1 Introduction
Nitrogen protection continues to attach a great deal of attention in a wide range of chemical fields, such as peptides, nucleoside, polymer and catalyst ligand synthesis [1]. Among the different means of the protection of amino group, N-tert-butoxycarbonylation has received the greatest attention due to extreme stability of the N-Boc group toward nucleophilic attack or alkali conditions and catalytic hydrogenation [2]. Various reagents and methodologies have been developed during the last years for the N-tert-butoxycarbonylation of amines. Most of them are carried out in the presence of an organic or inorganic base, for example: (Boc)2O in the presence of DMAP [3], 4-dimethylamino-1-tert-butoxycarbonyl pyridinium chloride/ tetrafluoroborate in aq. NaOH [4], 2-tert-butyloxy carbonyl oxyimine-2-phenylacetonitrile in the presence of Et3N in H2O-dioxane [5], tert-butyl-2-pyridyl carbonate in the presence of Et3N in H2O- DMF [6] or tert-butyl 1- chloroalkyl carbonates in the presence of K2CO3 in H2O-THF [7]. However, these methodologies have various drawbacks such as long reaction times, preparation of the tert-butoxycarbonylation reagents, and requirement of auxiliary substances (e.g., solvents and other reagents). Furthermore, the base-catalyzed reactions are often associated with the formation of isocyanate [8], urea [3] and N, N-di-Boc derivatives [9]. Moreover, the high toxicity of DMAP and reagents derived from it, limits their use [10]. These disadvantages can be avoided by electrophilic activation of (Boc)2O in the presence of acids. There are examples of other modified methods for N-tert-butoxycarbonylation of amines with H3PW12O40 [11], H2NSO3H [12], Zn(ClO4)2.6H2O [13], ZrCl4 [14], LiClO4 [15], Cu(BF4)2 [16], Montmorillonite K10 [17], sulfonic acid functionalized silica [18], I2 [19], indium (III)halides [20], HClO4-SiO2 [21] and TFE [22]. These procedures, although circumventing the problem associated with the formation of the above-mentioned side products, have certain drawbacks: the reactions require highly reactive or environmentally harmful catalysts and anhydrous conditions (the use of H2SO4 at 500 °C to prepare Yttria-Zirconia, ZrCl4 is highly moisture-sensitive and liberates HCl), use of solvents and extractive work-up to isolate the product from the catalyst. Moreover, most Lewis acids are decomposed or deactivated by the amines and amine derivatives. Even when the desired reactions proceed, greater than stoichiomethric amounts of Lewis acid are needed because the acids are trapped by nitrogen. Thus, the design of new, milder and effective methods for N-Boc protection still is an active topic in synthetic chemistry.
Currently, ionic liquids are extensively being used as green solvents for laboratory as well as industrial use due to their unique properties such as good solvating ability, variable polarity, negligible vapor pressure, and ease of recyclables [23]. In spite of advantages, there are few publications utilizing ionic liquids as green solvents for protection reactions. Moreover, the ionic liquids reported so far for protection reactions are mostly limited to salts of 1, 2-disubstituted imidazolium [24]. While considering ionic liquids as catalyst and their use in industrial processes, one major concern is cost. The cost of ionic liquid would be directly dependent on the price of the cations and anions that are used for their production [23]. Thus, the currently popular ionic liquids incorporating cations such as alkyl methyl imidazolium and dialkyl imidazolium as well as anions such as tetrafluoroborate and hexafluorophosphate are also expensive. Very recently, 1-methylimidazolium tertafluoroborate [(HMIm)BF4], has been exploited as an efficient catalyst for chemoselective N-Boc protection of various amines using (Boc)2O. However, ILs bearing perfluorinated anions have some disadvantages: (1) the presence of fluorine makes the disposal of spent ionic liquids (ILs) more complicated; (2) they may contain traces of halides arising from the preparation procedure. For these reasons, research on new ILs bearing inert low coordinating and non-fluorinated anions represents a field of intense investigation in the chemistry of ILs.
