1 Introduction
Xanthene derivatives posses a broad range of useful pharmacological activities such as antibacterial [1], anti-inflammatory [2], antiviral properties [3]. Furthermore, these heterocyclic compounds are used for photodynamic therapy [4]; such compounds are also utilized as antagonists of the paralyzing action of zoxazolamine. Some other compounds find applications in industries such as those of dyes [5], laser technology [6], and pH-sensitive fluorescent materials for visualization of biomolecules [7]. These compounds have been synthesized by many procedures. Some of the important method included trapping of benzynes by phenol [8], intermolecular phenyl carbonyl coupling reactions of aldehydes and 2-naphthol [9], cyclodehydrations [10], carbon monoxide [11], reaction of 2-naphthol with formamide for the synthesis of 14H-dibenzo-[a,j]xanthenes [12], aldehydes and cyclic 1,3-dicarbonyl compounds [13]. The most common synthesis routes to xanthenes included the reaction of 14H-dibenzo[a,j]xanthene by condensation of 2-naphthol and aldehydes in the presence of p-toluenesulfonic acid [14,15], molecular iodine [16], K5CoW12O40·3H2O/silica–gel/MW [17], LiBr/MW [18], amberlyst-15 [19], α-iodoacetates from alkenes/ammonium acetate/I2 [20], cation-exchange resins [21] as catalysts, Beckmann rearrangement products [22], isonitriles [23], silica sulfuric acid [24], sulfamic acid [25].
Recently, the use of acidic ionic liquids (ILs) has achieved considerable importance in organic synthesis, because of their application as eco-friendly solvents. The benefits of these compounds are low vapor pressure, non-inflammability, wide liquid range, reusability, high thermal stability, acidic catalytic activity, and the fact they can be used instead of conventional organic solvents [26–29]. The synthesis of heterocycles under solvent-free conditions has attracted much attention in synthetic organic chemistry. However, the above-mentioned methods for the synthesis of heterocyclic compounds suffer drawbacks such as long reaction times, low yield, expensive reagents, harsh reaction conditions, toxic organic solvents, and excess of catalysts.
In this research, we wish to report an efficient and green procedure for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes. Herein, we describe a new and very convenient protocol for the condensation of aromatic aldehydes with 2-naphthol to the corresponding 14-aryl-14H-dibenzo[a,j]xanthanes in the presence of Brønsted acidic ionic liquid [H-NMP][HSO4] as a homogeneous catalyst under solvent-free conditions. The catalyst could be recovered and recycled several times without significant loss of activity, and with high purity.
2 Results and discussion
Initially, in order to optimize the reaction conditions, we considered the reaction of 2-naphthol and 4-chloro-3-nitrobenzaldehyde in 2:1 ratio as a model substrate, in the presence of various catalytic amounts of [H-NMP][HSO4] as a homogeneous catalyst under solvent-free conditions at 110 °C (Scheme 1).

Synthesis of 14-(4-chloro-3-nitrophenyl)14H-dibenzo[a,j]xanthene.
The obtained results allowing us to determine the optimum amount of catalyst are presented in Table 1. In this reaction, the best results were obtained using 10 mol% of catalyst (as can be seen from Table 1, entry 4), while a higher amount of catalyst did not affect reaction times and yields (Table 1, entry 6).
Synthesis of 14-(4-chloro-3-nitrophenyl)14H-dibenzo[a,j]xanthene with different amounts of catalyst.
| Entry | Catalyst loading (mol%) | Time (min) | Yielda (%) |
| 1 | – | 120 | 0 |
| 2 | 2 | 25 | 30 |
| 3 | 5 | 20 | 65 |
| 4 | 10 | 8 | 95 |
| 5 | 15 | 7 | 95 |
| 6 | 20 | 7 | 95 |
a Isolated yield.
After optimization of the amount of catalyst, we studied the reaction of 2-naphthol, 4-chloro-3-nitrobenzaldehyde as a simple model by using an optimum amount of [H-NMP][HSO4] at different temperatures under solvent-free conditions (Table 2). It was found that the best result was obtained when the reaction was carried out at 110 °C in the presence of 10 mol% of ionic liquid (Table 2, entry 5).
