), 409 (M-dppt-Cl), 311 (dppt). M.p. > 300 °C.
2.4 Synthesis of Zn(dppt)2Cl2·2 H2O (2) from Zn(dppt)Cl2·0.5 H2O (1)
Dppt (112.5 mg; 0.363 mmol) was solubilized in 2 ml of CH2Cl2 and 1 ml of THF. To this yellow solution, (1) (54.4 mg; 0.121 mmol) was added as solid. A yellow solid started to precipitate immediately. The reaction solution was stirred at room temperature for 2 h and then filtered. The precipitate was washed with Et2O. C40H28N8Cl2Zn·2 H2O, yield 77 mg, 85% based on zinc chloride. Anal. Calc. for C40H28N8Cl2Zn·2H2O: C, 60.65; H, 4.04; N, 14.13. Found: C, 60.87, H, 3.89, N, 14.19%; UV–vis (EtOH) [λmax (nm) (ε (M–1 cm–1))]: 239 (31,134), 290 (41,680), 320 (sh); FT-IR (KBr) (cm–1): 534 (m), 598 (m), 697–879 (s, δC–H), 1009–1113 (s, Ar ring vibrations), 1258 (w), 1372 (s), 1401 (s), 1445 (s), 1508 (s), 1600 (m, νN=N), 1651 (m, νC=N), 3060 (m, μC–H); 1H NMR (acetone-d6, δ ppm, (J Hz)) 9.05 (qd, 2H, H6, 3J6–5 = 4.90, 4J6–4 = 1.70, 5J6–3 = 1.00), 8.86 (dt, 2H, H3, 3J3–4 = 7.80, 4J3–5 = 1.05, 5J3–6 = 1.00), 8.33 (td, 2H, H4, 3J4–5 = 7.80, 3J4–3 = 7.80, 4J4–6 = 1.70), 7.90 (qd, 2H, H5, 3J5–4 = 7.80, 3J5–6 = 4.90, 4J5–3 = 1.05), 7.82–7.44 (m, 20H, Ar–H), mass spectrum (FAB/NBA): 719 (M-Cl), 409 (M-dppt-Cl), 311 (dppt). M.p. > 300 °C.
2.5 X-ray structure determination
A colorless crystal, 0.66 × 0.20 × 0.14 mm3, was glued to a glass fibre. Intensity data were collected at room temperature with a Siemens SMART diffractometer equipped with a CCD two-dimensional detector [λ Mo Kα = 0.71073 Å]. Slightly more than one hemisphere of data was collected in 1271 frames with ω scans (width of 0.30° and exposure time of 10 s per frame). Data reduction was performed with SAINT software. Data were corrected for Lorentz and polarization effects, and an semi-empirical absorption correction based on symmetry equivalent reflections was applied by using the SADABS program [5]. Lattice parameters were obtained from least-squares analysis of all reflections.
The structure was solved by direct method and refined by full matrix least-squares, based on F2, using the SHELX-TL software package [6]. All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms were located with geometrical restraints in the riding mode.
Crystal structure analysis: monoclinic, space group P2(1)/c; dimensions a = 13.4000(2) Å, b = 10.4538(1) Å, c = 16.9513(2) Å, β = 90.359(1)°. V = 2374.51(5) Å3; Z = 4; total reflections collected: 16,183; independent reflections: 6161 (3701 Fo > 4σ(Fo)); a hemisphere of data was collected up to a 2θmax value of 59.62° (91% coverage). Number of variables: 283; R1 = 0.0456, wR2 = 0.0954, S = 1.001; highest residual electron density 0.357 e Å–3.
