1 Introduction
The lanthanide halides and their mixtures with alkali metal halides play a very important role in many modern technologies, such as metallic lanthanide or lanthanide-based alloy production [1,2], reprocessing of nuclear wastes [3], recycling of spent nuclear fuel [4,5], and in the lighting industry [6] (high-pressure discharge halide lamps). Such wide technological application of these compounds requires knowledge of their structural, physicochemical, and, in particular, their thermodynamic properties. The purity of the lanthanide halides is important to study their thermodynamic and physicochemical properties. These anhydrous salts commercially available still contain small amounts of water and cannot be used for this study. The synthesis of these salts is the preliminary step to cross [7–9]. The synthesis parameters of lanthanide halides (temperature, contact time, chemical composition…) therefore remain to be determined according to the nature of the lanthanide. The synthesis of lanthanide halides is a very long and difficult procedure. This reaction that occurs at high temperatures (of about 1150 K) is often accompanied by side reactions leading to the formation of oxyhalide LnOX (Ln = lanthanide and X = halide) [8–10]. It seems that such a situation is caused not only by the well-known difficult features of high-temperature experimentation, but also by the extreme reactivity of lanthanide halides. This reactivity necessitates the use of appropriate methods of synthesis and purification, chemical analysis, and manipulation. The present work is focused on praseodymium(III) chloride (PrCl3) synthesis by sintering chlorinating of praseodymium oxide (Pr6O11) with ammonium chloride (NH4Cl). It reports the influence of various synthesis parameters (temperature, contact time, and chemical composition) on the reaction yield. The optimum conditions for synthesis of PrCl3 were, thus, determined and discussed.
2 Experimental
PrCl3 was synthesised from Pr6O11 (Alfa Aesar, 99.9% (REO)) by sintering chlorinating with NH4Cl (Biochim, 99.5%). Pr6O11 and NH4Cl in well-defined proportions were homogenized in mortar and placed in an alumina crucible. The crucible with reaction charge was introduced into a quartz reactor and maintained under argon. The assembly was housed in a furnace programmed at the desired temperature. Temperature was measured with a Pt/Pt–Rh(10) thermocouple with 1-K accuracy. After the experiment, the product synthesized in solid form was dissolved in a buffered solution at pH = 5.6 (CH3COOH/CH3COONa mixture) heated to 80 °C for chemical analysis. The chemical analysis of the synthesized PrCl3 was performed by a complexometric titration with a standard EDTA solution, using xylenol orange as an indicator.
3 Results and discussion
3.1 Thermodynamic aspect of the reaction
The synthesis of PrCl3, from Pr6O11, by dry route in the presence of NH4Cl, can be described by the balanced equation:
| Pr6O11(s) + 22 NH4Cl (s,g) → 6 PrCl3 (s) + 22 NH3 (g) + 11 H2O (ℓ,g) + 2 Cl2(g) |
Using thermodynamic data found in literature [11,12], we have calculated the thermodynamic quantities for the standard enthalpy of reaction, the entropy of reaction and the standard Gibbs free energy of reaction. These quantities are also calculated according to the temperature and are presented in Figs. 1–3. Fig. 1 shows that the reaction enthalpy is negative over the entire temperature range used. The enthalpic jump observed is related to the vaporization of water and the reaction remains exothermic in this temperature range.
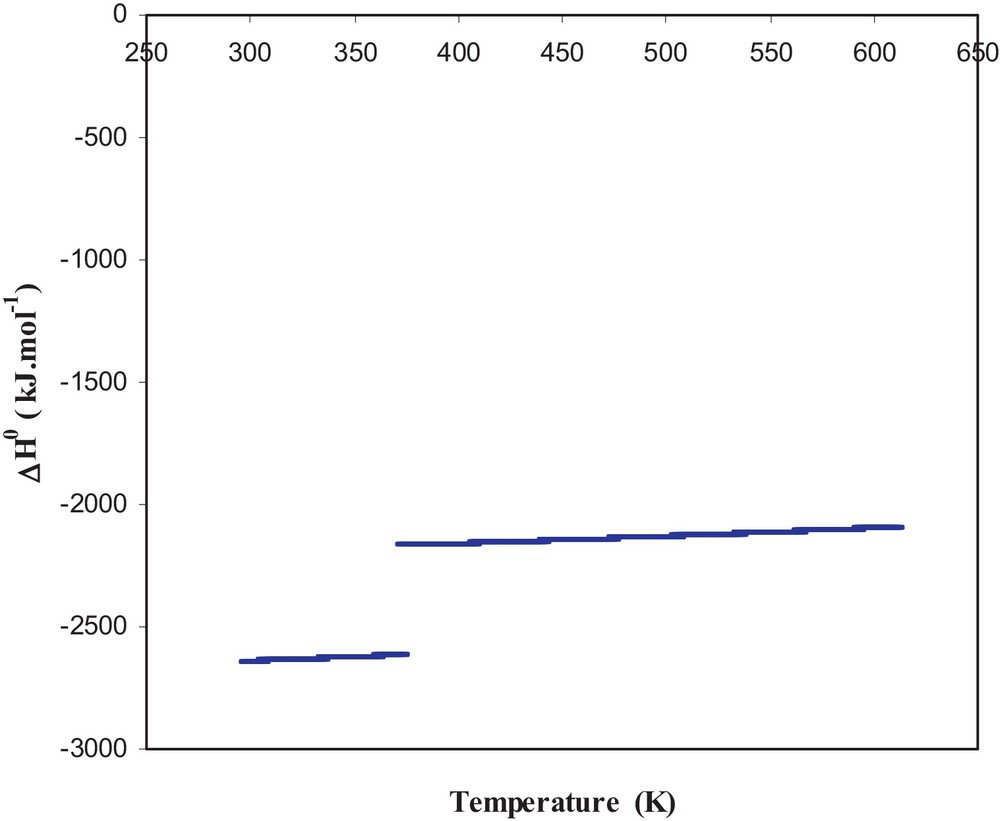
Standard enthalpy of reaction.