The synthesis of 14-(4-Chloro-3-nitrophenyl)14H-dibenzo[a,j] xanthene under different temperaturesa.
| Entry | Temperature | Yieldb (%) |
| 1 | 50 | 20 |
| 2 | 70 | 50 |
| 3 | 90 | 70 |
| 4 | 100 | 80 |
| 5 | 110 | 95 |
| 6 | 120 | 95 |
a The reaction was carried out in the presence of 10 mol% of ionic liquid as a catalyst.
b Isolated yield.
The efficiency of [H-NMP][HSO4] catalyst in comparison with that of various catalysts, including some ionic liquids, was also examined (Table 3). [H-NMP][HSO4] was found to be the most efficient of all the catalysts tested, in terms of reaction time, yield, and temperature (Table 3, entry 1 in compare with entries 2–7 for other catalysts and entries 8–10 for other ionic liquids).
Synthesis of 14-(4-nitrophenyl)-14H-dibenzo[a,j] xanthene with different catalysts.
| Entry | Catalyst | Time (min) | Yield (%) | Temperature (°C) | Ref |
| 1 | [H-NMP][HSO4] | 10 | 96 | 110 | – |
| 2 | Cellulose sulfuric acid | 120 | 90 | 110 | [34] |
| 3 | PFPAT | 180 | 96 | r.t | [1a] |
| 4 | Boric acid | 180 | 98 | 120 | [35] |
| 5 | SiO2–Pr–SO3H | 40 | 98 | 125 | [32] |
| 6 | Iodine | 150 | 85 | 90 | [36] |
| 7 | H2SO4/SiO2 | 60 | 97 | 125 | [37] |
| 8 | [Et3N–SO3H]Cl | 25 | 97 | 120 | [38] |
| 9 | [BMIm][BF4]–Mg(BF4)2 | 30 | 98 | 80 | [39] |
| 10 | [Hmim][HSO4] | 30 | 90 | 125 | [40] |
After optimization of the reaction conditions, the reaction of 2-naphthol with several aldehydes was carried out according to the general experimental procedure (Scheme 2). The corresponding products are summarized in Table 4. As can be seen in this Table, the best activity of aromatic aldehydes was shown with an electron-withdrawing group (such as –NO2) in para and meta positions. This can activate the carbon atom of the carbonyl group for the nucleophilic attack of the α position of 2-naphthol. The presence of an electron-donating group (such as–OH) decreased both the reaction rate and the yield of product, which can be explained by an inactivation of the carbonyl group via the resonance resulting from the non-bonded electron pairs of the OH group (Table 4, entry 11).
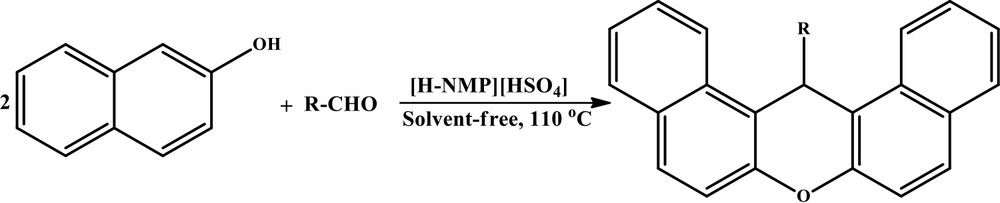
Synthesis of 14-aryl-14H-dibenzo[a,j]-xanthenes.