3 Results and discussion
3.1 Structure description
Complex (1) crystallizes in the monoclinic space group P2(1)/c with one molecule of Zn(dppt)Cl2 and one molecule of solvent (acetone) per asymmetric unit. Details of the data collection and refinement are provided in Table 1. Selected bond distances and bond angles are given in Table 2. An ORTEP [7] view of Zn(dppt)Cl2·(CH3)2CO where the acetone molecule has been omitted for clarity is presented Fig. 1. In Zn(dppt)Cl2·(CH3)2CO, Zn(II) is four coordinated. The Zinc atom is coordinated to the N atom of the pyridyl ring and to a N atom of the triazine ring and to two chlorine atoms. The coordination geometry is tetrahedrally distorted. The bond distances Zn–N(1) (2.073(2) Å) and Zn–N(12) (2.069(2) Å) are comparable with those observed in Zn-bipyridyl complexes exhibiting a distorted tetrahedral geometry, ranging from 2.064(3) to 2.113(2) Å [8]. The bond distances Zn–Cl(1) and Zn–Cl(2) are respectively, 2.2021(9) and 2.1816(9) Å. They are in accordance with those of related complexes having a NNCl2 coordination sphere around the metal center (NN being a bidentate ligand) and exhibiting values in the range 2.148(4) Å–2.331(8) Å [9]. The bite angles around the metal atom range from 78.83(8) to 122.80(4)°. The distortion from ideal tetrahedral geometry and from the ideal value of 109°28′ is a consequence of the opening of the two chlorine atoms due to the steric repulsion (Cl(1)–Zn–Cl(2) = 122.80(4)°) and of the small N(1)–Zn–N(12) angle (78.73(8)°). This last value is commonly observed in some Zn(II) complexes where the metal is coordinated to bidentate NN ligands in a distorted tetrahedral geometry [10,11]. The average Cl–Zn–N angle of 111.72° is also larger than the ideal value of 109°28′. The two phenyl rings at C9 and C10 of the triazine ring are not coplanar with the triazine plane as it is evidenced from the dihedral angles N(8)–C(9)–C(13)–C(14) and N(11)–C(10)–C(19)–C(24) which are –38.3° and –36.7° respectively. A similar distortion has been observed in 5,6-bis(4-methoxyphenyl)-3-(2′-hydroxyphenyl)-1,2,4-triazine (+41.0°) [12] and in some Ni(II) (–38.3°) [12] and Cu(II) complexes (+35.19°) of triazine derivatives [13]. In contrast, the pyridyl and the triazine rings appear almost coplanar as it is shown by the dihedral angle N(8)–C(7)–C(6)–C(5) equal to –2.7°. The coplanarity between the triazine ring and the phenol ring in 5,6-bis(4-methoxyphenyl)-3-(2′-hydroxyphenyl)-1,2,4-triazine has also been observed with a dihedral angle of +12.1° [12]. In Zn(dppt)Cl2·(CH3)2CO, the coplanarity between the two rings to whom the coordinating N atoms belong can be explained by the coordination of the Zinc atom while in 5,6-bis(4-methoxyphenyl)-3-(2′-hydroxyphenyl)-1,2,4-triazine it was explained by an intramolecular hydrogen bond between the O atom of the phenol ring and a N atom of the triazine ring [12].
