1 Introduction
Anhydrous emulsions, also known as non-aqueous or oil-in-oil emulsions, started to be developed in the early 1960s [1–4] and have since gained great interest as outlined in several recent review articles [5–7].
It was shown for the first time, by our group, that block copolymers are efficient steric stabilizers for non-aqueous emulsions formed by two non-miscible organic solvents, such as hexane/dimethylformamide (DMF) or cyclohexane/acetonitrile [8]. It could be demonstrated that, in contrast to aqueous emulsions where the stability is in general provided by low molecular weight ionic or non-ionic surfactants, anhydrous emulsions require preferably polymeric surfactants, such as block- or graft-copolymers.
As outlined by Crespy and Landfester [7], one of the most interesting features of anhydrous emulsions is the fact that they allow to perform any kinds of water-sensitive chemical reactions. Moreover, this type of emulsions has a broad application range in pharmaceutical and cosmetic formulations for handling of unstable or scarcely soluble drugs in controlled release systems and transdermal preparations. Of particular interest are therefore those emulsions comprising biocompatible oil components, as for instance, paraffin oil, ethylene glycols, silicon and triglyceride oils [9–12].
In this context, we recently became interested in block copolymers stabilized anhydrous emulsions based on poly(ethylene glycol) 400 (PEG 400) and Miglyol 812, a typical biocompatible and enzyme degradable medium chain triglyceride (MCT) [12]. Such drug loaded emulsions, with PEG 400 as dispersed phase stabilized by poly(butadiene)-b-poly(2-vinylpyridine) (PBut-b-P2VP) copolymers were developed in view of their topical application possibilities.
As an extension of this study, it was of interest to examine the colloidal characteristics of Miglyol 812-in-PEG 400 emulsions stabilized by a PBut-b-P2VP copolymer. Up to now, and to the best of our knowledge, only Amemiya et al. [13,14] have prepared this type of anhydrous emulsions of rather limited stability by using low molecular weight non-ionic surfactants.
An original aspect of the present study is that Miglyol 812/PEG 400 anhydrous emulsions, with PEG 400 as a water miscible continuous phase, opens the way to the design of water dispersible non-aqueous submicron sized emulsions, with the major advantage that no further addition of a stabilizer would be necessary.
In fact, the anhydrous emulsion is sterically stabilized by the PBut-b-P2VP copolymer and after water addition at low pH, the protonated P2VP sequence of the copolymer provides the electro-steric stabilization of the aqueous emulsion.
This concept of stimuli-responsive stabilization mechanism of emulsions could be of interest for drug release applications. Thus, a hydrolytically instable and/or scarcely water-soluble drug could be loaded in the anhydrous emulsions in order to provide its storage stability. Then just before administration, its dispersion would be possible in an aqueous medium at a pH around 1–2, for instance, like that existing in the stomach.
Therefore, the objective of this study was to examine in a first step the particle size, the stability and the rheological characteristics of the anhydrous Miglyol 812/PEG 400 emulsions as a function of the PBut-b-P2VP copolymer concentration. In a second step, the dispersion of these non-aqueous systems in water and the characteristics of the final aqueous emulsions will be investigated.
2 Materials and methods
2.1 Materials
The poly(butadiene)-b-poly(2-vinylpyridine) (PBut-b-P2VP) copolymer was synthesized by living anionic polymerization in THF according to the technique developed by Fontanille and Sigwalt [15]. The copolymer used in this study has the following characteristics: Mn (PBut) = 6900 g/mol; Mn (P2VP) = 5300 g/mol; and an polydispersity index Mw/Mn of 1.06. The configuration of the PBut sequence corresponds to 90 mol% 1,2 and 10 mol% 1,4 trans. In this study, the block copolymer will be designated as PBut0.55-b-P2VP0.45, where 0.55 and 0.45 are the PBut and the P2VP weight fractions.
Medium chain triglyceride (Miglyol 812), which is a blend of caprylic and capric triglyceride, was purchased from Axo Industry (Belgium) and it is characterised by a viscosity of 30 mPa·s, a refractive index of 1.45 and a specific mass of 0.95 g/cm3 at 20 °C. PEG 400, purchased from Fluka, has a refractive index of 1.47, a specific mass of 1.128 g/cm3 and a viscosity of 120 mPa·s.
