1 Introduction
In history, the quality of water used for human consumption is an indication for quality of life, and consequently, a determining factor for human welfare. Arsenic is a widespread ecotoxin; the presence of arsenic in water is a serious threat for more than 100 million persons in the world [1].
There have been many documented incidents of arsenic contamination in ground water around the world, most notably in countries such as Taiwan, Chile, Argentina, Hungary, Bangladesh, India, Pakistan, Thailand, Vietnam, China and the United States [2–5]. Arsenic contamination has also been invoked in many adverse health effects, including skin lesions, diabetes mellitus, chronic bronchitis, cardiovascular disease, peripheral neuropathy, adverse reproductive outcomes, and hematological effects [6,7]. Humans are clearly sensitive to arsenic carcinogenesis; prolonged exposure to arsenic damages the central nervous system and results in diverse types of cancer in liver, lungs, bladder, and skin [8,9]. To meet the urgent technology needs and keeping in view the severe calamity of arsenic, a number of technologies were explored, including electrocoagulation [10], polymer inclusion membrane process [11], mesoporous alumina sorption [12], activated mud [13] and ferrihydrite crystallization process [14]. However, these new techniques are in the developmental stages; consequently, innovative cost-effective treatment processes are urgently needed [15]. Recently, polyvinyl pyrrolidone K25 coated cassava peel carbon has been applied for the removal of arsenic [16].
Calixarenes are important macrocyclic compounds with high thermal stability and are known as interesting building blocks for the recognition of metal ions [17]. During the past three decades, immense interest in these compounds was stimulated by their simple large-scale synthesis, and intensive efforts have been expended in different ways in which they can be selectively functionalized at the narrow or wide rim [18,19]. Many examples display that their high selectivity for the substrates was due to the accurate arrangement of the binding sites.
Moreover, calixarenes have several advantages for their application as extractants, e.g., their aromatic core structure is stable against oxidation, and the association of oxoanion with multiple functionalities present on the aromatic moiety makes them splendid ionophores. This affiliation may arise due to the hydrogen bonding between substituents on the calixarene framework and one or more of the oxygen atoms on the periphery of the oxoanion [20]. Amberlite XAD series resins have been extensively used for the solid-phase extraction and often modified for designing chelating resins [21]. Amberlite XAD-4 is a cheap commercial cross-linked polymer which has fantantasic adsorptive properties for neutral small molecules onto its macroreticular structure and higher surface area. These structures provide excellent chemical, physical and thermal stability. Modification with calixarenes makes them the best choice for the removal of a variety of metal ions [22,23]. We have reported several polymeric calixarenes [24,25] and investigated their ionophoric properties. In a continuation of our previous studies, herein we describe the extended research work based upon the designing structures for the extraction of anions. The objectives of this study were to quantify arsenic(V) adsorption onto p-tert-butylcalix[8]areneoctamide impregnated Amberlite XAD-4 resin (4) and evaluate the sorption mechanism through various equilibrium sorption isotherms.
2 Results and discussion
Arsenic(V) ions are important because of their high toxicity and their presence in soils and waters.
For a molecule to be effective as a host, it is necessary that its structural features are compatible with those of the guest anions. The arsenic(V) (H2AsO4−/HAsO42−) ions have oxide moieties at their periphery. These oxides interact with potential sites of the host molecule for hydrogen bonding. It is known that calix[4]arenes with amino or amide functionalities on their lower rim are efficient extractants for oxoanions [30,31]. Thus, we were interested in synthesizing a p-tert-butylcalix[8]areneoctamide derivative (3) along with its impregnated resin (4) and exploration of their extraction properties for arsenic(V) ions. The present work determines the strategic requirements for two-phase extraction measurements.
2.1 Liquid-liquid extraction of arsenic(V) with ligand 3
A preliminary evaluation of the extraction efficiencies of 3 has been carried out by solvent extraction of arsenic(V) ions from water into dichloromethane at different pH values. The results are summarized in Fig. 1. From the extraction data, ligand 3 was found to be an effective extractant for the phase transfer of arsenic(V) anions. From Fig. 1, it is clear that maximum extraction (98%) occurs at pH 4; which shows that best interaction between ligand 3 and arsenic(V) ions occurs at this pH. The proposed interaction between arsenic(V) and ligand 3 is shown in Fig. 2.