Standard entropy of reaction.

Standard Gibbs free energy of reaction.
The entropic contribution is quite significant (Fig. 2). This was expected since the reaction is accompanied by a substantial disorder linked to the release of gaseous products (increasing number of moles of gas). The enthalpic and entropic contribution led to a negative free enthalpy of reaction. Fig. 3 shows that the free enthalpy of reaction decreases with temperature. So, an increase in temperature promotes the synthesis of PrCl3.
3.2 Characterization of reagents by TG–DTG
Pr6O11, and solid NH4Cl, constitute the raw material in the synthesis of PrCl3 by dry route. The thermal behavior of these reagents was investigated by TG–DTG.
3.2.1 Thermal analysis of ammonium chloride (NH4Cl)
The thermal decomposing process of pure NH4Cl was investigated by TG–DTG. The result showed that NH4Cl begins to lose weight at 188 °C; large loss of weight ends at 302 °C when NH4Cl is heated at the rate of 10 °C/min under N2 atmosphere (Fig. 4). For chlorination, NH4Cl directly participates in the chlorination reaction, and HCl decomposed from NH4Cl also contributes to the chlorination reaction for synthesizing PrCl3 [13].
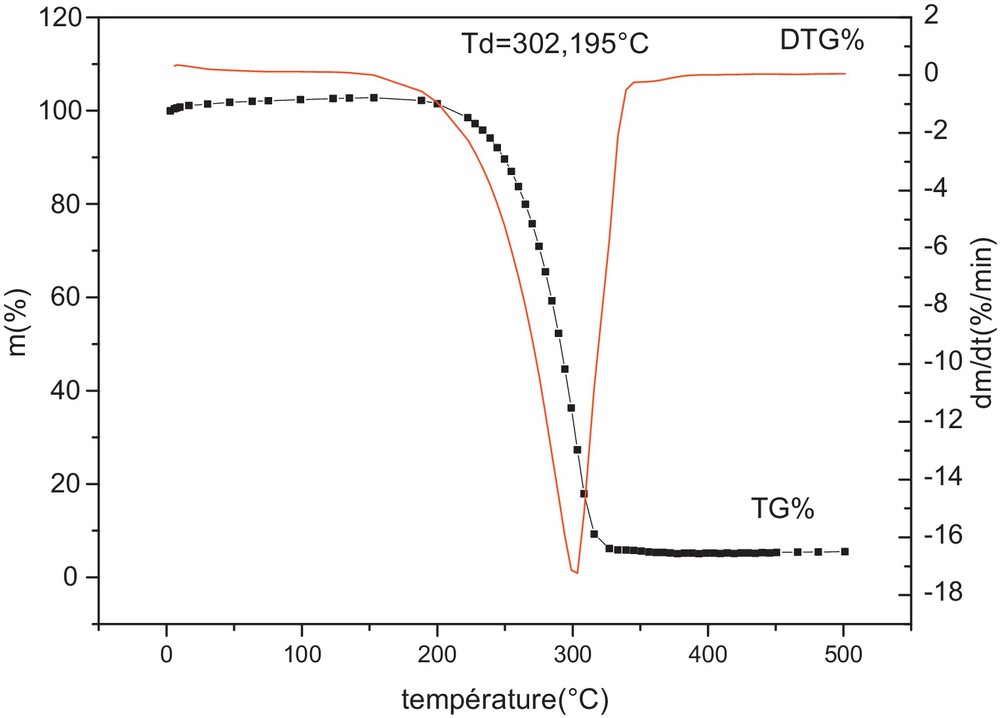
Curves TG–DTG of ammonium chloride (NH4Cl) obtained at 10 °C/min under nitrogen atmosphere.
