1 Introduction
Transition-metal catalyzed organic reactions are often considered to follow the principles of “Green Chemistry” due to the use of minimum energy, cleaner reagents or auxiliaries and minimization of waste. Nanocatalysts are considered to be a bridge between heterogeneous and homogeneous catalysis [1]. One of the attractive properties of the nanomaterials is that the active component has a high specific surface area, leading to an increase of the contact with the reactants [2]. Also, a higher surface area gives nanomaterials more active surface; they are hard to be separated. Therefore, it is important to design a recoverable and well-dispersed catalyst. Magnetite nanoparticles (MNPs) as catalysts are very promising due to their large specific surface area and their magnetic properties [3,4]. They can be collected very easily using a magnet for reusing it to prevent any loss of the catalyst. Recently, the chemists have focused on the catalytic aspects of nano-Fe3O4 to improve the protocols of catalytic activity [5–8].
Functional group protection and deprotection methods are important in the synthesis of the target molecule. The protection of amines is one of the most fundamental and useful transformations in organic synthesis, especially in peptide synthesis [9,10]. Several reagents and methods have been used for N-tert-butoxycarbonylation of amines [11–13]. Some of these methodologies suffer from a variety of drawbacks, such as long reaction times, low yields, high toxicity and the use of expensive reagents. Commercially available di-tert-butyl dicarbonate (diboc) is an efficient reagent for the clean and rapid introduction of the boc-protecting group [13–15].
Having the above facts in mind, we have introduced the nano-ferrous ferric oxide (nano-Fe3O4) as a highly efficient, reusable and heterogeneous Lewis acid catalyst for the selective N-tert-butoxycarbonylation of various amines, using diboc, at room temperature (Scheme 1). Interestingly, this method has none of the above-mentioned drawbacks for the N-boc protection of amines at all. The catalyst can be easily collected by a magnet and recovered without significant loss in the catalytic activity.

Selective tert-butoxycarbonylation of various amines using diboc catalyzed by nano-Fe3O4.
2 Results and discussion
At first, in this research project, the catalyst {nano-Fe3O4} was synthesized according to the previous literature [16,17]. The synthesized powder was studied by X-ray diffractometry (XRD), vibrating sample magnetometry (VSM), Fourier transform infrared spectroscopy (FTIR) and transmission electron microscopy (TEM). In order to prove that the magnetite was correctly synthesized, initially, its XRD pattern was recorded. As shown in Fig. 1a, the XRD patterns of magnetite Fe3O4 nanoparticles reveal peaks at 2θ ≈ 30.24°, 35.62°, 43.33°, 53.63°, 57.29° and 62.80°, respectively, which was confirmed by the reported value (JCPDS 01-1111). The average crystallite size D was calculated using the Debye–Scherrer formula: , λ being the X-ray wavelength, K is the Scherrer constant, β is the half-maximum peak width, and θ is the Bragg diffraction angle. The average size of the catalyst, thus, obtained from this equation was found to be about 9 nm, which is basically in accordance with the transmission electron micrographs (Fig. 2).

(a) XRD pattern of the nano-Fe3O4. (b) Vibrating sample magnetometry (VSM) spectrum of the nano-Fe3O4. (c) FTIR spectrum of the nano-Fe3O4.