Crystallographic data for Zn(dppt)Cl2·(CH3)2CO
| Formula | C23H20Cl2N4OZn |
| Fw | 504.70 |
| Space group | P2(1)/c |
| a (Å) | 13.4000(2) |
| b (Å) | 10.4538(1) |
| c (Å) | 16.9513(2) |
| α (°) | 90 |
| β (°) | 90.359(1) |
| γ (°) | 90 |
| V (Å3) | 2374.51(5) |
| T (°C) | 273(2) |
| dcalc (g cm–3) | 1.412 |
| Z | 4 |
| μ (Mo Kα) (cm–1) | 12.81 |
| θ range (°) | 1.52–29.81 |
| Total observations | 16,183 |
| Independent reflections | 6161 |
| R1(F) | 0.0456 |
| Rw(F2) | 0.0954 |
Selected bond distances (Å) and bond angles (°) for Zn(dppt)Cl2·(CH3)2CO
| Zn–N(12) | 2.069(2) | N(12)–Zn–N(1) | 78.73(8) |
| Zn–N(1) | 2.073(2) | Cl(1)–Zn–Cl(2) | 122.80(4) |
| Zn–Cl(1) | 2.2021(9) | N(12)–Zn–Cl(2) | 111.62(7) |
| Zn–Cl(2) | 2.1816(9) | N(1)–Zn–Cl(2) | 114.81(7) |
| N(12)–Zn–Cl(1) | 111.87(7) | ||
| N(1)–Zn–Cl(1) | 108.60(7) | ||
| N(8)–C(9)–C(13)–C(14) | –38.3 | ||
| N(11)–C(10)–C(19)–C(24) | –36.7 | ||
| N(8)–C(7)–C(6)–C(5) | –2.7 |
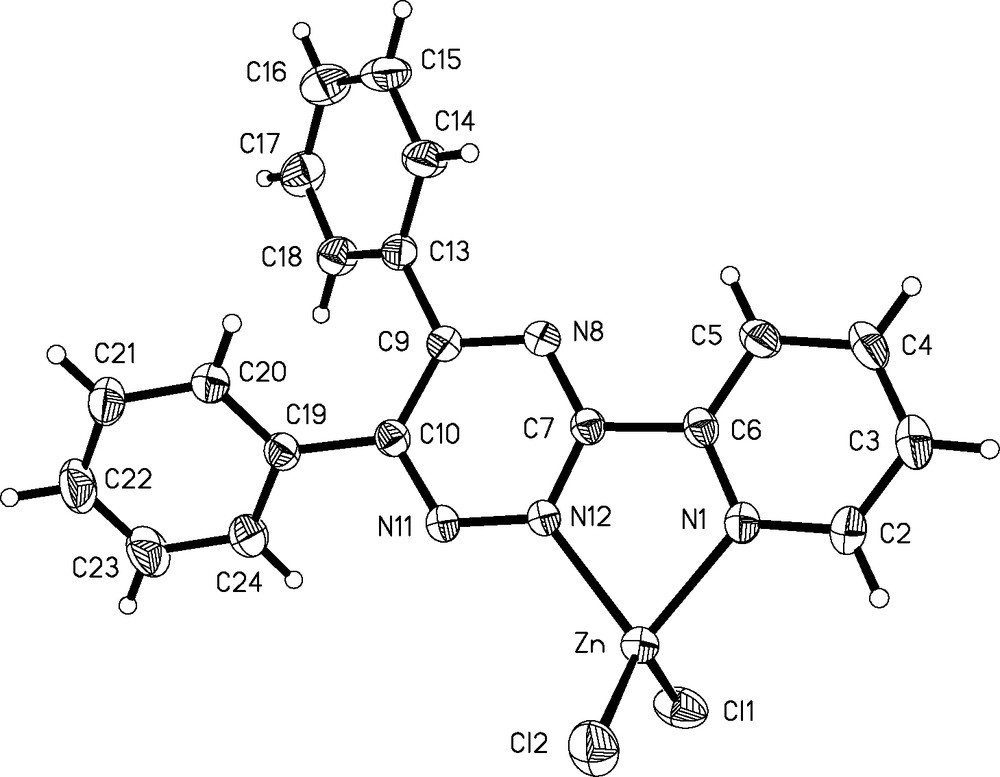
ORTEP view of Zn(dppt)Cl2·(CH3)2CO. The acetone molecule has been omitted for clarity.
3.2 Synthesis and characterization
The two zinc complexes Zn(dppt)Cl2·0.5 H2O (1) and Zn(dppt)2Cl2·2 H2O (2) were prepared in a one-step reaction, mixing together 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine and zinc chloride. (1) and (2) are insoluble in the reaction media and precipitate giving yields between 60% and 70%. The isolated 85% yield of (2) from (1) as precursor is larger than that of the conventional reaction of dppt with ZnCl2. (1) and (2) are respectively a white and a yellow solid which are scarcely soluble in common organic chlorinated solvents, CH2Cl2, CHCl3, and soluble in ethanol and acetone. (1) and (2) are air and moisture stable.
IR spectrum of 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine exhibits one stretching frequency for the C=N group and one stretching frequency for the N=N group: 1672 and 1614 cm–1 respectively [14]. In the IR spectra of the complexes (1) and (2), these stretching vibrations appear at lower values with respect to the ν(C=N) and the of the ν(N=N) of the free ligand, 1605 and 1575 cm–1 for (1) and 1651 and 1600 cm–1 for (2). These observations support the coordination of one of the N atoms of the triazine ring and the N atom of the pyridyl ring to the metal ion.
