1 Introduction
Our strategy for the synthesis of unnatural steroids involves the preparation of the benzocyclobutene 2 from phenylpropiononitrile 1 according to the Mukaiyama procedure [1]. For this cyclization reaction, a large excess of sodium amide (CAS number: 7782-92-5) is employed. We generally prepare this sodium amide in situ, by the addition at −33 °C of pieces of sodium to liquid ammonia (2.5 L for each mol of sodium) containing ∼0.6 mmol (2.5 g) of coarsely powdered Fe(NO3)3•9H2O for each mol of sodium [2]; and then phenylpropiononitrile 1 was added. After addition of diethyl ether, the ammonia was allowed to evaporate, and solid ammonium chloride was added. Then, classical work-up gave rise to 2 in 60% yield (Scheme 1).
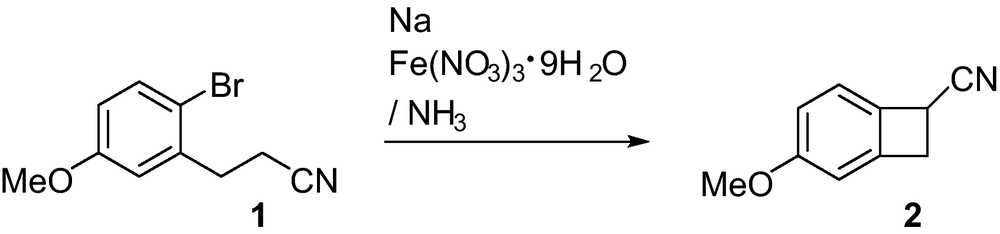
2 Results
During 10 years, we performed many successful experiments according to the Mukaiyama protocol and using undistilled ammonia from a commercial cylinder [3]. But, recently, instead of using iron(III) nitrate as the catalyst precursor, we employed anhydrous FeCl3 in the same amount. However, when this iron catalyst was employed, the hydrolysis appeared to be a very dangerous step. During the course of the hydrolysis, we observed several times the ignition of the mixture. Moreover, after usual work-up, the products 3 [4] and 4 [5] (Scheme 1) corresponding to a drastic reduction of 1 were obtained, and the formation of 2 was not observed. Clearly, in the presence of iron trichloride, sodium amide has not been formed and we have only, in the reaction mixture, the sodium metal dissolved in the liquid ammonia. The catalysis mechanism is uncertain, but appears to involve the rapid reduction of iron(III) to very finely divided metallic iron, which is probably the actual catalytic agent. Without catalyst, the sodium amide is formed very slowly [6]. Some old preparations describe the passing of dry ammonia gas over the molten sodium [7].
The first catalyst used for the preparation of sodium amide by addition of sodium in liquid ammonia, was divided platinum [8]. The use of iron salts, and specially iron(III) nitrate has been described for the first time in 1934 [9]. Since these reports, many preparative methods recommend the use of hydrated ferric nitrate, in particular in the various synthetic methods described in Organic Syntheses [2] or Org. Proc. Res. Dev [10]. However, for some reports including patents [11], and other procedures described in Organic Synthesis [12], it is recommended to use anhydrous ferric chloride [13]. Even a recent encyclopedia such as e-EROS indicated “preparative method: combination of ammonia, small quantities of an iron(III) salt and sodium…” [14]. In a very detailed study concerning the Birch reaction, industrial investigators showed that the ferric chloride catalyzes efficiently the reaction between sodium and alcohols in liquid ammonia [15]. Iron is a well-known transition metal playing an important role as catalyst in organic syntheses [16].
In summary, the nature of the iron salt has a huge influence on the reaction for the formation of sodium amide from liquid ammonia and sodium metal. Fe(NO3)3•9H2O led to the desired sodium amide in good yield; whereas, the use of FeCl3 appears to be dangerous and to give unexpected products.
3 Experimental Section
1H and 13C NMR spectra were recorded in CDCl3 solutions at 300, and 75 MHz, respectively. Chemical shift are reported in parts per million relative to CDCl3 (signals for residual CHCl3 in the CDCl3: 7.24 for 1H NMR and 77.16 (central) for 13C NMR). Carbon-proton couplings were determined by DEPT sequence experiments.
A 6 L three-neck flask equipped with a 6 cm egg-shaped magnetic bar, an acetone-dry ice reflux condenser, a pressure-equalizing addition funnel was charged with 4.5 L of liquid ammonia. One piece of sodium and then Fe(NO3)3•9H2O (500 mg) were added. When the solution turns from blue to gray, the remaining pieces of sodium (50 g, 2.18 mol) were gradually added with vigourous stirring. Then, the phenylpropiononitrile 1 (96 g, 0.40 mol) was slowly added and the stirring was continued during one night without reflux condenser. Then, diethyl ether (2 L) and ammonium chloride (400 g) were added and the mixture was allowed to stand until the ice on the outside of the flask was entirely melted. The mixture was cautiously poured on ice, stirred and, after usual work-up, the organic layer was dried using MgSO4. The crude product was then chromatographed on silica gel (petroleum ether/diethyl ether, 85/15) to give 2 [1] (38.2 g, 60%) as pale yellow crystals: mp 87 °C; 1H NMR (300 MHz, CDCl3), δ 3.45 (1H, dd, J = 14.1, 2.3 Hz), 3.59 (1H, dd, J = 14.1, 5.5 Hz), 3.76 (3H, s), 4.14 (1H, dd, J = 5.5, 2.3 Hz), 6.69 (1H, d, J = 2.1 Hz), 6.82 (1H, dd, J = 8.3, 2.1 Hz), 7.09 (1H, d, J = 8.3 Hz); 13C NMR (75 MHz, CDCl3), δ 28.0 (d), 35.6 (t), 55.5 (q), 108.8 (d), 115.2 (d), 120.0 (s), 123.8 (d), 130.4 (s), 143.7 (s), 161.1 (s).
When the anhydrous FeCl3 salt was employed, when the mixture was pourred on ice, the presence of some pyrophoric compounds result in the repeated ignition of the mixture. After hydrolysis, the crude product was chromatographed on silica gel to give 3-ethylanisole 3 (CAS number: 10568-38-4) (16%) and 3-ethylphenol 4 (CAS number: 620-17-7) (9%). 3-Ethylanisole 3: 1H NMR (300 MHz, CDCl3), δ 7.22–6.76 (m, 4H), 3.81 (s, 3H), 2.65 (q, J = 7.6 Hz, 2H), 1.26 (t, J = 7.6 Hz, 3H); 13C NMR (75 MHz, CDCl3), δ 159.8 (s), 146.0 (s), 129.4 (d), 120.4 (d), 113.8 (d), 110.9 (d), 55.2 (q), 29.0 (t), 15.6 (q). 3-Ethylphenol 4: 1H NMR (300 MHz, CDCl3), δ 7.15–6.7 (m, 4H), 5.25 (s, 1H), 2.60 (q, J = 7.6 Hz, 2H), 1.22 (t, J = 7.6 Hz, 3H); 13C NMR (75 MHz, CDCl3), δ 155.5 (s), 146.4 (s), 129.6 (d), 120.7 (d), 115.0 (d), 112.7 (d), 28.8 (t); 15.5 (q).