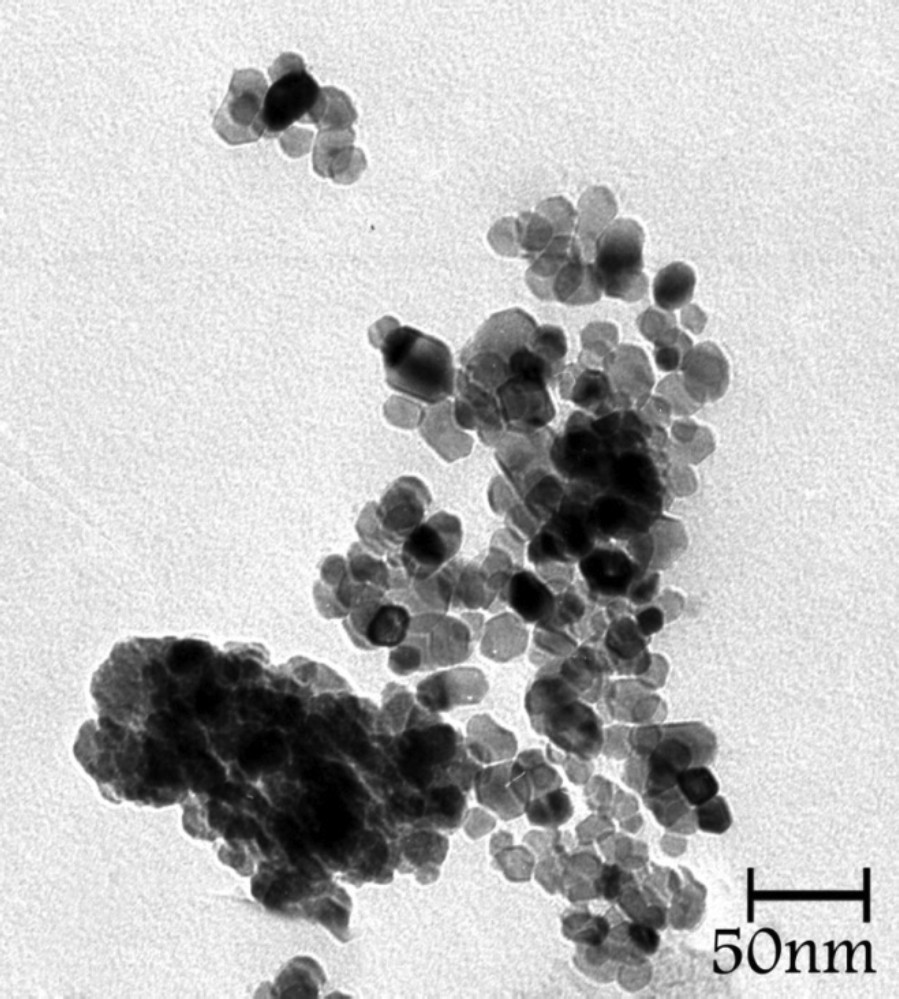
Transmission electron microscopy (TEM) of the nano-Fe3O4.
The magnetic properties of the catalyst were also calculated by VSM at 298 K. The magnetization curves reveal that the magnetization was saturated up to 60 emu/g at an applied field of 7000 Oe and confirm the super paramagnetic behavior of the catalyst at room temperature (Fig. 1b).
Finally, to further prove the composition of the catalyst, an FTIR spectrum of Fe3O4 was examined as shown in Fig. 1c, the peak at 582 cm−1 being related to the interactions of Fe–O bonds in the structure of nano-Fe3O4 (Fig. 1c).
After the preparation and characterization of the catalysts, to optimize the reaction conditions, the reaction of 4-methoxyaniline with diboc was tested as a model in the presence of 3 mol% of nano-Fe3O4 in various solvents, such as EtOH, H2O, CHCl3, CH2Cl2, CH3OH, CH3CN, n-hexane and EtOAc at room temperature. The results are summarized in Table 1. Diboc was soluble in all the tested solvents, except H2O. As it can be seen in Table 1, ethanol was the best solvent in this reaction.
The effect of various solvents on the reaction of 4-methoxyaniline (1 mmol) with diboc (1 mmol) catalyzed by 3 mol% of nano-Fe3O4 at room temperature.
| Entry | Solvent | Time (min)/Yielda (%) |
| 1 | EtOH | 15/99 |
| 2 | H2O | 130/95 |
| 3 | CHCl3 | 135/85 |
| 4 | CH2Cl2 | 60/95 |
| 5 | CH3OH | 85/70 |
| 6 | CH3CN | 30/90 |
| 7 | n-Hexane | 60/95 |
| 9 | EtOAc | 30/90 |
a Isolated yield
The model reaction was then carried out with different amounts of the catalyst (Table 2) and a higher yield and shorter reaction times were observed using 3 mol% of nano-Fe3O4 in ethanol at room temperature.
