1 Introduction
Multicomponent reactions (MCRs) play an important role in combinatorial chemistry because of the ability to synthesize target compounds with greater efficiency and atom economy by generating structural complexity in a single step from three or more reactants [1]. Moreover, MCRs offer the advantage of simplicity and synthetic efficiency over conventional chemical reaction [2].
In the past few decades, the synthesis of new heterocyclic compounds has been a subject of great interest because of their wide applicability [3]. Heterocyclic compounds occur very widely in nature and are essential to life [4]. Among a large variety of nitrogen-containing heterocyclic compounds, nitrogen heterocycles containing a phthalazine moiety are important because they show biological and pharmacological activities such as anticonvulsant, cardiotonic, and vasorelaxant, and also unique electrical and optical properties [5].
Moreover, one of the most important aspects in green chemistry is the use of ionic liquids (ILs) as greener solvents and catalysts. ILs have several advantages such as control of product distribution, enhanced rate and/or reactivity, ease of product recovery, catalyst immobilization, and reusability [6].
The synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives have been reported using Mg(HSO4)2 [7], [Bmim]Br [6], p-TSA [8], silica sulfuric acid [9], PPA–SiO2 [10], H2SO4/H2O–EtOH [11], H2SO4/[bmim]BF4 [11], Ce(SO4)2·4H2O [12] and starch sulfate [13], as catalysts.
In continuation of our research on new synthetic methods in organic synthesis [7,9,10,13], we develop the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives with three Brønsted acidic ionic liquids (BAILs) such as 2-pyrrolidonium hydrogensulfate ([Hnhp][HSO4]), (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate (Fig. 1) as catalysts (Scheme 1).

The structure of [Hnhp][HSO4], (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate, and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate.
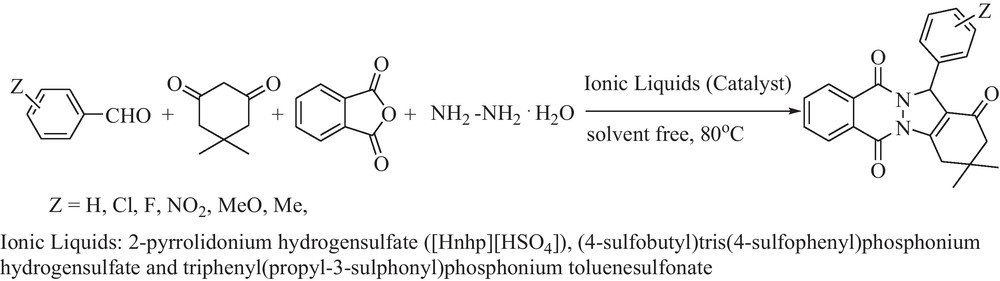
The preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives.
2 Results and discussions
In order to carry out preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives in a more efficient way, the reaction of dimedone (1 mmol), 2-chlorobenzaldehyde (1 mmol), hydrazine monohydrate (1.4 mmol), and phthalic anhydride (1.2 mmol) was selected as a model system under thermal solvent-free conditions to find optimization of reaction conditions. The preparation of 13-(2-chlorophenyl)-3,3-dimethyl-3,4-dihydro-1H-indazolo[1,2-b]phthalazine-1,6,11(2H,13H)-trione was studied at different reaction temperatures (25, 40, 60, 80, and 100 °C) and different amounts of acidic ionic liquids as catalyst (5, 10, 15, 20, 25, and 30 mol%) (Table 1). The reaction did not occur in the absence of catalyst (Table 1, Entry 1). The best result was obtained by using 5 mol% of [Hnhp][HSO4], 5 mol% of (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate and 5 mol% of triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate at 80 °C (Table 1).