2.2 Emulsion preparation
Non-aqueous emulsions, with PEG 400 as continuous phase and Miglyol 812 as dispersed phase, were prepared by varying the copolymer concentrations from 1 to 5 wt% with respect to the total emulsion volume, which has been fixed at 100 mL. The composition of the Miglyol 812/PEG 400 emulsions was 30/70 vol. The PBut-b-P2VP copolymer, used as polymeric stabilizer, was dissolved at 70 °C in PEG 400 at the desired concentrations. After complete dissolution, the Miglyol 812, also kept at 70 °C, was dispersed in the continuous PEG 400 phase with an addition rate of 2 mL/min under agitation at 20 000 rpm provided by an Ultra-Turrax T18 (IKA, Germany) homogenizer. In order to obtain submicron droplet sizes, this emulsion was further homogenized, at the same speed, for 10 min. At this stage, the emulsion temperature was around 60 °C and then, the temperature was slowly decreased to room temperature.
For the preparation of oil-in-water emulsions, two methods were used. In the first one, 10 mL of a non-aqueous emulsion were added to 10 mL H2O at pH 1 and the emulsification was carried out by gentle shaking. In the second one, the water was added to the non-aqueous emulsion and then agitated also by gentle shaking. Since, within the experimental error limits, identical droplets diameters were obtained for both methods, only the second one was used in the present study. After dispersion in acidic medium, the emulsion composition was 15/85 vol. with Miglyol 815 dispersed in a continuous phase formed by the mixture of PEG 400 and H2O at pH 1.
The stability of the emulsions was determined by following the creaming and/or sedimentation as a function of time. A characteristic stability indication is the t15 value, which is the time where 15% of the PEG 400 or of the Miglyol 812 volume has phase separated.
2.3 Dynamic light scattering (DLS)
DLS measurements, carried out with a Malvern Nano-ZS Zetasizer equipped with a 4 mW He–Ne laser operating at a wavelength of 532 nm, were made at a scattering angle θ = 173° and at a fixed temperature of 20 °C. The optics of this instrument is not in contact with the sample and hence, the detection optics is non-invasive. This so-called non-invasive back-scattering (NIBS) technology extends the range of sizes and concentration of samples that can be measured. Moreover, it reduces the multiple scattering effects by minimising the path length through which the scattered light has to pass. With the NIBS technology, samples up to a concentration of 40 w/v% can be measured. Using the Stokes–Einstein equation, with the viscosity of the continuous phase, the software package of the instrument calculates the hydrodynamic diameter (volume average) DV, the Z-average diameter, which is an intensity weighted average size and the polydispersity index (PDI) of the sample. The “data quality report” incorporated in the software indicated “good quality” for all the obtained data. For each experiment, the average of five consecutive measurements is indicated in tables and figures.
2.4 Rheological characterization
The rheological properties of emulsions were analysed using a rheometer AR 2000Ex-TA instrument with a cone and plate geometry sensor (40 mm diameter, 2° cone angle). The measurements were carried out at 20 °C with a gap distance of 0.05 mm. Prior to all measurements, the samples were treated at a shear rate of 50/s for 30 s and equilibrated for 2 min at 20 °C to standardize their history.
For the determination of viscoelastic properties, a frequency sweep test was performed at 20 °C with an applied torque of 10 μN·m and an excitation frequency in the ω range between 0.01 and 10 Hz. Pre-shear and equilibration steps were again applied prior to each test as described previously.
3 Results and discussion
3.1 Characteristics of the non-aqueous emulsions
In our preceding study [12], it was shown that PEG 400 and Miglyol 812 are almost completely immiscible as the reciprocal solubilities, e.g. of PEG 400 in Miglyol 812 and Miglyol 812-in-PEG 400, are below 1% vol. at room temperature. In addition to this requirement for the preparation of emulsions, it was demonstrated that the PBut and the P2VP sequences of the block copolymer are selectively soluble in Miglyol 812 and PEG 400, respectively [12]. Depending on its composition and molecular weight, this type of biocompatible block copolymer is therefore an efficient emulsifier and stabilizer of Miglyol 812/PEG 400 emulsions.
3.1.1 Emulsion stability and droplet size
Droplet size and size distribution, which have a major influence on the emulsion characteristics, were determined by DLS as a function of the copolymer concentration for a Miglyol 812/PEG 400-volume phase ratio of 30/70.
In Table 1 are listed, as a function of the copolymer concentration, the average particle sizes of the droplet, such as the Z-average and the volume average diameter Dv, as well as the polydispersity index (PDI). The stability of the emulsions is estimated by the t15 value, defined in the experimental section.