3.2.2 Thermal analysis of praseodymium oxide (Pr6O11)
Fig. 5 shows the curves TG–DTG obtained at 10 °C/min under nitrogen atmosphere with Pr6O11. The thermal analysis shows that Pr6O11 does not lose any weight and that during this process, the oxide mass does not vary with temperature. This observation is in agreement with literature information [14].

Curves TG–DTG of praseodymium oxide (Pr6O11) obtained at 10 °C/min under nitrogen atmosphere.
3.3 Determination and optimization of the synthesis parameters
In this work, we studied the influence of various synthesis parameters (temperature, contact time and chemical composition) on the reaction yield.
3.3.1 Influence of the contact time
The study of the influence of contact time on the reaction yield was achieved by varying it from 10 to 120 min, while the other two parameters were kept constant: stoichiometric proportions of reagents, in the molar ratio Pr6O11:NH4Cl = 1:22 and experimental temperature T = 250 °C.
The choice of the first parameter (contact time) is important for the rest of the experiments, because it allows us to determine the time required to attain equilibrium and have optimum yield.
The amount of PrCl3 obtained at the end of each experiment was determined by a complexometric titration with standard EDTA. We realized three assays for each experiment. Next, we calculate the reaction yield. The reaction yield, noted R, is defined by:
The mass of product obtained is the mass synthesized. Theoretical mass of product is the mass corresponding to a yield of 100%. It must therefore be calculated from the mass of the reagents.
The experimental results for the effect of the contact time on the reaction yield are graphically represented in Fig. 6. From the results, we observe an increase in the reaction yield R (%) versus time up to 60 min. Beyond this value, we find that the yield stabilizes at an average of 64.48%. The optimum contact time is, therefore, t = 60 min.
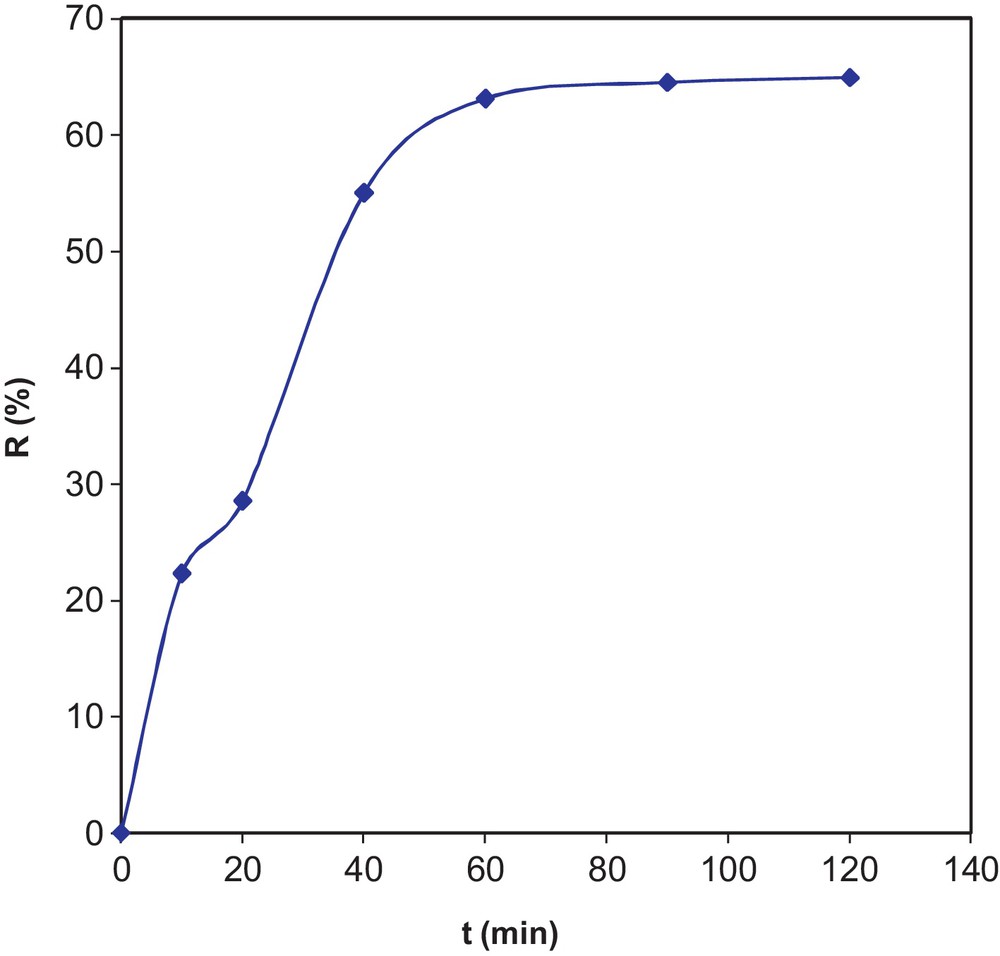
Effect of the contact time on variations of yield.