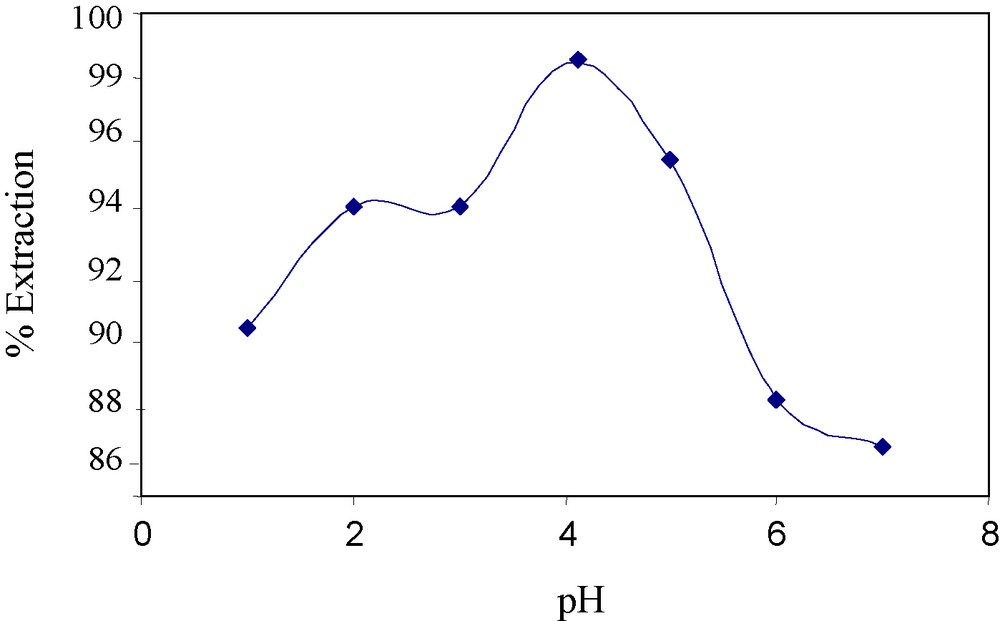
Effect of pH on the extraction of arsenic with resin 4 in liquid–liquid extraction with ligand 3.
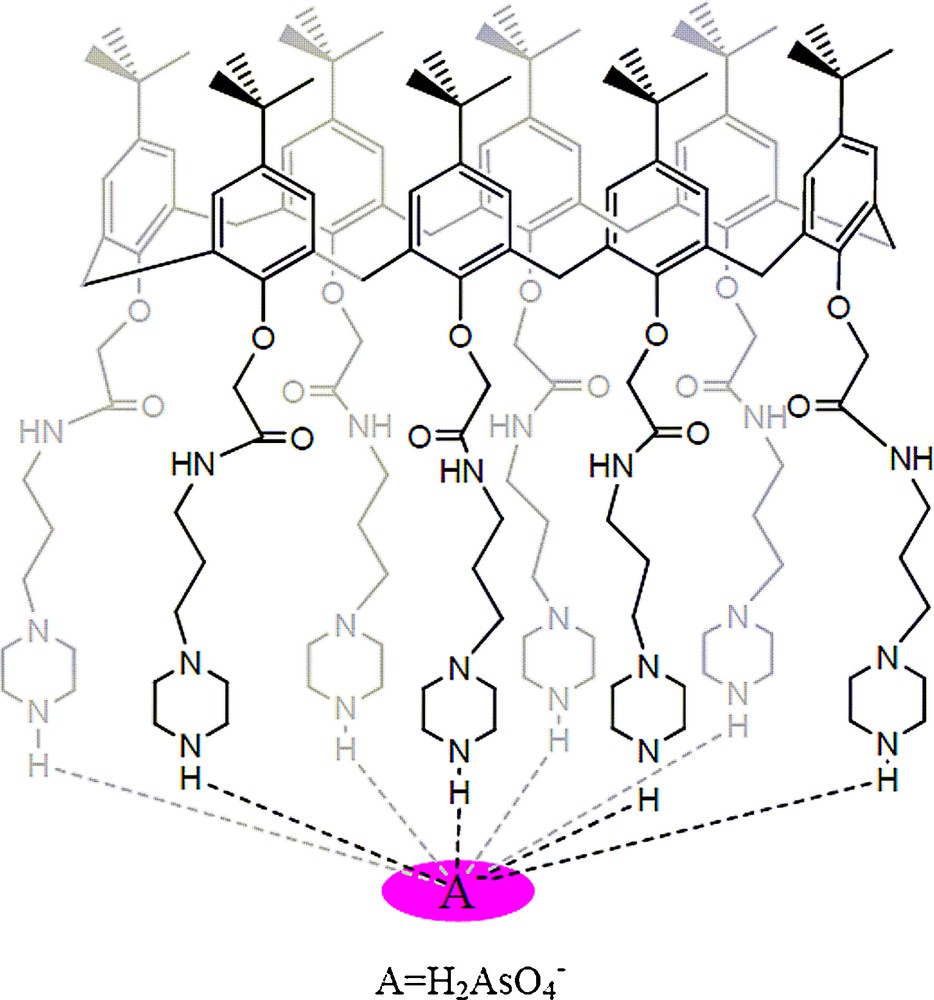
Proposed interactions of 3 with H2AsO4.
