1 Introduction
The photochemistry and photophysics of semiconductors, in which TiO2 is one of the most efficient and promising materials, have gained sufficient attention from the discoveries of Honda and Fujishima in 1972 [1,2] because of its strong oxidation and reduction abilities. Both extensive fundamental studies and practical applications have been undertaken [3]. One potential application of semiconductors is for light energy conversion devices such as photocatalysts and photosensitizers in wet solar cells. Considerable efforts have been focused on modifying TiO2 to extend the photoresponse to visible light and to enhance the efficiency of energy conversion. The photoresponse to visible light could be improved by organic dyes [4]. On the other hand, the efficiency can be controlled by photoinduced hole and electron recombination [5]. This control is similar to that of electron transfer reactions [5], where magnetic fields can affect the recombination process of cation and anion radicals [6]. Similarly the photocatalytic reactions with TiO2 should be expected to be influenced by magnetic fields. There are, however, few reports for such magnetic field effects on photocatalytic reaction. In 1983, Kiwa reported the magnetic field effects on photosensitized electron transfer reactions in the presence of TiO2 and CdS loaded particles [7]. In the report, a suspension of TiO2 and CdS particles was used and H2 evolution was found to decrease in the presence of magnetic field strengths below 0.4 T. The effect was not observed for direct photolysis with TiO2 and CdS, but observed only in the presence of Ru(bpy)32+. Recently, we studied magnetic field effect on the photocatalytic reactions with platinized TiO2 particles. In this communication, we report the yield of acetone, generated from the photocatalytic decomposition of tert-butanol with platinized TiO2 particles, decreased with increasing magnetic field strength from 0 to 1.5 T.
2 Experimental
The platinized TiO2 particles were prepared by photodeposition of platinum on TiO2 according to a method similar to that of Kraeutler and Bard [8]. As a typical example, anatase powder (1 g, Kanto Chemical Co.) and H2PtCl6 6H2O (10 wt.%) were placed in reaction vessel and 50 ml of an H2O/EtOH (1:1) mixture was added. Ar was bubbled through the suspension for 30 min to eliminate dissolved air. To commence the photocatalytic deposition, the suspension was irradiated with a 500 W deep UV lamp for 30 min under continued stirring. After irradiation, the suspension was filtered by membrane filter (> 0.45 μm) and washed fifth times with deionized water. The precipitate was dried in an electric oven at 200 °C for 24 h. To control the size of particles, the precipitate was sieved through a strainer (mesh 100 μm). The molar ratio of platinum to titanium (Pt/Ti) was measured with a JEOL JSX3200 energy dispersive X-ray fluorescence spectrometer and obtained to be 0.04.
The platinized TiO2 particles (typically 50 mg) and tert-butanol (neat, 3 ml) were placed in a quartz cell (10 mm) with a PTFE-rubber septum. The slurry was continuously agitated by Ar bubbling. Each solution was irradiated for 3 h with a 500 W deep UV lamp at room temperature. The lamp intensity was continually measured by a power meter and its fluctuation was within 1%. The reaction products were analyzed by a gas chromatograph (GC) and a gas chromatograph mass spectrometer (GC–MS). Quantitative analysis was carried out by GC as follows: A Shimadzu GC-14B GC coupled with a Shimadzu AOC-20i auto-injector was used with a Carbowax-PEG capillary column (15 m, 0.25 mm ID, 0.25 μm df). The chromatograph was recorded using a Shimadzu chromatopac C-R6A integrator. The yield was determined using decane as an internal standard.
Magnetic fields (B) of up to 1.5 T were provided by a Tokin SEE-10W electromagnet. The lowest magnetic field, generated by applying a counter-current to cancel the residual field, was less than 0.05 mT. Hereafter, the experiments under the lowest field are denoted as those in the absence of a magnetic field.
3 Results and discussion
3.1 Photocatalytic reaction
In order to measure magnetic field effects on the photocatalytic reactions with platinized TiO2 particles, we selected the decomposition reaction of alcohol with photocatalysis, as it is a simple and well-studied reaction mechanism [9]. In the present study, we carried out the photocatalytic decomposition of neat tert-butanol. In the absence of a magnetic field, the suspension of platinized TiO2 particles and tert-butanol was irradiated. Acetone and methane were generated as the main products.