One-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using a catalytic amount of [H-NMP][HSO4] as a Brønsted acidic ionic liquid under solvent-free conditions at 110 °C.
| Entry | Aldehyde | Product | Time (min) | Yielda | Mp (°C) | Mp (°C) [Ref.] |
| Found | Reported | |||||
| 1 | 12 | 94 | 183–184 | 182–183 [31] | ||
| 2 | 20 | 87 | 212–213 | 214–215 [31] | ||
| 3 | 20 | 96 | 289–290 | 287–288 [31] | ||
| 4 | 17 | 90 | 229–230 | 227–228 [31] | ||
| 5 | 20 | 91 | 250–253 | – | ||
| 6 | 15 | 87 | 213–214 | 214–215 [31] | ||
| 7 | 10 | 95 | 212–213 | 210–211 [31] | ||
| 8 | 10 | 96 | 308–309 | 310–311 [31] | ||
| 9 | 8 | 95 | 232–234 | – | ||
| 10 | 20 | 92 | 240–241 | 242–243 [32] | ||
| 11 | 20 | 80 | 138–140 | 139–140 [33] | ||
| 12 | 17 | 87 | 197–198 | 198 [42] | ||
| 13 | 19 | 85 | 172–173 | 174–176 [41] | ||
| 14 | 17 | 85 | 228–230 | 227–228 [31] | ||
| 15 | 25 | 82 | 204–205 | 202–203 [31] |
a Isolated yield.
In this study, 14-aryl-14H-dibenzo[a,j]xanthenes were prepared using the model reaction of 2-naphthole and various aromatic aldehydes in the presence of a homogeneous catalytic amount of [H-NMP][HSO4] (10 mol %) at 110 °C under solvent-free conditions. After separation from the product, the catalyst is removable as a by-product by washing with water, and easily recycled to catalyze the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes with excellent yields. The product yields (%) were calculated as the amount of experimental xanthene (g)/amount of theoretical xanthene (g) × 100.
We also examined the model reaction being conducted with concentrated sulfuric acid (98% w/w) as a catalyst under the same conditions to compare ionic liquids with hazardous H2SO4 (0.5 mmol) under solvent-free conditions at 110 °C. The reaction was completed in 65 min with 70% yield. The corrosive properties of sulfuric acid are accentuated by the highly exothermic reactions in which it is implicated. The danger is greater with concentrated sulfuric acid and also this is not good from the green chemistry viewpoint. The catalyst cannot be reused and recycled for further reactions and is not environmentally safe.
In order to reuse the catalyst after the full separation of the solid products (3a) from water, the water-containing Brønsted acidic ionic liquids (BAILs is soluble in water) were evaporated under vacuum and the catalyst was recycled several times without any decrease in its catalytic activity; the yields ranged from 94% to 89% (Fig. 1).

Reusability of the catalyst.
The structures of the products were confirmed by spectroscopic and physical determinations such as IR, 1H NMR, 13C NMR, and UV. The infrared spectra of the 14-(phenyl)14H-dibenzo[a,j]xanthene exhibit a band at 1248 cm−1, assigned to ν(C–O). In the 1H NMR spectra, the signal around δ = 6.98–8.41 ppm is assigned to the protons of the aromatic rings [ν(CH = CH)], the signal of the aliphatic rings ν(CH) appears at 6.5 ppm, and in the 13C NMR spectrum the signal around δ = 117.34–148.74 ppm is assigned to the carbons of the aromatic rings (CH = CH), and the signal of the aliphatic rings ν(CH) is observed at 38.07 ppm.
2.1 Proposed reaction mechanism
A proposed mechanism for the formation of 14-aryl-14H-dibenzo[a,j]xanthenes is presented in Scheme 3 [34]. As can be seen, the Brønsted acidic ionic liquid with hydrogen bonding can activate the carbonyl group of the aldehyde to decrease the energy of the transition state. Then 2-naphthol attacks the carbonyl group of the activated aldehyde to afford intermediate (1), and the reaction likely proceeds via initial formation of the carbonium ion (2). Then, the oxonium species (4) is formed in the cyclization reaction of intermediate (3), which undergoes dehydration to afford the desired product (5).

The proposed reaction mechanism.
3 Conclusions
In this new method, we have described the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using 2-naphthol with different kinds of aromatic aldehydes. The use of [H-NMP][HSO4] as a highly efficient, inexpensive, non-toxic, easy-workup, and reusable catalyst makes the present procedure eco-friendly and economically acceptable. Furthermore, its low cost, its thermal conditions, the fact that the catalyst is easy to recycle, the short reaction times needed make this solvent-free method relevant from the green chemistry viewpoint.