2.1.1 Effect of pH
Arsenic(V) speciation is affected by the solution pH through the following equilibrium:
| H3AsO4 ↔ H2AsO4− + H+ pKa1 = 2.3 | (1) |
| H2AsO4− ↔ HAsO42− + H+ pKa2 = 6.8 | (2) |
| HAsO42− ↔ AsO43− + H+ pKa3 = 11.6 | (3) |
From Eqs. (1)–(3), the arsenic(V) species occur mainly in the form of H2AsO4− in the pH range between 3 and 6, while a divalent anion HAsO42− dominates at higher pH values (such as between pH 8 and 11). Thus, it is evident that the adsorption of arsenic(V) by calixarene is pH dependent. In aqueous solutions having a lower pH (pH 1–3) the arsenic(V) ion will be primarily in its protonated form H3AsO4. Because of the distribution of arsenic(V) in water which is in the form of H3AsO4, it is clearly indicated that the calixarene derivative that has been modified with cationic form at low pH is also suitable to adsorb arsenic(V) species in water. Beside this, the monoanion (H2AsO4−) will have a smaller free energy of hydration then its dianionic form HAsO42−. As a result, there is a smaller loss in hydration energy as H2AsO4− is transferred from the aqueous phase into the dichloromethane phase. An additional advantage of H2AsO4− over HAsO42−is that, for the former, only one sodium ion needs to be coextracted to maintain charge balance, whereas for HAsO42− two sodium ions are extracted, with additional loss of hydration energy.
These interactions are attributed to the hydrogen bonding and electrostatic interaction between amino/amide groups and the oxygens of arsenic(V) ions [32], which is supported by IR spectrum shown in Fig. 3. The IR spectrum clearly shows the shift of amidic carbonyl peak from 1667 to 1673 cm−1 and sec-amine (N-H) peak from 3300 to a broad shoulder at 3442 cm−1.
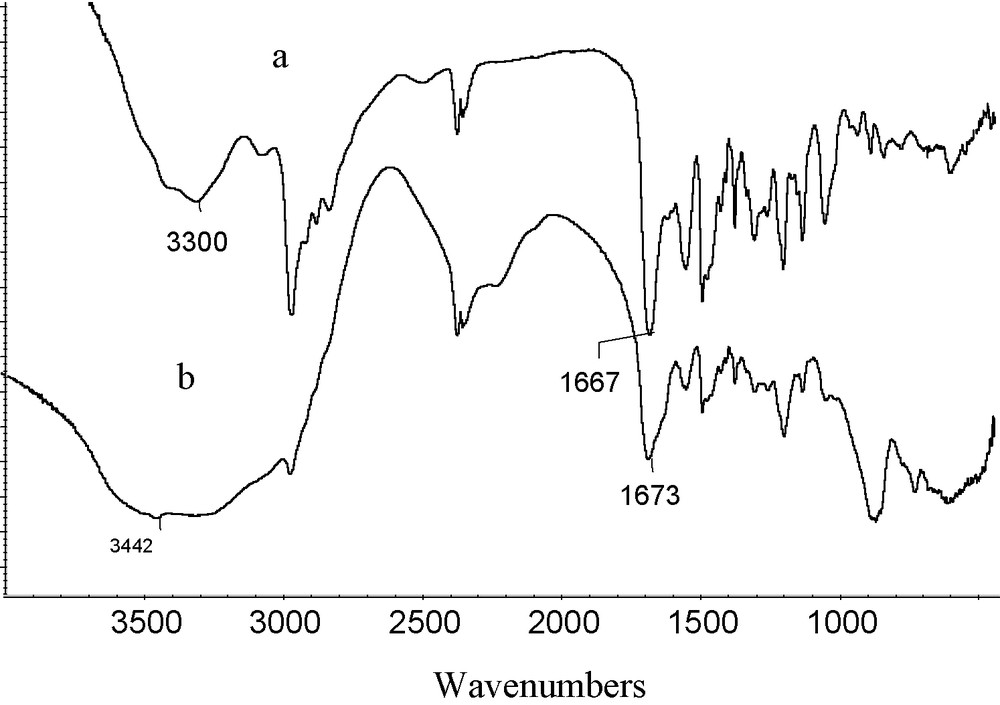
IR-Studies of (a) Ligand 3 and (b) its arsenic(V) complex.
2.2 Sorption studies (solid-phase extraction)
Sorption is a well-known equilibrium separation process in manipulating the data and understanding the surface phenomena. The arsenic(V) sorption experiments were also carried out with impregnated resin 4. The equilibrium isotherms for the investigated arsenic solutes onto 4 have been studied in the pH range of 1–7. Maximum sorption up to 99% was found at pH 4 as given in the Fig. 4. Increased sorption at low pH may be due to the presence of fewer −OH ions which may otherwise compete with arsenic(V) anions for the available adsorption sites.