Z-average droplets diameter, Dv, PDI and stability index t15 for freshly prepared non-aqueous emulsions as a function of copolymer concentrations at 20 °C.
| [PBut0.55-b-P2VP0.45] (wt%) | Z-average (nm) | Dv (nm) | PDI | t15 (h) |
| 1 | 201 | 198 | 0.140 | 12 |
| 3 | 133 | 130 | 0.140 | 20 |
| 140a | 135a | 0.200a | – | |
| 5 | 123 | 121 | 0.150 | 25 |
a Re-dispersion by gentle shaking after two months of storage.
The results shown in Table 1 confirm that submicron sized emulsions in the range of 100 to 200 nm are obtained and that, as expected, the droplet size decreases with increasing copolymer concentration and consequently the t15 value, which is representative of the stability, increases as a function of this parameter. Moreover, it has been noticed that the re-dispersion by gentle shaking is possible even after storage times of 2 months at room temperature with no major modification of the initial emulsion characteristics.
3.1.2 Rheological behaviour
The rheological characteristics of oil–water emulsions have been studied quite extensively over the last decades and it could be shown that they are influenced by quite a number of parameters, such as the viscosity of two phases in presence, the volume fraction of dispersed phase, the droplet size and size distribution and the viscoelastic properties of the emulsifier film adsorbed at the liquid/liquid interface [16]. On the contrary, the rheology of non-aqueous emulsions was only scarcely studied up to now. It was therefore of interest to examine the viscosity and the viscoelastic characteristics of the present Miglyol 812/PEG 400 emulsions. For instance, the viscosity in function of shear rate provides important information concerning the flow behaviour of the emulsions and their shear stability, which are required for practical applications.
Fig. 1 shows the viscosity of PEG 400 and the Miglyol 812/PEG 400 emulsions as a function of shear rate at different copolymer concentrations.

Viscosity vs. shear rate at 20 °C for PEG 400 and Miglyol 812/PEG 400 emulsions.
If PEG 400, the continuous phase of the emulsions, shows a typical Newtonian behaviour, a slight tendency of shear thinning with increasing shear rate is observed for all the emulsion samples. A viscosity increase as a function of the copolymer concentration can furthermore be observed as a consequence of the droplet size reduction. A quantitative evaluation of the shear stability may be given by the ratio η400/η100, corresponding to the ratio of the apparent viscosities at 400 and 100 s−1. An almost constant value of 0.78 ± 0.02 was found for the three emulsion samples.
These results were completed by the determination of the viscoelastic characteristics of the emulsions and in particular of the loss modulus G′′, which is dominant over the storage modulus G′. The values of G′ are in the range of 4.1 to 8.4 Pa and are given in Table 3.
G′ and G′′ values for non-aqueous and for oil-in-water emulsions at a frequency of 10 Hz and 20 °C.
| [PBut0.55-b-P2VP0.45] (wt%) | G′(Pa) | G′′ (Pa) | ||
| Non-aqueous emulsions | Oil-in-water emulsions | Non-aqueous emulsions | Oil-in-water emulsions | |
| 1 | 4.1 | (0.8)a | 14.8 | (1.1)a |
| 3 | 5.4 | 1.1 | 19.8 | 1.8 |
| 5 | 8.4 | 1.3 | 32.5 | 2.4 |
a Relative large error range.
The G′′ values, determined in the angular frequency range of 0.01 to 10 Hz, are given in Fig. 2 for the three emulsion samples.