Effect of different amounts of the catalyst on the reaction of 4-methoxyaniline (1 mmol) with diboc (1 mmol) at room temperature.
| Entry | Mol% of catalyst | Time (min)/Yielda (%) |
| 1 | 1 | 60/70 |
| 2 | 3 | 15/99 |
| 3 | 6 | 15/99 |
| 4 | 10 | 15/99 |
a Isolated yield
To assess the efficiency and scope of the catalyst, the reaction of diboc with various amines was studied under optimal conditions (Section 4.3). The results were summarized in Table 3. All amines, including aromatic and aliphatic, as well as halogens on their aromatic rings afforded the desired tert-butoxycarbonylated products in high to excellent yields (80–99%) in acceptable reaction times (10–200 min). As shown in Table 3, when diboc reacted with aromatic amines with the electron-releasing substituents, the yield was increased and the reaction time was decreased in accordance with the effect of the electron-withdrawing substituents.
Protection of amines (1 mmol) with diboc (1 mmol) using nano-Fe3O4 (3 mol %) as catalyst in ethanol (5 mL) at room temperature.
| Entry | Amine | Time (min)/Yielda (%) | Reference |
| 1a | Ph-NH2 | 20/99 | [13a,d] |
| 1b | 4-Cl-Ph-NH2 | 180/99 | [13b] |
| 1c | 4-Br-Ph-NH2 | 120/80 | [13b] |
| 1d | 4-OMe-Ph-NH2 | 15/99 | [13c] |
| 1e | 4-OH-Ph-NH2 | 20/99 | [13c] |
| 1f | 2-OH-Ph-NH2 | 30/95 | [13b] |
| 1g | 2-Me-Ph-NH2 | 120/80 | [13b] |
| 1h | 3-Me-Ph-NH2 | 100/85 | [13b,d] |
| 1i | 3-Br-Ph-NH2 | 200/95 | [13a] |
| 1j | (PhCH2)2NH | 10/95 | [13a] |
| 1kb | (4-NH2Ph)2CH2 | 60/80 | [13a] |
| 1l | Ph-CH2NH2 | 200/95 | [13c] |
| 1mb | Benzene-1,2-diamine | 60/95 | [13a] |
| 1nb | 4-Methylbenzene-1,2-diamine | 45/99 | [13a] |
| 1o | Naphthalen-1-amine | 180/99 | [13c] |
| 1p | Azepane | 20/95 | [13a] |
| 1qb | Piperazine | 25/95 | [13a] |
| 1rb | Cyclohexane-1,2-diamine | 15/99 | [13a] |
a Isolated yield
b This reaction was performed using 2 mmol of diboc.
Furthermore, we compared the turnover number (TON) value of our catalyst with some reported catalysts in the reaction (1 mmol) of aniline with diboc (1 mmol) (Table 4). As shown in Table 4, nano-Fe3O4 was better than the reported catalysts in terms of TON.
Comparison of the results of the reaction of aniline (1 mmol) with diboc (1 mmol) catalyzed by nano-Fe3O4 with those obtained by the reported catalysts.
| Catalyst/condition | Catalyst amount (mol%) | Time (min)/Yielda (%) | TON | Reference |
| Nano-Fe3O4/EtOH/rt | 3 | 20/99 | 33 | –b |
| Thiourea/toluene/60 °C | 10 | 40/95 | 9.5 | 11 |
| NH2SO3H/solvent-free/rtc | 5 | 10/96 | 19.2 | 12 |
| La (NO3)3 6H2O/solvent-free/35 °C | 5 | 2/100 | 20 | 16 |
a Isolated yield.
b Our work.
c In this reaction, 5 mmol of aniline were reacted with 5 mmol of diboc.