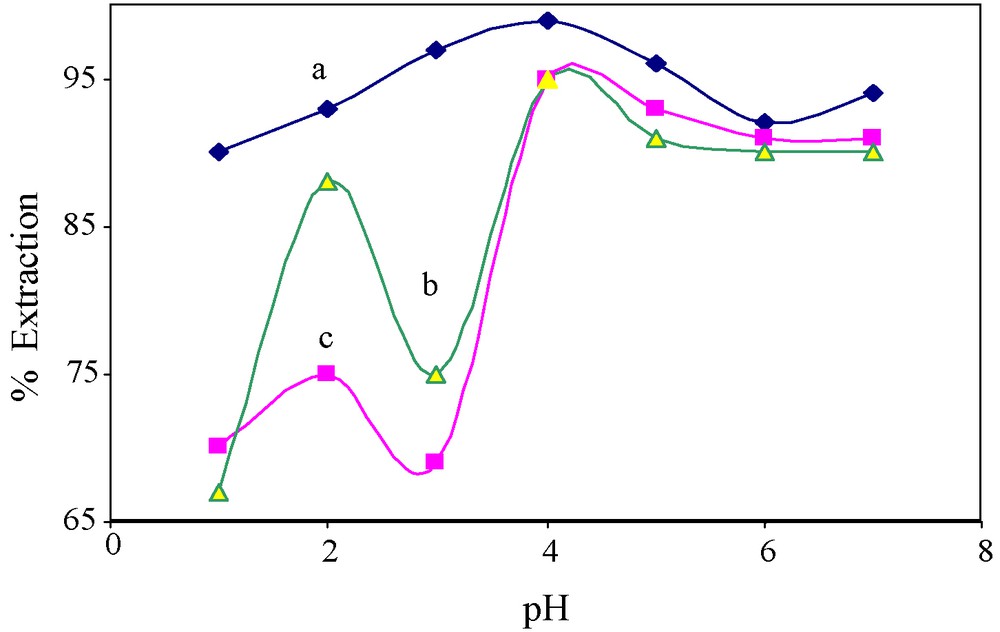
Effect of pH on the extraction of arsenic with resin 4 in column sorption experiments of various samples: (a) Standard (b) Municipal and (c) Ground water.
2.2.1 Isotherm studies
Several adsorption isotherms have been developed to evaluate the data obtained. Langmuir and Freundlich model are most common. Langmuir model suggest monolayer coverage, favorability, unfavorability, reversibility and irreversibility of the process in terms of a dimensionless separation factor RL. Multi layer formation can be checked by the Freundlich isotherm which was also applied for arsenic(V) sorption. Langmuir and Freundlich isotherms have special significance to explain the ideal case of physisorption or chemisorption on surface containing finite number of identical sites. Some of the non-ideal effects can be explained by a number of mathematical expressions. Mostly, applied models for mathematical expression are by Temkin and Flory-Huggins [33,34].
To find out the degree of surface coverage of the sorbate on the sorbent, the Flory-Huggins isotherm was studied. Similarly, to evaluate the energy distribution pattern and linearity of heat of sorption due to sorbate/sorbent interactions, the Temkin isotherm has been applied to the data. Equilibrium studies on adsorption give information about the nature of adsorption and maximum adsorption capacity.
2.2.2 Sorption capacity
The sorption capacity for the column was calculated numerically by using Eq. (2).
| (2) |
| (3) |
Q, Cad, and t total are the volumetric flow rate, adsorbed concentration and total flow time, respectively. Similarly, unadsorbed arsenic(V) concentration in the column at equilibrium can be calculated by Eq. (4).
| (4) |
The experimental data obtained was manipulated in terms of equilibrium isotherms.
2.2.3 Langmuir model
The data obtained from the Langmuir model, Fig. 5, shows the maximum sorption capacity of the column (i.e., 289 mg g−1) and the separation factor shows that the sorption is favorable as its value is less than 1, Table 1. The Langmuir isotherm and separation factor RL can be expressed by the Eqs. (5) and (6).