2 Results and discussion
Herein we report that (1, 1, 3, 3-tetra-methylguanidine [TMG])[Ac] [26] is an excellent catalyst for the N-tert-butoxycarbonylation of various structurally different amine derivatives (Scheme 1) and the results are summarized in Table 1 [27]. In an initial study, aniline (1 mmol) was treated with (Boc)2O (1 mmol) and [TMG][Ac] (0.018 g, 10 mol %) as a catalyst to isolate the corresponding mono N-Boc derivative within 5 min in 98% yield. The efficiency of our protocol was evaluated using a variety of structurally diverse amines (1a–q). The reactions were completed after 5–30 min, affording good to excellent yields (Table 1). Both primary and secondary amines worked well. In each case, only the mono N-Boc protected product was found. No isocyanide or urea formation was detected (by NMR of crude products). Aromatic amines having various substituents such as OH and NO2 were converted to their N-Boc derivatives efficiently. Previously, it was found to be difficult to prepare N-Boc derivatives from an aromatic amine containing an electron-withdrawing group [27]. The method can be applied to the conversion of sterically hindered tert-butylamine. N-Boc protection catalyzed with [TMG][Ac] is highly chemoselective: the amino group is protected exclusively in the presence of alcoholic (entries 1j–l) or phenolic (entry 1 h). In the case of phenylglycinol 1j, ephedrine 1k and N-benzylethanol 1l no O-Boc-derivatives, oxazolidinone derivatives or symmetrical carbonates were observed. In the presence of [TMG][Ac], acting as a mild catalyst, the selective monoprotection of 1, 2-diamino benzene 1o can be achieved with 1 equiv. of (Boc)2O in reasonably good yield. However, under conventional conditions, the reaction of 1, 2-diamino benzene with (Boc)2O at r. t. afforded the N, N-di-Boc product in 95% yield after 24 h [28]. The use of (Boc)2O in the presence of 0.5 equiv. of DMAP and 1,2-diamino benzene afforded 1,3-di-Boc benzimidazolidinone in 50% yield and 50% of starting amine was recovered [3]. In a further study, hydrazine (1m), amino acid ester (1p) and p-toluensulfonamide (1q) were converted to the corresponding mono N-Boc derivatives under similar reaction conditions in good yields.

N-Boc protection of amines catalyzed by [TMG][Ac].
N-Boc protection of amines with [TMG][Ac].
| Entry | Substrate | Time (min) | Product | Yield 3% |
| a | 5 | 98 | ||
| b | 10 | 95 | ||
| c | 6 | 94 | ||
| d | 10 | 98 | ||
| e | 15 | 94 | ||
| f | 8 | 96 | ||
| g | 8 | 98 | ||
| h | 6 | 98 | ||
| i | 30 | 98 | ||
| j | 10 | 98 | ||
| k | 15 | 94 | ||
| l | 10 | 93 | ||
| m | 15 | 94 | ||
| n | 30 | 97 | ||
| o | 6 | 98 | ||
| p | 30 | 94 | ||
| q | 30 | 94 |
At present, we do not know how the ionic liquid catalyzed this reaction. The key features of the ionic liquid that is to be used as catalyst are those which determine how it will interact with potential solutes. This is commonly recorded as polarity. This term takes into account all the possible microscopic properties responsible for all the interactions between catalyst and solute molecules (e.g. Columbic, directional, inductive, dispersion, hydrogen bonding). However, NMR studies seem to indicate, in protic ammonium ionic liquid such as [TMG][Ac], that the nitrogen proton is not labile and, therefore, cannot be viewed as a conventional Bronsted acid [29]. Based on polarity scale parameters, [TMG][Ac] is protic and is a highly polar ionic liquid and its hydrogen bond donating ability is much higher than the molecular solvent. So, this ionic liquid can catalyze by the electrophilic activation of (Boc)2O to form a zwitterionic species, making the carbonyl group susceptible to nucleophilic attack by the amine. Successive elimination of CO2 and tert-BuOH results in the formation of N-Boc derivatives and regenerates [TMG][Ac] (Scheme 2). The insolubility of catalyst in diethyl ether allows for easy separation of the product by simple filtration; [TMG][Ac] was reused with only a gradual decrease in its activity. For example, the reaction of aniline (1a) and (Boc)2O in the presence of [TMG][Ac] afforded the corresponding N-Boc in 98, 98 and 96% isolated yield over three cycles.