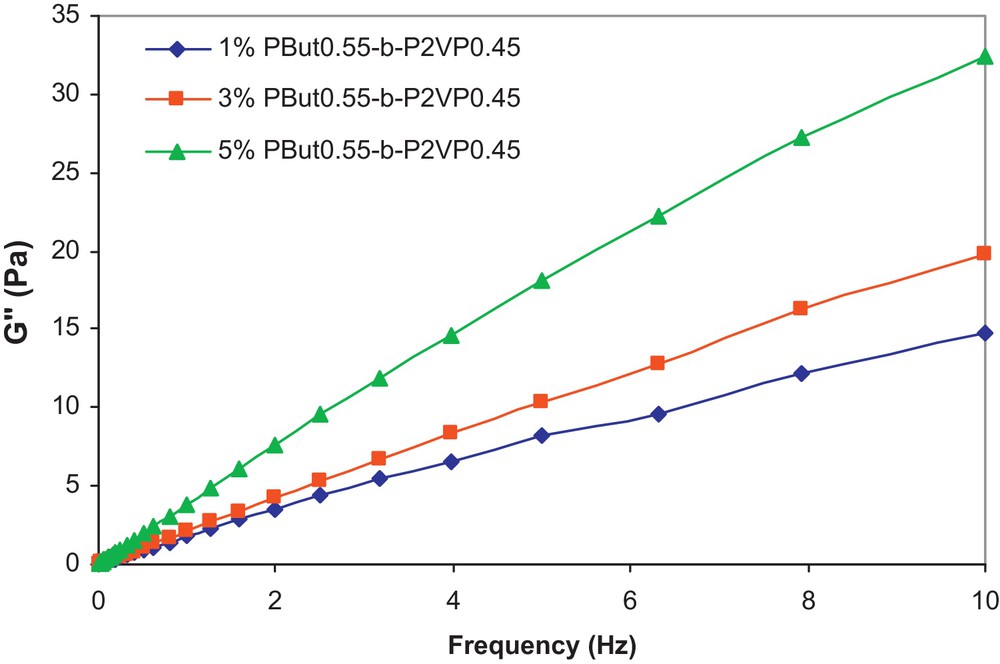
G′′ vs frequency at 20 °C for Miglyol 812/PEG 400 emulsions.
From this figure, it can be noticed that the G′′ values of the emulsions increase with the copolymer concentration. Moreover, as indicated by Niraula et al. [17], it is of interest for cosmetic applications of emulsions to examine the variation of G′′ versus G′, at different frequencies, which is indicative of the stability and the spreadability of a given formulation. This correlation is shown in Fig. 3 as a function of copolymer concentration and frequency.
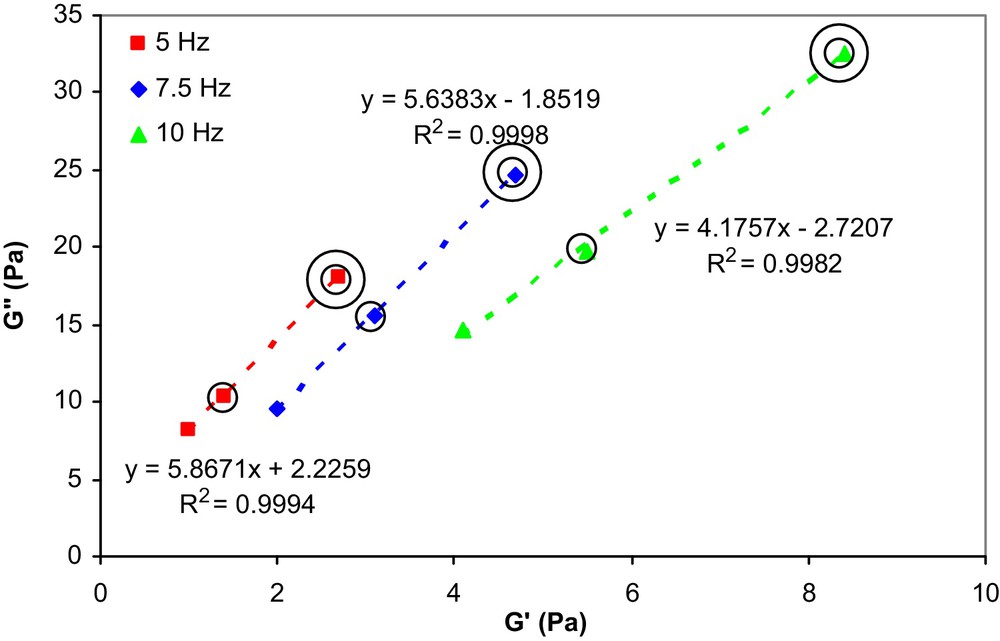
G′′ vs. G′ as a function of copolymer concentration and frequency at 20 °C for Miglyol 812/PEG 400 emulsions stabilized by 1 (without circle), 3 (one circle) and 5 (two circles) wt% copolymer, respectively.
From the fact that the G′′ response is higher than G′ at all measured frequencies and copolymer concentrations, it is evident that the viscous properties dominate over the elastic properties of these emulsions which is a practical advantage concerning their spreadability.