| (5) |
| (6) |
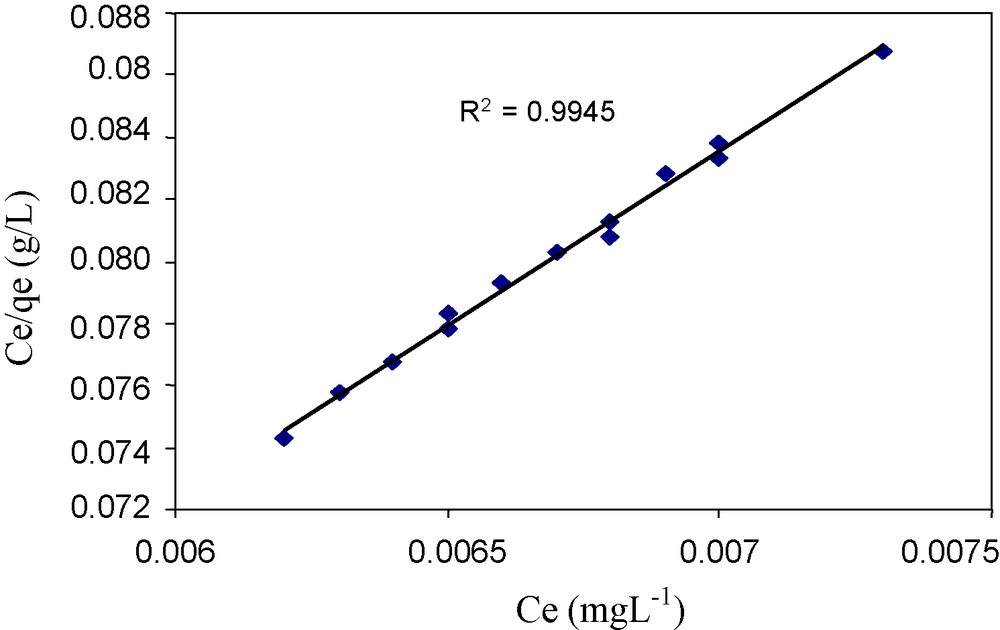
Langmuir sorption isotherm.
Various model parameters obtained in column sorption experiments.
| Langmuir isotherm | Freundlich isotherm | Flory-Huggins isotherm | Temkin isotherm | |||||||||
| qo (mg g−1) | RL | R2 | Kf (mg g−1) | n | R2 | KFH | nFH | R2 | ΔG0 (kj mol−1) | b (kj mol−1) | a | R2 |
| 289.2 | 0.045 | 0.99 | 0.3 | 53.2 | 0.99 | 1.7 | 1.3 | 0.99 | −1.24 | 10.22 | 2.5 | 0.99 |
Uptake efficiency of the impregnated resin is compared with already reported resins for the extraction of arsenic given in Table 2 [35–40]. It has been observed that the impregnated resin is more powerful in efficiency (i.e., 289 mg g−1); this may be due to the greater surface area for interaction.
Comparison between maximum sorption capacities of reported resins with the impregnated resin (present work).
| Adsorbent | Concentration range | pH | Model used | Capacity (mgg−1) | References |
| Activated Bauxsol | 7.03–220.9 mM | 4.5 | Langmuir | 7.64 | [35] |
| Monoclinic hydrous Zirconium oxide (ZR resin) | 1 × 10−3 M | 4–6 | Langmuir | 89.90 | [36] |
| Untreated GAC | 0.10–30.0 mg L−1 | 4.7 | Langmuir | 0.04 | [37] |
| Zirconium loaded chelating resin (ZR-LDA) | – | 4.0 | Langmuir | 49.15 | [38] |
| Chitosan | 400 mgL−1 | 4.0 | Langmuir | 58.00 | [39] |
| Titanium dioxide-loaded Amberlite XAD-7 resin | 0–5 mM | 1–5 | Langmuir | 4.72 | [40] |
| Ligand 3 impregnated Amberlite XAD-4 | 250 mg/L | 4.0 | Langmuir | 289.00 | Present study |
2.2.4 Freundlich model
Similarly, multilayer sorption can be explained by the Freundlich model given in Fig. 6. Although the correlation coefficient for the model shows good relations, the value of Kf and n are not in agreement; which indicates that the sorption process does not undergo heterogeneous or multilayer formation Eq. (7).
| (7) |

Freundlich sorption isotherm.
2.2.5 Flory-Huggins model
The Flory-Huggins model (Fig. 7), explains the surface coverage of the sorbate on the sorbent (Eq. 8). KFH was further manipulated to calculate the Gibb's free energy of spontaneity of sorption using Eq. (9). The negative value of ΔG0 shown in Table 1 indicates that the sorption of arsenic(V) ions onto impregnated resin is feasible, spontaneous and exothermic in nature. A high value of R2 shows excellent applicability of this model to the sorption of arsenic(V) ions onto the resin.