The 1H chemical shift are displayed in Table 3. The 2D COSY 1H NMR spectrum of (2) is shown in Fig. 2. In the spectra of (1) and (2), the protons of the pyridyl rings appear as a set of four associate resonances well separated from the protons of the two phenyl rings. In (1) and (2), the coordination of dppt to Zn2+ produces a broadening and a displacement of the resonances down field with respect to the uncoordinated dppt ligand, as it is clearly shown in Table 3. The protons of the two phenyl rings appear in the remaining part of the spectra as two sets of multiplets, without being too much affected upon complexation. In (2), the two coordinated dppt ligands are magnetically equivalent and are symmetrically coordinated to the Zn(II) center. The 1H–1H connectivities within the 2-pyridyl-1,2,4-triazine moieties were established by evaluating the COSY spectra, by measuring the different coupling constants in the 1D spectra and according literature data for the free dppt ligand [15], for Fe(II) [16], Ru(II) [17], and Re(V) [18] complexes. For illustration, complex (2) is discussed to explain the assignments. Lines are too broad in the spectrum of (1) to allow the measurement of any coupling constant. The H5 proton (δ = 7.93 ppm) exhibits three correlations with H3, H4 and H6 that are all related by further cross peaks with H4 and H6, H3 and H6, and H3 and H4 respectively. H5 is first coupled with H4: 3J5–4 = 7.80 Hz. The second and third coupling constants are observed with H6: 3J5–6 = 4.90 Hz and with H3: 4J5–3 = 1.05 Hz, leading to a quadruplet of doublet signal shape. H6 (δ = 9.06 ppm) is coupled with H5: 3J6–5 = 4.90 Hz, with H4: 4J6–4 = 1.70 Hz and with H3: 5J6–3 = 1.00 Hz explaining the quadruplet of doublet shape of the H6 signal. It then appears that the two coupling constants 4J5–3 and 5J6–3 (1.10 and 1.05 Hz respectively) are very closed and that the two coupling constants 3J5–4 = 7.80 Hz and 3J3–4 = 7.80 Hz are equal. That could explain the multiplicity (doublet of triplet) of the signal observed for H3 (δ = 8.88 ppm), that is first correlated to H4 with a coupling constant 3J3-4 = 7.80 Hz, and then with H5 and H6 with the almost same coupling constant. The remaining H4 (δ = 8.37 ppm) proton is first correlated via a triplet to H5 and H3 with 3J4–5 = 7.80 Hz and 3J4–3 = 7.80 Hz. The triplet is then split in a triplet of doublet due to the connectivity with H6: 4J3–6 = 1.70 Hz.