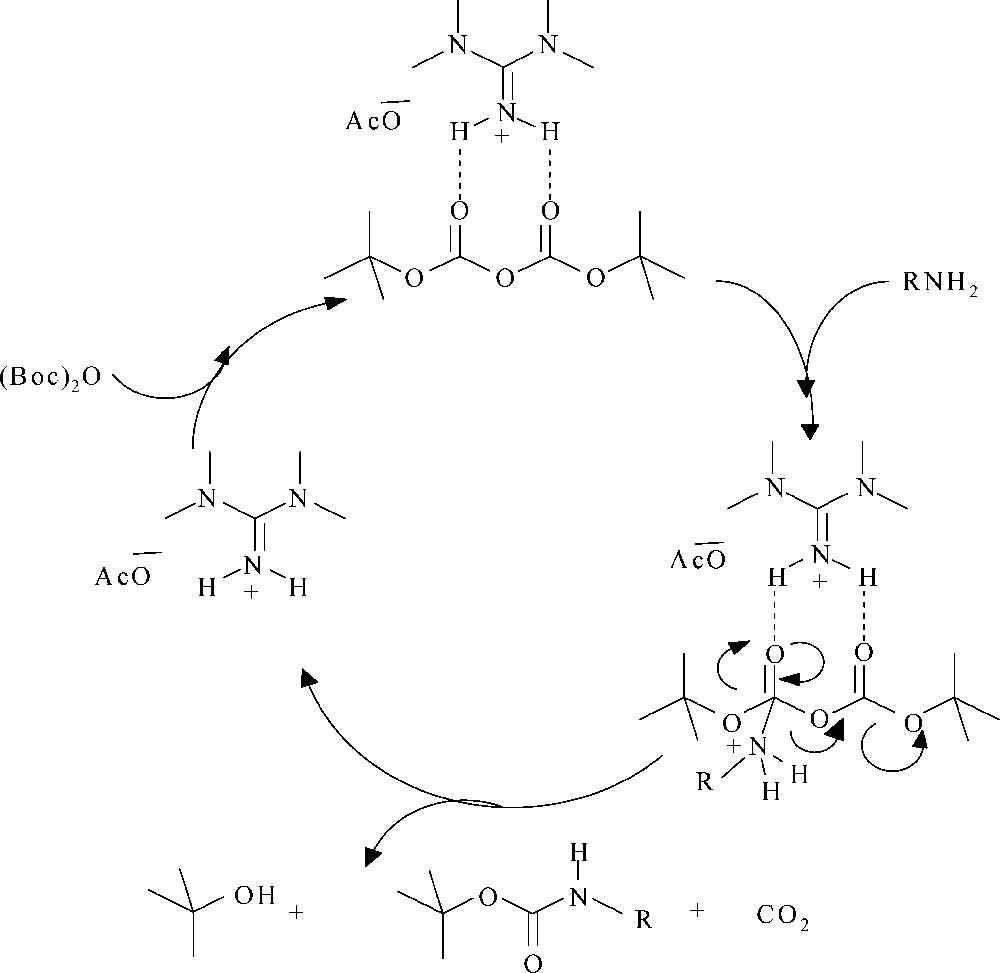
Proposed mechanism for GIL catalyzed N-Boc protection.
Also, the efficiency of our protocol was evaluated using N-Boc protection of some amines in different, tetra-methylguanidinium salts (Guanidine ionic liquid [GILs] 1–4) (Scheme 3). Surprisingly [TMG][Ac] was found to be the best in terms of reaction times and yield (Table 2). The effect of amount of the catalyst was examined and the results are summarized in Table 3. As shown in Table 3, in the case of aniline, 10 mol % of catalyst give N-Boc product with 96% yield in 5 min, while decreasing the catalyst leading up to 5 mol % it gives lower yield of 70%. With 20 mol% catalyst, the yield and the time of reaction did not improve. Accordingly, 10 mol % catalysts are considered optimal for the N-Boc protection reaction. The performance of IL and various previously used catalysts are shown in Table 4.