Optimization the amount of the acidic ionic liquids, 2-pyrrolidonium hydrogensulfate (IL1), (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate(IL2), triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate (IL3), as catalysts at different temperature in the reaction of dimedone (1 mmol), 2-chlorobenzaldehyde (1 mmol), hydrazine monohydrate (1.4 mmol) and phthalic anhydride (1.2 mmol) under solvent-free conditions.
| Entry | Catalyst (mol%) | Temperature (°C) | Time (min) | Yield (%)a | ||||
| IL1 | IL2 | IL3 | IL1 | IL2 | IL3 | |||
| 1 | 0 | 80 | 60 | 60 | 60 | 0 | 0 | 0 |
| 2 | 5 | 80 | 5 | 4 | 9 | 88 | 91 | 93 |
| 3 | 10 | 80 | 8 | 8 | 13 | 87 | 84 | 83 |
| 4 | 15 | 80 | 6 | 9 | 11 | 87 | 87 | 91 |
| 5 | 20 | 80 | 6 | 8 | 12 | 78 | 90 | 85 |
| 6 | 30 | 80 | 9 | 6 | 12 | 83 | 89 | 87 |
| 7 | 5 | 25 | 25 | 18 | 30 | 10 | 19 | 17 |
| 8 | 5 | 40 | 23 | 10 | 25 | 32 | 42 | 25 |
| 9 | 5 | 60 | 9 | 9 | 18 | 83 | 68 | 60 |
| 10 | 5 | 100 | 5 | 6 | 7 | 82 | 92 | 91 |
a Yields refer to isolated pure products.
Using these optimized reaction conditions, the scope and efficiency of these procedures were explored for the synthesis of a wide variety of substituted 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives. The results are summarized in Table 2. As shown in Table 2, the direct four-component reactions worked well with a variety of arylaldehydes including those bearing electron-withdrawing and electron-donating groups such as Me, OMe, Cl, F, Br and NO2, and the desired compounds were obtained in high to excellent yields. This methodology offers significant improvements such as simplicity in operation, and green aspects by avoiding expensive or corrosive catalysts.
Four-component synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives.
| Entry | Z | Time (min) | Yield (%)a | M.p. (°C)/Lit. M.p. (°C) [Ref] | ||||
| IL1 | IL2 | IL3 | IL1 | IL2 | IL3 | |||
| 1 | H | 7 | 6 | 8 | 88 | 93 | 90 | 206–208/(207–209) [10] |
| 2 | 2-Cl | 5 | 4 | 9 | 88 | 91 | 93 | 263–265/(264–266) [10] |
| 3 | 4-Cl | 7 | 8 | 10 | 83 | 87 | 91 | 261–263/(259–261) [10] |
| 4 | 2,4-(Cl)2 | 11 | 6 | 15 | 79 | 89 | 87 | 218–220/(219–221) [10] |
| 5 | 4-F | 9 | 8 | 10 | 86 | 90 | 89 | 220–222/(224–226) [10] |
| 6 | 4-MeO | 6 | 5 | 6 | 83 | 89 | 92 | 219–221/(218–220) [10] |
| 7 | 4-Me | 6 | 5 | 7 | 90 | 95 | 92 | 228230/(226–228) [10] |
| 8 | 3-NO2 | 8 | 7 | 10 | 70 | 85 | 80 | 271–273/(270–272) [10] |
| 9 | 3,4-(MeO)2 | 7 | 6 | 7 | 82 | 90 | 92 | 184–186/(185–186) [11] |
| 10 | 2-Me | 8 | 7 | 11 | 85 | 88 | 86 | 240–242/(241–243) [10] |
| 11 | 3,4,5-(MeO)3 | 5 | 6 | 6 | 88 | 92 | 90 | 232–234/(232–234) [10] |
| 12 | 3-Cl | 9 | 8 | 9 | 84 | 89 | 85 | 203–205/(204–206) [10] |
| 13 | 4-OH-3-MeO | 7 | 5 | 8 | 90 | 92 | 87 | 250–252/(250–252) [10] |
| 14 | 4-NO2 | 11 | 9 | 12 | 78 | 83 | 85 | 220/(215–217) [6] |
| 15 | 2-MeO | 7 | 8 | 9 | 90 | 88 | 89 | 240–242/(242–243) [6] |
a Yields refer to isolated pure products. The molar ratio of the starting reactants was chosen as: dimedone (1 mmol), arylaldehydes (1 mmol), hydrazine monohydrate (1.4 mmol), phthalic anhydride (1.2 mmol). The reaction was carried out in an oil bath at 80 °C.