4 Experimental
4.1 Materials
All commercially available reagents were used without further purification and purchased from Merck Chemicals Ltd in high purity. The used solvents were purified by standard procedures.
4.2 Apparatus
IR spectra were obtained as KBr pellets on a Perkin Elmer 781 spectrophotometer and on a Nicolet Impact 400 FTIR spectrophotometer. 1H NMR and 13C NMR were recorded in CDCl3 as the solvent on a Bruker DRX-400 spectrometer with tetramethylsilane as an internal reference. UV–vis spectra obtained with a PerkinElmer 550 were recorded in CDCl3 solvents. Melting points, obtained with a Yanagimoto micro melting point apparatus, are uncorrected. The purity determination of the substrates and reaction monitoring were accomplished by TLC on silica-gel polygram SILG/UV 254 plates (Merck).
4.3 General procedure for the synthesis of [H-NMP][HSO4]
1-Methyl-2-pyrolidone (0.2 mol) was charged into a 250-mL three-necked flask containing a magnetic stirrer. Then an equimolar amount of concentrated sulfuric acid (98 wt %) was added dropwise slowly into the flask at 80 °C for 12 h. The mixture was washed by ether three times to remove non-ionic residues and dried under vacuum by a rotary evaporator to obtain the viscous clear [H-NMP][HSO4] compound [30]. The pH of the resulted ionic liquid (10% w/v) was determined and the obtained pH was equal to 1.2.
4.4 A typical procedure for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes
A mixture of aldehyde (1 mmol, 0.15 g), 2-naphthol (2 mmol, 0.288 g), [H-NMP][HSO4] (10 mol %, 0.19 g) was left under solvent-free conditions at 110 °C for the appropriate time indicated in Table 2. The progress of the reactions was monitored by TLC (ethyl acetate/petroleum ether 3:7). After completion of the reaction, the reaction mixture was cooled, and the mixture was extracted with (2 × 10 ml of CH2Cl2) and [H-NMP][HSO4] was removed as a by-product by washing with water. The extract was dried on CaCl2 and evaporated to give the crude product, which was then purified by recrystallization from ethanol. Products were characterized by comparison of their physical and spectral data with those of authentic samples [31,32].
14-(Phenyl)14H-dibenzo[a,j]xanthene (3a): pale yellow solid, mp 183–184 °C (mp 182–183 °C) [31], IR (KBr)/ν(cm−1): 3061, 1624, 1592, 1513, 1458, 1410, 1248, 1078, 962, 805, 744; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.50 (s, 1H, CH), 6.98–7.01 (t, 1H, J = 7.6, Ar), 7.13–7.17 (t, 2H, J = 7.6, Ar), 7.40–7.43 (t, 2H, J = 7.6, Ar), 7.48–7.54 (m, 4H, Ar), 7.56–7.60 (t, 2H, J = 7.2, Ar), 7.81–7.92 (d, 2H, J = 8.8, Ar), 7.82–7.84 (d, 2H, J = 8.0, Ar), 8.39–8.41 (d, 2H, J = 8.8, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 148.74, 145.03, 131,48, 131.07, 128.88, 128.82, 128.5, 128.29, 126.81, 126.41, 124.26, 122.72, 118.04, 117.34, 38.07, UV (CH2Cl2)/λmax (nm): 244, 230.
14-(4-Chlorophenyl)14H-dibenzo[a,j]xanthene (3b): yellow solid, mp 289–290 °C (mp 287–288 °C) [31], IR (KBr)/ν(cm−1): 3067, 1624, 1591, 1514, 1585, 1431, 1243, 1085, 961, 808, 743; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.48 (s, 1H, CH), 7.10–7.12 (d, 2H, J = 8.4, Ar), 7. 41–7.47 (m, 4H, Ar), 7.48–7.50 (d, 2H, J = 8.8, Ar), 7.57–7.61 (t, 2H, J = 7.6, Ar), 7.80–7.82 (d, 2H, J = 8.8, Ar), 7.84–7.86 (d, 2H, J = 8.0, Ar), 8.32–8.34 (d, 2H, J = 8.4, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 148.51, 145.01, 131.39, 131.31, 131.20, 130.18, 129.83, 129.19, 128.91, 127.60, 125.14, 123.77, 118.21, 117.50, 39.87; UV (CH2Cl2)/λmax (nm): 244, 230.