From the qualitative analysis, the following reactions are considered to occur. The electron (e–) and hole (h+) were generated by irradiation of TiO2 (Eq. (1)). Oxidation of tert-butanol occurs via the photo-generated hole to form its cation radical (Eq. (2)). The cation radical decomposes to a 2-propanol cation and a methyl radical (Eq. (3)). Another possibility is the cation radical decomposes to a 2-propanol radical and a methyl cation. As the 2-propanol cation mass fragment is much larger than the methyl cation, from the GC–MS analysis of tert-butanol, we were able to conclude the decomposition of a 2-propanol cation radical to a 2-propanol radical and a methyl cation is less dominant than reaction 3. Subsequently the 2-propanol cation is reduced by an electron to form a 2-propanol radical (Eq. (4)). Finally, a disproportionation reaction occurs between the generated 2-propanol and methyl radicals to form acetone and methane (Eq. (2)).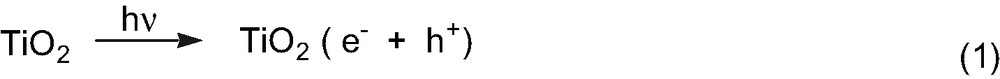



3.2 Magnetic field effect
In the absence and presence of an external magnetic field of 1.5 T, the photocatalytic decomposition reaction of tert-butanol with the platinized TiO2 particles was performed. The observed yields of acetone (Y) are listed in Table 1. The relative magnetic field effect observed at 1.5 T, R(1.5 T), is represented as follows:
Yields of acetone and the relative magnetic field effect (R(B)) observed for the photocatalytic decomposition reaction of tert-butanol by platinized TiO2 particles in the absence and presence of an external magnetic field (1.5 T)
| Run | Y(B)/mmol a | ||
| B = 0 T b | B = 1.5 T | ||
| 1 | 0.0553 | 0.0534 | 0.966 |
| 2 | 0.0571 | 0.0551 | 0.965 |
| 3 | 0.0578 | 0.0569 | 0.984 |
| 4 | 0.0579 | 0.0562 | 0.971 |
| 5 | 0.0571 | 0.0550 | 0.963 |
a Average values for the yields were obtained from five or 10 separate chromatograms for each reaction solution Experimental errors were within ± 0.001.
b The lowest magnetic field generated by a countercurrent for canceling the residual field was less than 0.05 mT. The experiments under the lowest magnetic field are denoted as those in the absence of a magnetic field.
The R(1.5 T) values obtained are also listed in Table 1. This Table shows the yield of acetone decreases by about 3–4% in the presence of a magnetic field of 1.5 T. Similar magnetic field effects have been reported by Kamochi et al. [10] for photocatalytic decomposition reactions of methanol–water solution using platinized TiO2 particles. The yield of photo-generated gas (H2 and CO2) decreased gradually with increasing magnetic field strength from 0 to 4 T.
In order to clarify the mechanism of the magnetic field effect, we measured the magnetic field dependence of the relative magnetic field effect R(B) for fields between 0 and 1.5 T. The observed R(B) values are plotted against B in Fig. 1. It is clear the R(B) value gradually decreases with increasing B from 0 to 1.5 T. From these results, the observed magnetic field effects may occur during either the reencounter of free radicals [6] or the recombination of electrons and holes.