1H NMR data for the Zn(II) complexes (1) and (2) and for the free dppt ligand. Chemical shifts values in ppm, measured in acetone-d6, and coupling constants values in Hz
| Compound | H6 | H5 | H4 | H3 | Phenyl rings |
| Dppt | 8.88 (d) | 7.94 (t) | 8.09 (t) | 8.67 (d) | 7.77–7.50 (m) |
| (1) | 9.11 (br) | 8.23 (t) | 8.64 (t) | 9.09 (br) | 7.91–7.57 (m) |
| (2) | 9.06 (qd) | 7.93 (qd) | 8.37 (td) | 8.88 (dt) | 7.83–7.49 (m) |
| 3J6–5 = 4.90 | 3J5–4 = 7.80 | 3J4–5 = 7.80 | 3J3–4 = 7.80 | ||
| 4J6–4 = 1.70 | 3J5–6 = 4.90 | 3J4–3 = 7.80 | 4J3–5 = 1.05 | ||
| 5J6–3 = 1.00 | 4J5–3 = 1.05 | 4J4–6 = 1.70 | 5J3–6 = 1.00 |
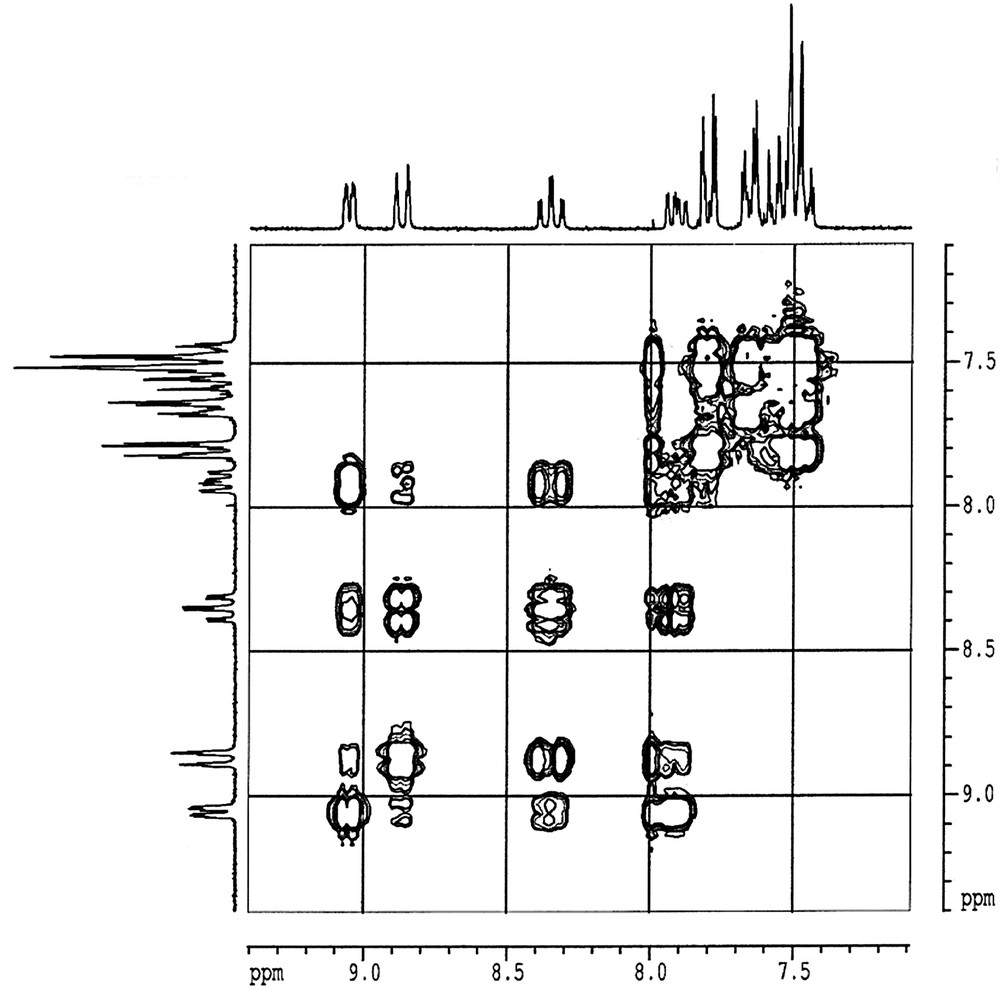
2D COSY 1H NMR spectrum of (2) in acetone-d6.
Chemical ionization mass spectrum of (1) exhibits two fragments, one corresponding to the loss of one chloride ion: [Zn(dppt)Cl]+ at m/z = 409, and the other one being the fragment of the free dppt at m/z = 311. For (2), the FAB mass spectrum is mainly characterized by three fragments. The first one, at m/z = 721, characterizes the loss of one chloride ion leading to the fragment [Zn(dppt)2Cl]+. The loss of one dppt ligand associated with the loss of one chloride ion is also observed with fragments [Zn(dppt)Cl]+ appearing at m/z = 409. The last fragment obtained is the free dppt ligand at m/z = 311. These results are in agreement with the two proposed chemical formula for (1) and (2).
In summary, we have synthesized and fully characterized two Zn(II) complexes of the 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. The different spectral characterizations coupled with the X-ray diffraction for (1) have shown the coordination of one ligand in (1) and the symmetrically coordination of two ligands in (2) through the N atom of the pyridyl ring and one of the N atom of the triazine ring. The lability of the two chlorine atoms and the possibility of coordinating a second ligand to the Zinc atom in Zn(dppt)Cl2 (1) will lead us to study the combination effects potentially produced by a mixture of the isolated Zn(dppt)Cl2 complex reported herein and different drugs on the killing or inhibiting the growth of living cells.
4 Supplementary material
The CIF format file for Zn(dppt)Cl2·(CH3)2CO has been sent to Cambridge Crystallographic Data Center, 12 Union road, Cambridge CB21EZ, UK as supplementary materials No. SUP 238194 and can be obtained by contacting the CCDC (quoting the article details and the corresponding SUP number).