14-(2-Chlorophenyl)14H-dibenzo[a,j]xanthene (3c): white solid, mp 212–213 °C (mp 214–215 °C) [31], IR (KBr)/ν(cm−1): 3059, 1625, 1592, 1461, 1400, 1243, 1032, 959,808; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.81 (s, 1H, CH), 6.92 (m, 2H, Ar), 7.40–7.44 (m, 3H, Ar), 7.48–7.51 (d, 2H, J = 8.8, Ar), 7.61–7.64 (m, 5H, Ar), 8.74–8.76 (d, 2H, J = 8.4, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 148.95, 143.59, 131.83, 130.91, 130.15, 129.61, 129.06, 128.67, 127.95, 127.88, 126.94, 124.45, 123.49, 118.12, 118.01, 34.65; UV (CH2Cl2)/λmax (nm): 246, 230.
14-(2-Nitrophenyl)14H-dibenzo[a,j]xanthene (3d): yellow solid, mp 213–214 °C (mp 214–215 °C) [31], IR (KBr)/ν(cm−1): 3057, 1625, 1593, 1500, 1397, 1346, 1305, 817, 750; 1H NMR (CDCl3, 400 MHz)/δ ppm: 7.08 (t, 1H, J = 8.0, CH), 7.23–7.27 (t, 1H, J = 7.2, Ar), 7.42–7.46 (t, 2H, J = 7.2, Ar), 7.42–7.49 (m, 3H, Ar), 7.57–7.63 (m, 3H, Ar), 7.81–7.84 (m, 4H, Ar), 8.53–8.55 (d, 2H, J = 8.4, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 149.4, 147.03, 140.87, 134.14, 132.26, 131.72, 130.97, 129.47, 128.73, 127.59, 127.41, 124,92, 124,67, 122.58, 118.03, 117.58; UV (CH2Cl2)/λmax (nm): 244, 230.
14-(3-Nitrophenyl)14H-dibenzo[a,j]xanthene (3e): yellow solid, mp 212–213 °C (mp 210–211 °C) [31], IR (KBr)/ν(cm−1): 3075, 1592, 1527, 1500, 1397, 1346, 1252, 1080, 958, 812, 748; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.62 (s, 1H, CH), 7.28–7.32 (t, 1H, J = 7.6, Ar), 7.43–7.47 (t, 2H, J = 7.6, Ar), 7.51–7.53 (d, 2H, J = 8.8, Ar), 7.60–7.64 (t, 2H, J = 7.2, Ar), 7.81–7.87 (m, 6H, Ar), 8.30–8.32 (d, 2H, J = 8.4, Ar), 8.42 (s, 1H, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 148.77, 148.21, 146.94, 134.27, 131.04, 129.57, 129.49, 129.07, 127.25, 124.59, 122.71, 122.04, 121.69, 118.13, 115.87, 37.71; UV (CH2Cl2)/λmax (nm): 246, 228.
14-(4-Nitrophenyl)14H-dibenzo[a,j]xanthene (3f): pale yellow solid, mp 308–309 °C (mp 310–311 °C) [31], IR (KBr)/ν(cm−1): 3068, 1593, 1515, 1341, 1243, 824, 745; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.61 (s, 1H, CH), 7.43–7.47 (t, 2H, Ar), 7.50–7.53 (d, 2H, Ar), 7.59–7.63 (t, 2H, Ar), 7.68–7.70 (d, 2H, Ar), 7.84–7.89 (t, 4H, Ar), 8.00–8.02 (d, 2H, Ar), 8.28–8.30 (d, 2H, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 152.01, 148.77, 146.29, 131,06, 129.6, 129.07, 128.97, 127.19, 124.59, 123.87, 122.04, 118.07, 115.76, 37.87; UV (CH2Cl2)/λmax (nm): 248, 228.