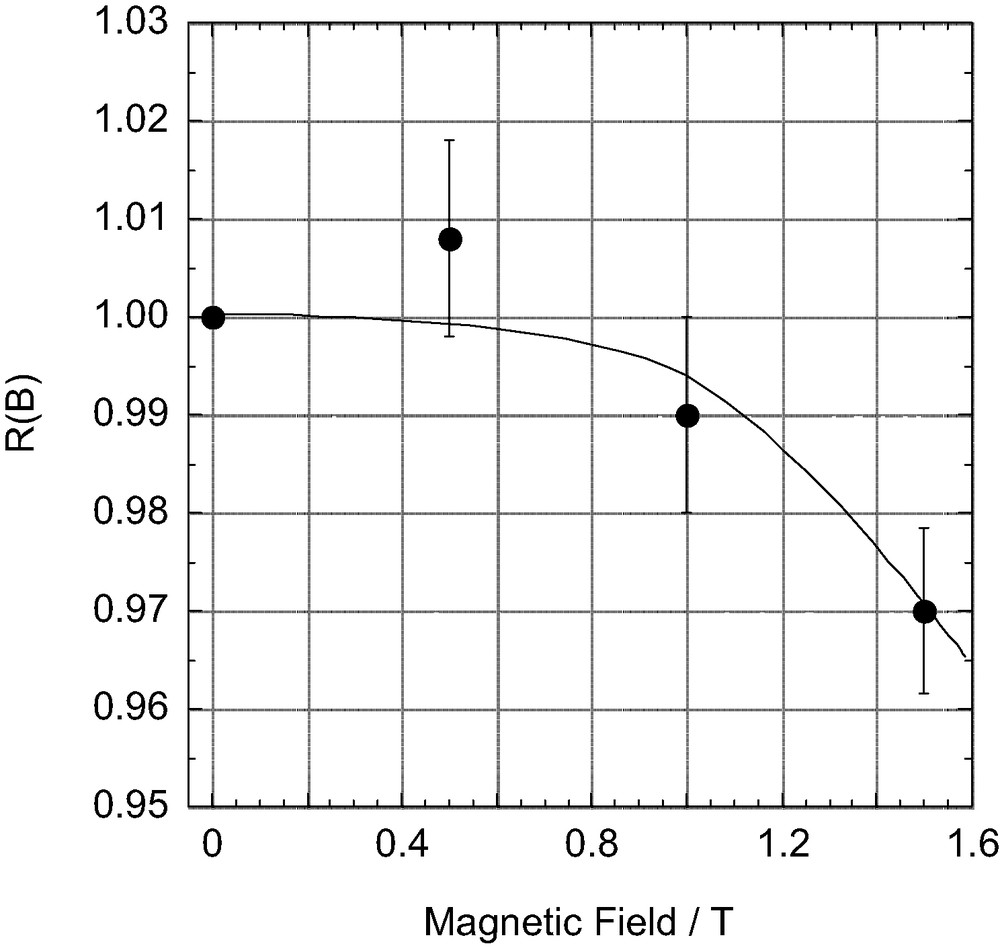
Magnetic field dependence of the relative magnetic field effect (R(B) = Y(B)/Y(0 T)) observed at room temperature for the photocatalytic decomposition reaction of tert-butanol by platinized TiO2 particles in the absence and presence of external magnetic fields of varying magnitude.
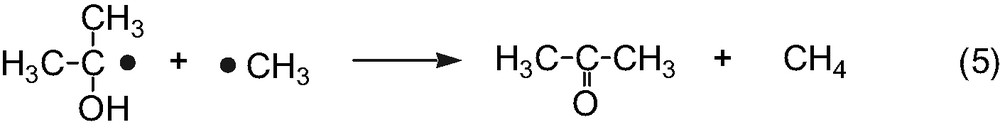
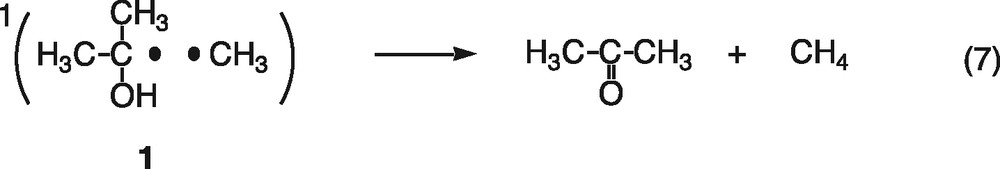
First, consider magnetic field effects on the reencounter of free radicals. The triplet and singlet radical pairs (1, 2) are generally formed in the ratio of three to one, when the free 2-propanol and methyl radicals reencounter (Eq. (6)). The singlet radical pair (1) immediately reacts to form the disproportionation products of acetone and methane, cage products (Eq. (7)). On the other hand, since the triplet radical pair (2) cannot react directly, the radicals either escape from the triplet pair (Eq. (8)) or undergo spin-state mixing to produce a singlet pair (1) (Eq. (9)). The spin-state mixing can be affected by the magnetic field. In the case of the hyperfine coupling and the relaxation mechanisms (HFCM and RM) of the triplet radical pair, the yield of cage products is expected to decrease with increasing magnetic field [6]. Moreover, magnetic field effects caused by the HFCM are usually saturated below 50 mT. As shown in Fig. 1, however, the yield of acetone decreases with increasing magnetic field and no saturation is observed below 1.5 T. These results indicate that the magnetic field effects are not due to the HFCM but due to the RM, if the present magnetic field effects occur during the reencounter of free radicals.