We also compared the results of the present ILs with other catalysts reported in the literature such as Mg(HSO4)2 [7], [Bmim]Br [6], p-TSA [8], silica sulfuric acid [9], and PPA–SiO2 [10] for synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives (Table 3). The Table 3 clearly demonstrates that 2-pyrrolidonium hydrogensulfate, (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate are effective catalysts in terms of reaction time and yield of obtained product (Table 2, Entry 2) relative to other reported catalysts.
Comparison the results of 2–pyrrolidonium hydrogensulfate, (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate with other catalysts reported in the literature for synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives.
| Entry | Catalyst (mol%) | Conditions | Time (min)/Yield (%) [Ref] |
| 1 | Mg(HSO4)2 (11.44)a | Solvent-free, 100 °C | 4/88 [7] |
| 2 | p-TSA (30)a | Solvent-free, 80 °C | 15/88 [8] |
| 3 | Silica sulfuric acid (6.5)a | Solvent-free, 100 °C | 10/85 [9] |
| 4 | PPA–SiO2 (5)a | Solvent-free, 100 °C | 12/81 [10] |
| 5 | [Bmim]Br (228)b | Sonication (40 kHz, 200 W) | 10/91 [6] |
a Based on the three-component reaction of dimedone, 2-chlorobenzaldehyde, phthalhydrazide.
b Based on the four-component reaction of dimedone, 2-chlorobenzaldehyde, hydrazine monohydrate, phthalic anhydride.
The proposed mechanism for the cyclic catalytic reaction was suggested according to the literature [10]. First, the reaction of phthalic anhydride (1) with hydrazine monohydrate (2) at room temperature produced the phthalhydrazide (3). Next, in the catalytic cycle, the Knoevenagel type coupling of arylaldehyde (4) with active methylene compounds such as dimedone (5) give 2-benzylidene-5,5-dimethylcyclohexane-1,3-dione (6) [14]. Then, the subsequent 1,4-conjugate addition of phthalhydrazide to the activated intermediate (7) followed by cyclization and elimination of water affords the corresponding products (Scheme 2).

The catalytic mechanism for the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives using 2-pyrrolidonium hydrogensulfate as selected ionic liquid.
In green organic synthesis, the recovery of the catalysts is more important. Thus, the reusability of the ILs as catalyst was studied. After the completion of the reaction, the mixture was cooled to room temperature and 5 mL of water was added to the reaction mixture. The ionic liquid was dissolved in water, and filtered for separation of the crude product. The separated product was washed twice with water (2 × 5 mL). For recovering the ionic liquids, water containing ILs was evaporated, and the remaining viscous liquid was washed with CH2Cl2 (5 mL) and dried under reduced pressure. The recovered ILs was tested for studying its catalytic activity in the subsequent run without adding fresh catalyst. The ILs were tested for five runs. It was seen that the ILs as catalyst displayed very good reusability without any considerable loss of their activities (Fig. 2).

Reusability of ionic liquids as catalyst.
3 Conclusions
In summary, an efficient protocol for the one-pot preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives from the four-component condensation reaction of dimedone, arylaldehyde, hydrazine monohydrate and phthalic anhydride using a environmental friendly and reusable ionic liquids, 2-pyrrolidonium hydrogensulfate, (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate, as catalysts were described. The reactions were carried out under thermal solvent-free conditions with short reaction time and produced the corresponding products in good to excellent yields. Also the catalysts could be successfully recovered and recycled at least for five runs without significant loss in activity. The one-pot nature and the use of homogeneous ionic liquids as an eco-friendly catalysts make it an interesting alternative to multistep approaches.