14-(4-Chloro-3-nitrophenyl)14H-dibenzo[a,j]xanthene (3 g): yellow solid, mp 232–234 °C, IR (KBr)/ν(cm−1): 3054, 1623, 1592, 1530, 1462, 1350, 1246, 952, 812, 742; 1H NMR (CDCl3, 400 MHz)/δ ppm: 7.27 (s, 1H, CH), 7.29–7.31 (d, 1H, J = 8.4, Ar), 7.45–7.52 (m, 4H, Ar), 7.62–7.65 (m, 3H, Ar), 7.84–7.89 (t, 4H, J = 9.2, Ar), 8.03–8.04 (d, 1H, J = 2.0, Ar), 8.24–8.26 (d, 2H, J = 8.4, Ar); UV (CH2Cl2)/λmax (nm): 246, 232.
14-(2,4-Dichlorophenyl)-14H-dibenzo[a.j]xanthene (3 h): pale yellow solid, mp 229–230 °C (mp 227–228 °C) [31], IR (KBr)/ν(cm−1): 3061, 1622, 1591, 1514, 1464, 1430, 1401, 1247, 1103, 1072, 960, 864, 836, 807, 743; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.77 (s, 1H, CH), 6.90–6.92 (d, 1H, Ar), 7.28–7.32 (m, 2H, Ar), 7.43–7.50 (m, 4H, Ar), 7.61–7.65 (t, 2H, J = 7.6, Ar), 7.81–7.85 (t, 4H, J = 9.0, Ar), 8.65–8.67 (d, 2H, J = 8.0, Ar); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 148.9, 142.25, 132.8, 132,71, 132.6, 130.92, 130.59, 129. 3, 128.78, 128.41, 127.06, 124.57, 123.17, 118.11, 117.46, 34.24; UV (CH2Cl2)/λmax (nm): 248, 230.
14-(2,3-Dichlorophenyl)-14H-dibenzo[a.j]xanthene (3i): white solid, mp 250–253 °C, IR (KBr)/υ(cm−1): 3057, 1625, 1593, 1514, 1459, 1405, 1401, 1251, 1107, 1070, 962, 869, 813, 807, 746; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.85 (s, 1H, CH), 6.87–6.89 (d, 1H, J = 8.0, Ar); 7.09–7.11 (d, 2H, J = 8.8, Ar), 7.32–7.35 (d, 1H, J = 9.2, Ar), 7.44–7.51 (m, 4H, Ar), 7.62–7.85 (t, 4H, J = 9.2, Ar), 8.69–8.67 (d, 2H, J = 8.4, Ar); UV (CH2Cl2)/λmax (nm): 248, 230.
14-(3-Hydroxyphenyl)-14H-dibenzo[a,j]xanthene (3j): Pale Pink solid, mp 172–173 °C (mp 174–176 °C) [32]; IR (KBr)/ν(cm−1): 3068,1593, 1513, 1401, 1251, 1242, 810; 1H NMR (CDCl3, 400 MHz)/δ ppm: 3.58 (s, 3 H), 6.42 (s, 1 H), 6.47 (d, 1H, J = 8.2), 7.05 (t, 2H, J = 8.4), 7.13 (d, J = 7.5 Hz, 1 H), 7.39 (t, 2H, J = 7.2), 7.43 (d, 2H, J = 9.0), 7.53 (t, 2H, J = 7.6), 7.72–7.82 (m, 4H), 8.36 (d, 2H, J = 8.7).
14-(4-Hydroxyphenyl)-14H-dibenzo[a,j]xanthene (3k): Pink solid, mp 137–138 °C (mp 138–140 °C) [33]; IR (KBr)/ν(cm−1): 3404,1592, 1511, 1401, 1250, 1242, 816; 1H NMR (CDCl3, 400 MHz)/δ ppm:4.97 (br s, 1H, OH), 6.43(s, 1H, CH), 6.55–8.36 (m, 16H, Ar-H); 13C NMR/(CDCl3, 100 MHz)/δ ppm: 37.41, 115.70, 118.00, 118.39, 123.10, 124.62, 127.23, 129.11, 129.20, 129.78, 131.53, 131.81, 137.90, 149.11, 154.24.