| (8) |
| (9) |
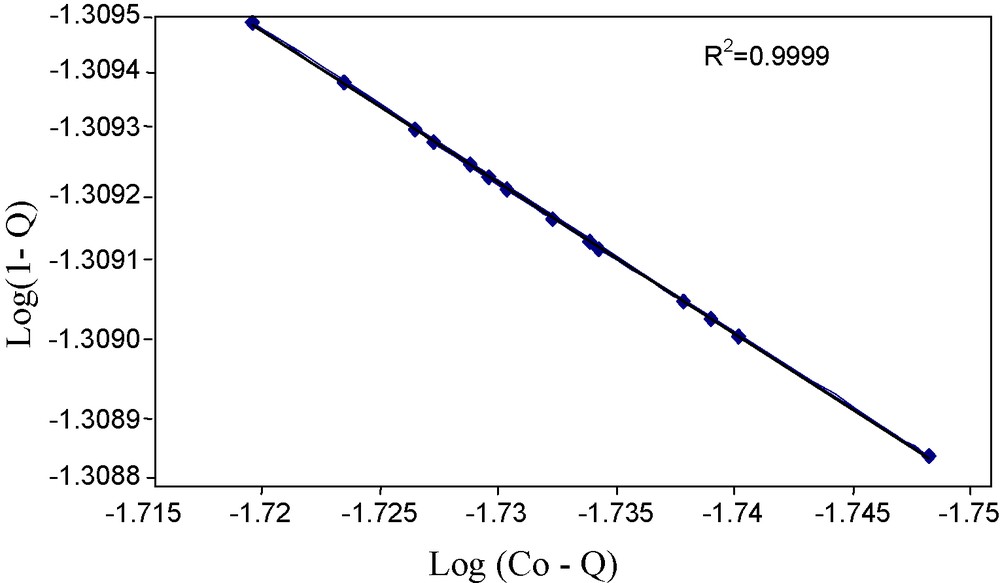
Flory-Huggins sorption model.
2.2.6 Temkin model
To evaluate the indirect interaction between sorbate and sorbent as well as to find the heat of sorption, we applied the Temkin isotherm Eq. (10), graphically presented in Fig. 8.
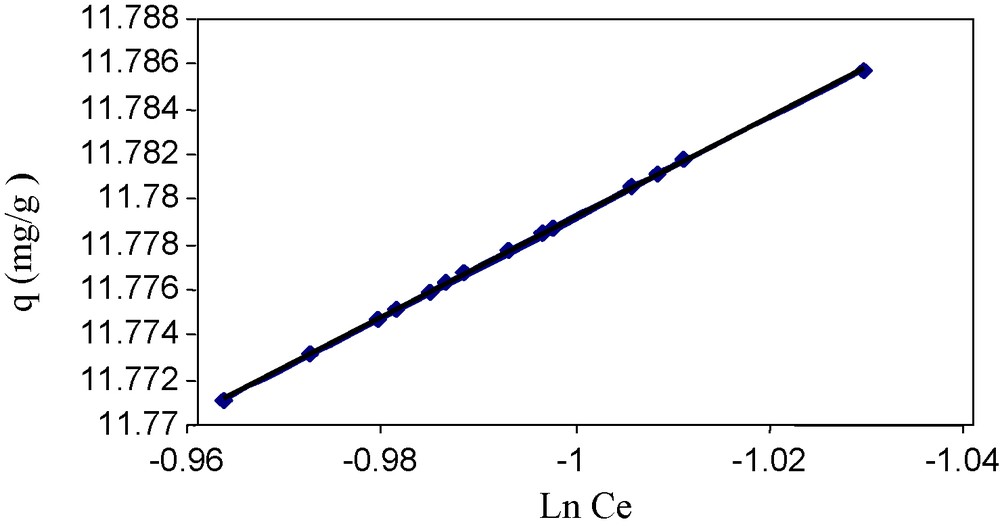
Temkin isotherm model.
The value of the correlation coefficient is significant (i.e., 0.99) which indicates that the sorption process is feasible and compatible with this model. Similarly, the values of b which is 10 KJ mol−1 is high showing greater interaction between the sorbate and the sorbent, and is in the range of 8–16 KJ mol−1. Thus, the nature of the exothermic reaction for the sorption has confirmed as was calculated by the Flory-Huggins model in terms of Gibb's free energy.
| (10) |
where b is the Temkin isotherm constant showing heat of sorption (KJ mol−1), R is gas constant, a is the isotherm constant.
The column could be used precisely up to 10 cycles with quantitative recovery of 99%. After 10 cycles there is reduction in the recovery of arsenic(V) ions Fig. 9.