In a plausible mechanism shown in Scheme 2, carbonyl groups of diboc would be activated by nano-Fe3O4 as a Lewis acid. Fe3O4 was clearly considered as a Lewis acid and studied in the previous literature [18]. Then, the nucleophilic nitrogen of amine would attack the carbonyl group. After opening and closing the π bond of the carbonyl group in diboc, the amine was protected and one molecule of CO2 and tert-butyl alcohol were obtained as the side products.
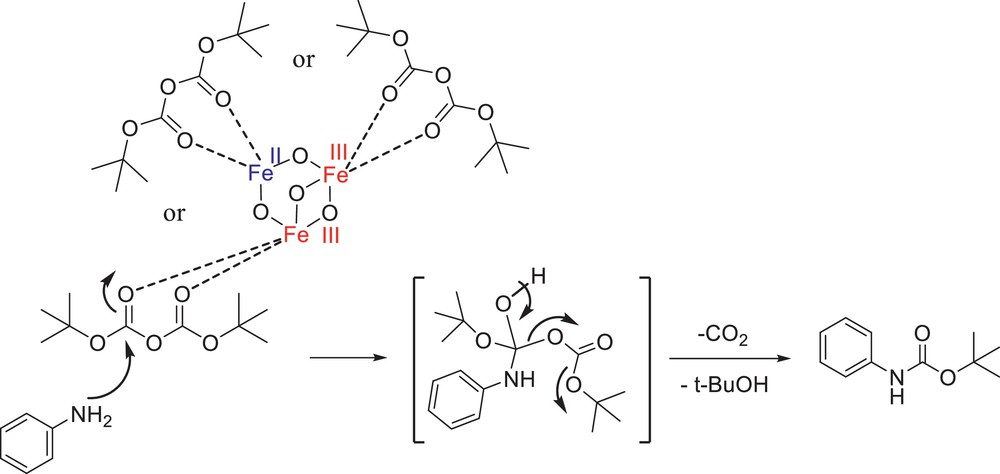
Purposed mechanism for the protection of amines with diboc using nano-Fe3O4 as a catalyst.
In another study, the recyclability of the catalyst was examined in the reaction of 4-methoxyaniline with diboc. After completion of the reaction, the catalyst was collected by a magnet and separated from the product and from the remaining starting materials. The recovered catalyst was washed with ethanol, dried and reused for the next run. The catalyst was recovered and reused for six times without any significant changes in the yield and the reaction time (Table 5).
Recovery and reuse of the nano-Fe3O4 as the catalyst in the reaction of 4-methoxyaniline (1 mmol) with diboc (1 mmol) in EtOH at room temperature.
| Run | Time (min) | Yielda (%) |
| 1 | 15 | 99 |
| 2 | 15 | 99 |
| 3 | 15 | 98 |
| 4 | 15 | 96 |
| 5 | 16 | 95 |
| 6 | 16 | 94 |
a Yield of the purified product.
3 Conclusion
In summary, we have introduced nano-Fe3O4 as an efficient, green and heterogeneous catalyst for the chemoselective N-boc protection of amines at room temperature in ethanol for the first time. The promising points of this method are safety, low cost, ease of separation and reusability of the catalyst, minimization of chemical wastes, mild reaction conditions, high yields, simple experimental procedure, short reaction times and compliance with the green chemistry protocols.
4 Experimental
4.1 General
All the chemicals were purchased from Merck, Fluka or Acros Chemical Companies and used without any further purification. The products were identified by comparison of their 1H NMR, IR spectrum and TLC with those of authentic samples. The progress of the reactions was monitored by TLC using silica gel SIL G/UV 254 plates. The 1HNMR (90 MHz) was run on a Jeol FX90Q NMR spectrometer (δ in ppm). The infrared spectrum of the compounds was recorded with a PerkinElmer PE-1600-FTIR device. The crystal structure of synthesized Fe3O4 (magnetite) was determined using an X-ray diffractometer (Italstructure ADP2000 XRD diffractometer) at ambient temperature. The magnetic measurements were carried out in a vibrating sample magnetometer (VSM-4 inch, Daghigh Meghnatis Kashan Co., Kashan, Iran) at room temperature. TEM analyses were performed with a Philips model CM 10 instrument.