14-(3-Methoxy)14H-dibenzo[a,j]xanthene (3l): Pale pink solid, mp 173–174 °C (mp 174–176 °C) [40]; IR (KBr)/ν(cm−1): 3067, 3014, 2932, 1582, 1454, 1431, 1400, 1247, 1050, 964, 806, 743; 1H NMR (CDCl3, 400 MHz)/δ ppm: 3.63 (s, 3H, OCH3), 6.45 (s, 1H, CH), 6.51 (dd, 2H, J = 7.5, ArH), 7.02–7.46 (m, 6H, ArH), 7.55 (t, 2H, J = 7.5, ArH), 7.78 (t, 4H, J = 7.5, ArH), 8.39 (d, 2H, J = 7.5 Hz, ArH).
14-(4-Methoxy)14H-dibenzo[a,j]xanthene (3 m): pink solid, mp 204–205 °C (mp 202–203 °C) [31], IR (KBr)/ν(cm−1): 3062, 1594, 1509, 1458, 1398, 1247, 960, 814, 744; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.45 (s, 1H, CH), 6.67–6.69 (d, 2H, J = 8.4, Ar), 7.40–7.44 (m, 4H, Ar), 7.47–7.49 (d, 2H, J = 8.8, Ar), 7.56–7.60 (t, 2H, J = 7.2, Ar), 7.78–7.80 (d, 2H, J = 8.8, Ar), 7.82–8.84 (d, 2H, J = 8.0, Ar), 8.38–8.40 (d, 2H, J = 8.4, Ar); 13C NMR/(CDCl3, 100)/δ ppm: 37.14, 55.06, 113.87, 117.55, 118.04, 122.73,123.60, 124.25, 126.40, 126.79, 128.77, 128.84, 129.20, 131.11, 131.45, 137.42, 148.68, 157.86; UV (CH2Cl2)/λmax (nm): 246, 230.
14-(3-Methyl)14H-dibenzo[a,j]xanthene (3n): White solid, mp 196–197 °C (mp 197–198 °C) [41], IR (KBr)/ν(cm−1): 3018, 2922, 1623, 1591, 1513, 1457, 1401, 1249, 961, 806, 806, 743; 1H NMR (CDCl3, 400 MHz)/δ ppm: 2.14 (s, 3H, CH3), 6.42 (s, 1H, CH), 6.76 (d, 1H, J = 7.5, ArH), 7.03 (t, 1H, J = 7.5 Hz, ArH), 7.20–7.41 (m, 4H, ArH), 7.43 (d, 2H, J = 8.75, ArH), 7.54 (t, 2H, J = 7.5, ArH), 7.75 (t, 4H, J = 10, ArH), 8.35 (d, 2H, J = 7.5, ArH).
14-(4-Methyl)14H-dibenzo[a,j]xanthene (3o): pale yellow solid, mp 228–230 °C (mp 227–228 °C) [31], IR (KBr)/ν(cm−1): 3069, 1623, 1591, 1531, 1458, 1399, 1244, 961, 810, 741; 1H NMR (CDCl3, 400 MHz)/δ ppm: 6.46 (s, 1H, CH), 6.95–6.97 (d, 2H, J = 8.0, Ar), 7.39–7.43 (m, 4H, Ar), 7.47–7.50 (d, 2H, J = 9.2, Ar), 7.56–7.60 (t, 2H, J = 9.2, Ar), 7.78–7.80 (d, 2H, J = 8.8, Ar), 7.82–8.84 (d, 2H, J = 8.0, Ar), 8.39–8.41 (d, 2H, J = 8.8, Ar); 13C NMR/(CDCl3, 100)/δ ppm: 20.91, 37.64, 117.46, 118.02, 122.72, 124.22, 126.77, 128.11, 128.77, 128.79, 129.19, 131.08, 131.46, 135.91, 142.14, 148.68; UV (CH2Cl2)/λmax (nm): 247, 232.
Acknowledgement
The authors are grateful to University of Kashan for supporting this work by Grant No. 159148/9.