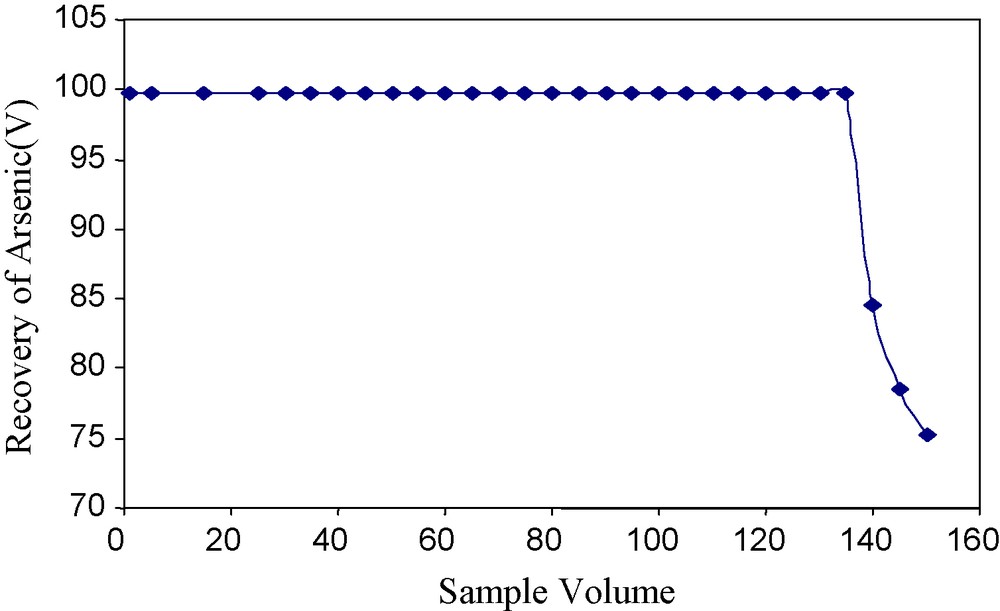
Effect of variation of sample volume on the recovery of arsenic(V).
2.2.7 Field study
A study regarding the application of resin on real samples was preformed by collecting municipal and ground water samples from 12 different sites of Hyderabad city in Pakistan. Their conductivity and total dissolved salts (TDS) contents were determined before and after the treatment with resin through column (Table 3).
Analytical study data of real samples before and after treatment with resin 4.
| Name of samplea | TDS (mg/L) (BE)b | TDS (mg/L) (AE)b | Conductivity) (μs/cm)(BE)b | Conductivity (μs/cm)(AE)b | Total Arsenic (ppm) |
| Ms-1 | 475 | 380 | 970 | 820 | 0.046 |
| Ms-2 | 236 | 180 | 481 | 350 | 0.009 |
| Ms-3 | 256 | 190 | 523 | 470 | 0.007 |
| Ms-4 | 107 | 100 | 227 | 151 | 0.005 |
| Ms-5 | 318 | 280 | 646 | 502 | 0.002 |
| Ms-6 | 64 | 48 | 130 | 77 | 0.007 |
| Ms-7 | 130 | 100 | 288 | 153 | 0.004 |
| Gs-8 | 458 | 377 | 935 | 781 | 0.004 |
| Gs-9 | 88 | 60 | 177 | 110 | 0.028 |
| Gs-10 | 23 | 18 | 47.7 | 31 | 0.001 |
| Gs-11 | 116 | 88 | 236 | 159 | 0.008 |
| Gs-12 | 128 | 91 | 265 | 183 | 0.001 |
a Ms and Gs stands for municipal and ground water samples.
b TDS stands for total dissolved salts BE and AE stands for before and after extraction.
Among these real samples, only two (i.e., Ms-1 and Gs-9) have been found to contain total arsenic above the permissible limits of US EPA [41]. Thus, these samples with higher TDS were passed twice at optimum flow rate through resin 4 in the column. It has been observed that the resin 4 has shown quite remarkable efficiency (up to 90%) for removal of total arsenic from the real samples (Fig. 4) at a wide range of pH, i.e., from pH 4 to pH 8. But in comparison with the standard sample, resin 4 has shown a small decrease (∼5–7%) in its efficiency which may be due to the presence of a high concentration of TDS (Table 4).
Interference of coexisting ions on the percent removal of arsenic in real samples.
| Name of samplea | Conc. BE (ppm) and AE (%) | |||||||||||||
| Total Arsenic | Sulphates | Phosphate | Chloride | Fluoride | Potassium | Calcium | ||||||||
| BE | AE | BE | AE | BE | AE | BE | AE | BE | AE | BE | AE | BE | AE | |
| Ms-1 | 0.046 | 94 | 2.926 | 0.89 | 1.57 | 15.9 | 2.465 | 1.9 | 0.137 | 1.0 | 63 | 0.3 | 12 | 0.4 |
| Gs-9 | 0.028 | 92 | 1.27 | 1.44 | – | – | 2.534 | 6.6 | 0.471 | 4.6 | 6.4 | 0.3 | 10 | 0.3 |
a Both these samples were above the limit of recommended value [41].