4 Experimental
4.1 General
All reagents were purchased from Merck and Aldrich and used without further purification. All yields refer to isolated products after purification. 2-Pyrrolidonium hydrogensulfate [15], (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate [16] and triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate [17] were prepared according to literature procedure. Products were characterized by comparison of spectroscopic data (IR, 1H-NMR, 13C NMR spectra) and melting points with authentic samples. The NMR spectra were recorded on a Bruker Avance DPX 300 MHz instrument. The spectra were measured in CDCl3 relative to TMS (0.00 ppm). IR spectra were recorded on a JASCO FT-IR 460plus spectrophotometer. All of the compounds were solid and solid state IR spectra were recorded using the KBr disk technique. Melting points were determined in open capillaries with a BUCHI 510 melting point apparatus. TLC was performed on silica gel polygram SIL G/UV 254 plates.
4.2 General procedure for the synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives
Hydrazine monohydrate (1.4 mmol), phthalic anhydride (1.2 mmol) reacted at room temperature until a white precipitated of phthalhydrazide was obtained (< 2 min). Then, dimedone (1 mmol), aromatic aldehyde (1 mmol), and 2-pyrrolidonium hydrogensulfate (0.009 g, 5 mol%, 0.05 mmol) or (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate (0.036 g, 5 mol%, 0.05 mmol) or triphenyl(propyl-3-sulphonyl)phosphonium toluenesulfonate (0.026 g, 5 mol%, 0.05 mmol) as catalyst was added to the mixture and stirred in an oil bath at 80 °C for the appropriated times (Table 2). After completion of the reaction, it was cooled to room temperature. Then, 5 mL of water was added to the mixture. The ionic liquid was dissolved in water, and filtered for separation of the crude solid product. The separated product was washed twice with water (2 × 5 mL). The solid product was purified by recrystallization procedure in ethanol. For recovering the ionic liquids, the water containing ILs was evaporated and the remaining viscous liquid was washed with CH2Cl2 (5 mL) and dried under reduced pressure.
All of the products are known. We selected 1H-NMR, 13C NMR and IR spectroscopic data for two known compounds as below:
- • 3,4-dihydro- 3,3-dimethyl-13-phenyl-2H-indazolo[2,1-b]phthalazine- 1,6,11(13H)-trione (Table 2, Entry 1). [M.p.: 206–208 °C] 1H-NMR (300 MHz, CDCl3): δ = 1.20 (s, 3H), 1.21 (s, 3H), 2.34 (s, 2H), 3.27 (dd, J = 1.7, 18.5 Hz, 1H), 3.44 (d, J = 18.6 Hz, 1H), 6.47 (s, 1H), 7.31–8.36 (m, 9H) ppm; 13C NMR (75 MHz, CDCl3): δ = 28.4, 28.6, 34.6, 38.1, 50.8, 64.8, 118.7, 127.2, 127.6, 127.9, 128.6, 128.9, 129.3, 133.7, 134.6, 136.3, 150.8, 154.4, 156.2, 192.3 ppm; IR (KBr, cm−1): 3027, 2959, 1662, 1618, 1469, 1421, 1360, 1306, 1273, 1145, 1074, 1026, 754, 698.
- • 13-(4-Hydroxy-3-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-indazolo[1,2-b]phthalazine-1,6,11(13H)-trione (Table 2, Entry 13). [M.p.: 250–252 °C] 1H-NMR (300 MHz, CDCl3): δ = 1.23 (s, 6H), 2.36 (s, 2H), 3.23 (d, J = 19.0 Hz, 1H), 3. 44 (d, J = 18.9 Hz, 1H), 3.91 (s, 3H), 5.33(br, 1H), 6.40 (s, 1H), 6.77–7.07 (m, 3H), 7.86 (s, 2H), 8.27–8.35 (m, 2H) ppm; 13C NMR (75 MHz, CDCl3): δ = 28.4, 28.8, 34.6, 38.1, 51.0, 56.0, 64.8, 111.0, 114.6, 118.6, 119.2, 127.7, 128.0, 128.2, 129.0, 129.2, 133.5, 134.5, 146.0, 146.4, 150.7, 156.1, 192.3 ppm; IR (KBr, cm−1): 3408, 2958, 1660, 1600, 1493, 1359, 1270, 1234, 1135, 1030, 790, 627.
Acknowledgments
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.