Structure of GILs.
N-Boc protection using different TMG ionic liquid.
| Entry | Substrate | Product | Ionic liquid | Time (min) | Yield 3% |
| a | GIL 1 (10mol%) | 5 | 96 | ||
| GIL 2 (10mol%) | 5 | 98 | |||
| GIL 3 (10mol%) | 10 | 96 | |||
| GIL 4 (10mol%) | 10 | 96 | |||
| b | GIL 1 (10mol%) | 2 h | 98 | ||
| GIL 2 (10mol%) | 12 h | 65 | |||
| GIL 3 (10mol%) | 12 h | 45 | |||
| GIL 4 (10mol%) | 24 | 55 |
Effect of amount of catalyst on yield.
| Amount of catalyst (mol %) | Yield % |
| 20 | 98 |
| 10 | 98 |
| 5 | 70 |
Compared performance of various catalysts in the N-Boc protection of aniline.
| Entry | Catalyst | Time | Solvent | Yield |
| 1 | Iodine | 30 min | Neat | 95 [28] |
| 2 | Yttria-Zirconia | 14 h | CH3CN | 90 |
| 3 | Zn(ClO4)2.6H2O | 12 h | CH2Cl2 | 92 |
| 4 | HClO4-Silica | 5 min | Neat | 100 |
| 5 | Cyclodextrin | 2.5 h | H2O | 75 [27] |
| 6 | Uncatalyzed | 48 h | – | 60 [26] |
| 7 | Uncatalyzed | 30 min | H2O | 95 [29] |
| 8 | Sulfonic acid functionalized silica | 45 min | CH2Cl2 | 83 |
In conclusion, [TMG][Ac] shows promising results for the monoprotection of various aliphatic, aromatic amines, 1,2-diamine, heterocyclic amine, hydrazide, aminoalcohols, amino acid esters and p-toluensulfonamide as N-Boc derivatives in good to excellent isolated yields. In contrast to the existing methods using potentially hazardous catalysts, additives and solvents, this new method offers the following competitive advantages: mild and operationally simple, lower catalyst loading, environmentally benign catalyst under solvent free conditions, high chemoselectivity, wide substrate scope and no side reactions.
3 Experimental
3.1 Synthesis of TMG based ionic liquid
Protic ionic liquids, especially those based on the TMG are synthesized through simple neutralizing of equimolar TMG with acids [25].
3.2 General procedure for chemoselective N-Boc protection of amines
To a magnetically stirred mixture of (Boc)2O (1 mmol) and [TMG][Ac] (0.018 g, 0.1 mmol), amine (1 mmol) was added and the mixture was stirred at ambient temperature. The reaction was monitored by TLC. After the completion of reaction, diethyl ether was added, the resulting suspension was filtered and solid residue washed with diethyl ether (5 mL). The ionic liquid could be reused for further reaction. The products were isolated after evaporation of the diethyl ether to yield the highly pure N-Boc derivatives. 1H NMR and 13C NMR were consistent with the assigned structures and were compared with those reported in the literature ([19] and reference cited therein). Spectral data for selected products [15]: 2l: 1H-NMR (500 MHz, CDCl3): δ = 1.51 (s, 9H), 2.18 (bs, 1H, OH), 3.44 (m, 2H), 3.74 (m, 2H), 4.50 (s, 2H), 7.27-7.32 (m, 3H), 7.37 (m, 2H); 13C-NMR (125 MHz, CDCl3): δ = 28.8 (CH3), 50.12 (CH2), 52.40 (CH2), 62.2 (CH2), 80.94(C), 128.0 (CH), 128.9 (CH), 138.68 (CH), 147.19 (C) 157.64 (C = O). 2 g: 1H-NMR (500 MHz, CDCl3): δ = 1.5 (s, 9H), 3.37 (t, 4H), 3.52 (t, 4H); 13C-NMR (125 MHz, CDCl3): δ = 28.1 (CH3), 43.7 (C), 66.6 (CH2), 79.9 (CH2), 155 (CO).
Acknowledgments
This research was supported by the National Research Council of the Islamic Republic of Iran as a National Research project under the number 984.