3 Conclusion
From the present study, it can be concluded that the impregnated resin 4 has applicability in both the laboratory and the environment. It has remarkable efficiency for the sorption of arsenic(V) from standard as well as real samples. It is highly efficient as compared to ligand 3 alone, which may be due to the greater surface area provided by the framework of Amberlite resin to the ligand for interaction with arsenic oxoanions. The resin 4 can be prepared easily from available cheap raw material. Another advantage is that it is regenerable and can be used several times.
4 Experimental
4.1 Reagents
High purity sodium hydrogen arsenate heptahydrate (Na2HAsO4·7H2O) purchased from Merck (Darmstadt, Germany) was used to prepare 1 gL−1 arsenic stock solution. Amberlite XAD-4 resin (styrene-divinylbenzene copolymer), surface area 725 m2g−1, pore diameter 4 nm and bead size 20–50 mesh was supplied by Fluka (Buchs, Switzerland). Concentrated HCl, HNO3 were analytical reagent grade from Merck (Darmstadt, Germany) and were checked for possible trace contamination. Calibrations were prepared for each analytical session using certified stock standard solution of arsenic 1000 ppm, Fluka Kamica (Bushs, Switzerland). Solutions of Na2HAsO4.7H2O were prepared daily. All glassware and polyethylene bottles were thoroughly washed and then soaked overnight in 5 M HNO3, and rinsed with deionized water before use. All aqueous solutions were prepared with deionized water that had been passed through a millipore Milli-Q Plus water purification system.
4.2 Instrumentation
pH meter (Metrohm Ltd Switzerland) with glass electrode was used to measure the pH. Total arsenic was determined by Perkin–Elmer Analyst 700 atomic absorption spectrometer equipped with a deuterium background corrector and a MHS-15 hydride generation system, Perkin-Elmer Corp. Perkin-Elmer (Shelton, CT, USA). The operating parameters for working of hollow cathode lamp were set as recommended by the manufacturer. IR spectra were recorded on a Thermo Nicollet AVATAR 5700 FTIR spectrometer using KBr pellets in the spectral range 4000–400. Interference study was performed on 861 Advanced Compact ion chromatograph equipped with Metrosep A Supp 4–250 column (Metrohm Ltd Switzerland).
4.3 Synthesis procedure
5,11,17,23,29,35,41,47-octa-tert-butyl-49,50,51,52,53,54,55,56-octa-(2-piprazinoethyl-amino)carbonylmethoxycalix[8]arene (3) and its impregnated resin (4) as illustrated in Scheme 1 were prepared and characterized according to the reported methods [26–28].

Synthesis of 5,11,17,23,29,35,41,47-octa-tert-butyl-49, 50,51,52,53,54,55,56-octa-(2-piprazinoethylamino)carbonylmethoxycalix[8]arene (3) and its impregnated resin (4).
4.4 Extraction procedures
4.4.1 Liquid–liquid extraction
Liquid–liquid extraction experiments were performed using Pederson's experimental procedure [29]. 10 mL of 1 × 10−4 M aqueous Na2HAsO4.7H2O solution (pH of arsenic(V) solution has been maintained by 0.01 M KOH/HCl) and 10 mL of 1 × 10−3 M solution of 3 in CH2Cl2 was vigorously agitated in a stoppard 25 ml flask with a mechanical shaker for 2 min, then magnetically stirred in a thermostatic water-bath at 25 °C for 1 h, and finally left standing for an additional 30 minutes so that the two phases separate distinctively. The effect of pH has been studied in the range of pH 1–7. The concentration of arsenic(V) ions remaining in the aqueous phase was then determined by atomic absorption spectrometer. Blank experiments indicated that no arsenic(V) extraction occurred in the absence of 3. The percent extraction (E (%)) has been calculated as:
| (1) |
4.5 Sorption experiments
Column sorption (Solid–liquid extraction) experiments were conducted at room temperature over the pH range of 1–7. A glass column (1.0 cm I.D × 10.0 cm length) filled with 0.25 g of impregnated resin 4 was used. It was cleaned by passing ethanol, 4 M HCl solution and double distilled water. Column was conditioned to the desired pH with 0.01 M KOH/HCl solutions. Arsenic(V) solution was passed under the gravitational force through the column at a flow rate of 2 mL/min. The analyte in the eluents was determined as discussed above.
4.6 Stability of the column
The stability of the column was studied using 0.25 g of resin. The adsorbed arsenic(V) was eluted using 15 ml of 6 M HCl.
Acknowledgment
We are extremely greatful to the Higher Education Commission (PIN No. 063-142830-Ps3-234/HEC/Sch/2006) Islamabad, The Scientific and Technical Research Council of Turkey (TUBITAK), Ministry of Science and Technology, Islamabad, [Project No -12(106-B)/2004-ASA (IL)] and the National Center of Excellence in Analytical Chemistry, University of Sindh, Jamshoro-Pakistan, for the financial support of this work.