3.3.2 Influence of the stoichiometry
The influence of excess NH4Cl on the reaction yield was studied by varying the stoichiometry in moles from Pr6O11:NH4Cl = 1:22 to Pr6O11:NH4Cl = 1:66, while maintaining the two other parameters constant (T = 250 °C and t = 60 min).
The experimental results for the effect of the stoichiometry on the reaction yield are represented in Fig. 7. From the results, we observe an increase in the reaction yield R (%) with excess NH4Cl up to the molar ratio Pr6O11:NH4Cl = 1:44. Beyond this value, we find that the yield stabilizes at an average of 76.46%. The optimal proportions in moles are, therefore, Pr6O11:NH4Cl = 1:44.
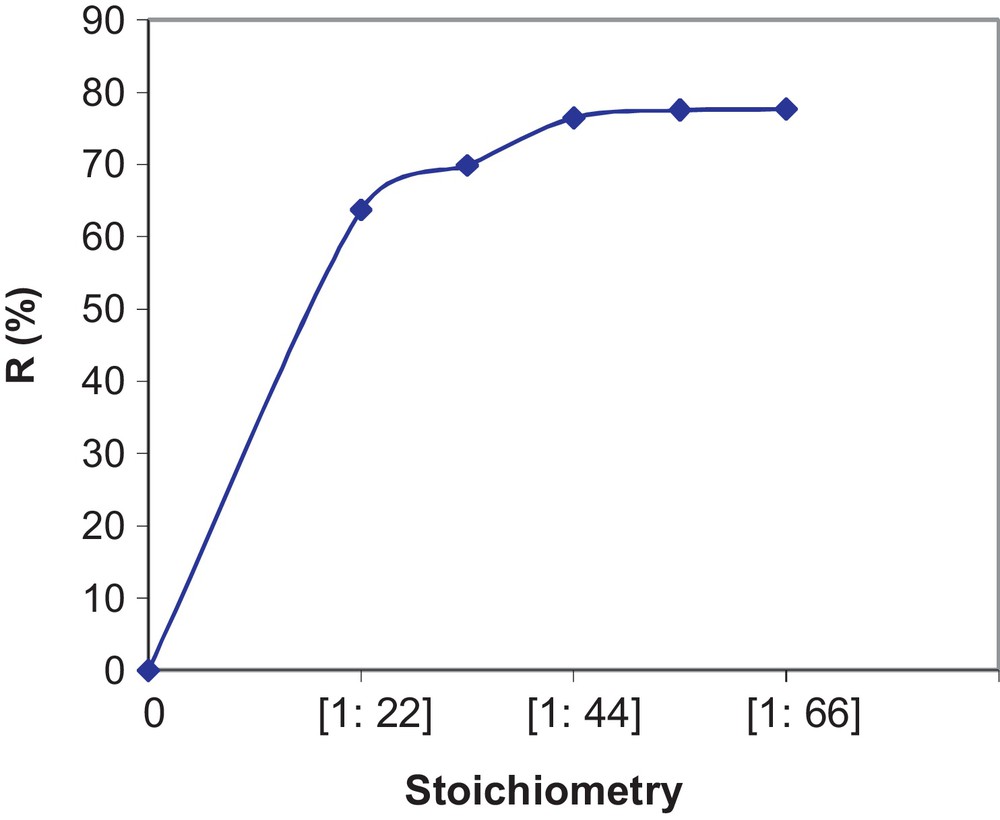
Effect of the stoichiometry on variations of yield.
3.3.3 Influence of the temperature
The influence of the temperature on the reaction yield was studied by varying it from 250 to 450 °C while the other two parameters were kept constant (stoichiometry in moles Pr6O11:NH4Cl = 1:44 and t = 60 min).
The experimental results for the effect of the temperature on the reaction yield are represented in Fig. 8.

Effect of the temperature on variations of yield.
From the results, we observe an increase in the reaction yield R (%) versus temperature up to 400 °C. Beyond this value, the yield decreases slightly. This is probably due to the loss of mass of NH4Cl related to its high sublimation at high temperatures. The excess of this reagent cannot compensate for these losses. The maximum yield corresponds to 91.36%. The optimum temperature is, therefore, T = 400 °C.
4 Summary
Taking into account the above observations, we can conclude that the optimum conditions for the synthesis of PrCl3 are: temperature, T = 400 °C, contact time, t = 60 min and stoichiometry in moles Pr6O11:NH4Cl = 1:44, for which the reaction yield reached 91.36%.
Acknowledgements
One of us (LR) acknowledges support under a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of the Wroclaw University of Technology.