4.2 General procedure for the synthesis of nano-ferrous ferric oxide (nano-Fe3O4)
First, 3 mL of FeCl3 (2 mol L−1 dissolved in 2 mol L−1 HCl) were added to 10.33 mL of double distilled water, and 2 mL of Na2SO3 (1 mol L−1) were added to the former solution dropwise in 1 min under magnetic stirring. When the color of the solution turned back from red to light yellow, the solution was added to 80 mL NH3·H2O solution (0.85 mol L−1) under vigorous stirring. After 30 min, the magnetite precipitates were washed to pH < 7.5 by distilled water [16,17].
4.3 General procedure for the N-boc protection of amines
A round-bottom flask (10 mL), which contains EtOH (5 mL), was charged with a solution of diboc (1–2 mmol), nano-Fe3O4 (3 mol%, 0.007 g) and the amine (1 mmol). The mixture was stirred at room temperature for the appropriate time (Table 3). After completion of the reaction, the catalyst was collected by a magnet and separated from the solution of product and the remaining starting materials. After drying and evaporation of the solvent, the resulting solid was recrystallized from n-hexane or ethyl acetate (5 mL) to give the pure product. The recovered catalyst was washed with EtOH, dried and reused for the next run. The catalyst was recovered and reused for six times without any significant changes in the yield and the reaction time.
4.4 Selected spectral data of the products
4.4.1 tert-Butyl 4-chlorophenylcarbamate (1b) [13b]
IR (KBr): 3304, 2961, 1700, 1593 cm−1; 1H NMR (90 MHz, CDCl3), δ 1.49 (s, 9H), 6.49 (s, 1H), 7.19–7.35 (m, 4H).
4.4.2 tert-Butyl 4-methoxyphenylcarbamate (1d) [13c]
IR (KBr): 3420, 2970, 1693, 1535 cm−1; 1H NMR (90 MHz, CDCl3), δ 1.47 (s, 9H), 3.73 (s, 3H), 6.42 (s, 1H), 6.65–6.96 (m, 2H), 7.15–7.32 (m, 2H).
4.4.3 tert-Butyl 4-hydroxyphenylcarbamate (1e) [13c]
IR (KBr): 3360, 2969, 1705, 1535 cm−1; 1H NMR (90 MHz, CDCl3), δ 1.48 (s, 9H), 5.35 (s, 1H), 6.36 (s, 1H), 6.60–6.82 (m, 2H), 7.02–7.35 (m, 2H).
4.4.4 tert-Butyl m-tolylcarbamate (1h) [13b,d]
IR (KBr): 3327, 2960, 1698, 1533 cm−1; 1H NMR (90 MHz, CDCl3), δ 1.52 (s, 9H), 2.30 (s, 3H), 6.62–7.36 (m, 5H).
4.4.5 tert-Butyl4,4′-methylenebis (4,1-phenylene) dicarbamate (1n) [13a]
IR (KBr): 3325, 2960, 1683, 1539 cm−1; 1H NMR (90 MHz, CDCl3): δ 1.49 (s, 18H), 3.85 (s, 2H), 6.42 (s, 2H), 7.02–7.35 (m, 8H).
Acknowledgements
The authors gratefully acknowledge the partial support of this work by the Research Affairs Office of Bu-Ali Sina University (Grant number 32-1716 entitled “Development of chemical methods, reagents and molecules”), and the Center of Excellence in Development of Chemical Method (CEDCM), Hamedan, Islamic Republic of Iran.