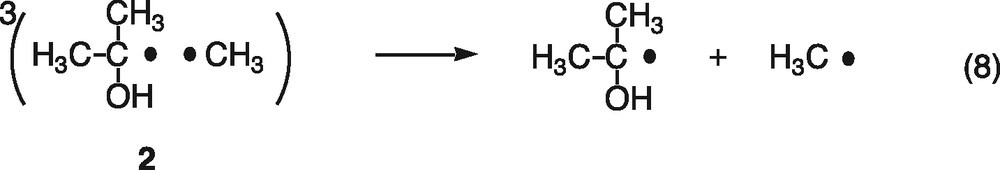
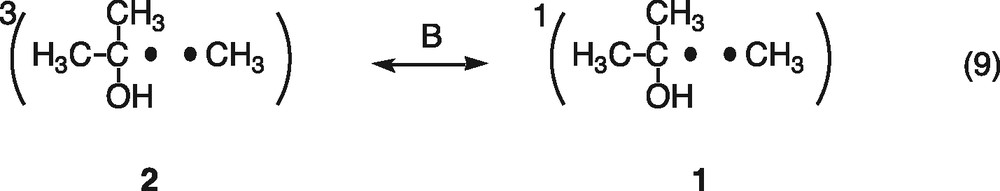
Another possible explanation is acceleration of recombination between electrons and holes by external magnetic fields. The geminated singlet pairs of electrons and holes, generated by irradiation of the platinized TiO2 particles, recombine with one another (Eq. (10)) but can also partially react with tert-butanol (Eq. (2)) and isopropanol cations (Eq. (4)). The singlet pairs of electrons and holes undergo spin-state mixing to produce triplet pairs (Eq. (11)). The triplet pairs of electrons and holes cannot recombine and they react with tert-butanol and isopropanol cations on the surface of the semiconductor (Eqs. (2) and (4)). The spin-state mixing can be blocked by magnetic fields through the HFCM or RM. This means that the singlet–triplet spin conversion is blocked and the recombination of singlet pairs of electrons and holes is accelerated by the magnetic field.
| (10) |
| (11) |
In order to elucidate the mechanism of the observed magnetic field effects, we carried out photocatalytic decomposition reaction using ultra fine colloidal TiO2 particles (< 50 nm). Upon irradiation of ultra fine TiO2 particles in neat tert-butanol, acetone and methane were similarly generated, but the yield of acetone was found to increase with increasing magnetic field strength from 0 to 1.5 T [11]. As the magnetic field effects observed with platinized TiO2 particles are in the opposite sense to those observed with the ultra fine colloidal TiO2 particles, we can conclude that the magnetic field effects observed in both cases are not caused by the reencounter of free radicals generated by the photocatalytic decomposition reaction of tert-butanol, as this should be the same for both particle types.
4 Conclusion
In the present study, we found a magnetic field effect on the photocatalytic reaction of tert-butanol with platinized TiO2 particles. The yield of acetone decreased with increasing magnetic field strength from 0 to 1.5 T. At this stage, the effect may be explained by a magnetically induced acceleration of the recombination of electrons and holes.
Acknowledgments
M.W. thanks partial support from Yamada Science Foundation, Research Foundation for Opto-Science and Technology, and Shimadzu Science Foundation.