3.2 Water diluted anhydrous emulsions
An original aspect of this study is that the previous Miglyol 812/PEG 400 anhydrous emulsions, with PEG as a water miscible continuous phase, are directly water dispersible without phase inversion of the emulsions. An additional advantage of this concept is due to the fact that the PBut-b-P2VP copolymer, initially used as the emulsifier and steric stabilizer of the non-aqueous emulsions, becomes, by protonation of the P2VP sequence, an electro-steric stabilizer for the Miglyol 812/(PEG 400-water) emulsions at low pH. This approach, schematically illustrated by Fig. 4, opens the way for the design of water dispersible non-aqueous emulsions, with the major advantage that no further addition of stabilizer would be necessary.

Schematic representation of the dilution of non-aqueous emulsion with water at pH = 1.
As a first point, it was necessary to verify if the dilution process involves any change in the droplet size of Miglyol 812 dispersed phase. In this dilution step, the viscosity of the PEG 400/water continuous phase (41/59 vol. ratio) was taken into account for the particle size calculation, according to the Stokes–Einstein equation. This value, 70 Pa·s, was determined as a function of the shear rate at 20 °C.
A typical example of the size distribution before and after water dilution is illustrated in Fig. 5.

Size distribution curves for the non-aqueous Miglyol 812/PEG 400 emulsion stabilized by 5 wt% copolymer and the corresponding water diluted, at pH = 1, oil-in-water emulsion stabilized by 2.5 wt% copolymer at 20 °C.
From this figure, it appears that the dilution process with water induces only a slight size increase of the Miglyol 812 droplets and of the polydispersity index. However, their overall size distribution remains in the submicron range.
The characteristics of the water diluted emulsions, such as droplet size, zeta potential and stability index t15 are summarized in Table 2.
Z-average droplets diameter, Dv, PDI, zeta potential ZP and stability index for freshly prepared oil-in-water emulsions as a function of copolymer concentrations at 20 °C.
| [PBut0.55-b-P2VP0.45]a (wt%) | Z-average (nm) | Dv (nm) | PDI | ZP (mv) | t15 (h) |
| 1 | (225)b | (227)b | (0.250)b | 5.5 | 1 |
| 3 | 206 | 210 | 0.200 | 9.5 | 3 |
| 5 | 189 | 201 | 0.250 | 12.3 | 5 |
a Copolymer concentration before dilution; after dilution the copolymer concentrations are 0.5, 1.5 and 2.5 wt% respectively.
b Relative large error range.
As already shown by the size distribution curves (see Fig. 5), these results, concerning the droplet size, confirm that a slight size increase could not be avoided in the dilution process, which leads to a decrease of the stabilizer concentration in the emulsion. Therefore, due to the limited stability of the oil-in-water emulsions stabilized with 0.5 wt% PBut-b-P2VP copolymer, their DLS characteristics present a relative large error range. Of practical interest, however, is the fact that the submicron size distribution, around 200 nm, is maintained after dilution with water at pH 1 for the other samples. Despite of the possible droplet–droplet interactions for such concentrated emulsions, the NIBS technology allows this type of comparative study.
Furthermore, the positive zeta potential, which increases with the copolymer concentration, is a direct proof that the P2VP sequence of the copolymer is at least partially protonated [18].
The rheological and viscoelastic characteristics of the water diluted emulsions were determined as a function of the shear rate and angular frequency. The shear stability of water diluted emulsions is illustrated in Fig. 6.

Viscosity vs shear rate at 20 °C for PEG 400-water (41/59 volume ratio) and water diluted emulsions.
From this figure, it can be observed that emulsions have a typical Newtonian behaviour, as indicated by the ratio η400/η100, which is close to 1. A viscosity reduction by a factor of around 10 is observed with respect to non-aqueous emulsions, as shown in Fig. 1.
The viscoelastic properties, G′ ad G′′ values, are listed in Table 3 for non-aqueous emulsions and for water diluted emulsions at a frequency of 10 Hz.
As a preliminary remark concerning this table, it has to be noticed that the direct comparison of non-aqueous and water diluted emulsions is somehow limited due to the fact that the dilution process involves mainly an increase in the volume fraction of the continuous phase and consequently a decrease of the dispersed one and of the copolymer concentration with respect to the total volume. These changes involve a reduction of the viscosity with respect to that of the precursor non-aqueous emulsions. A similar trend is observed for the viscoelastic characteristics G′ and G′′ of the oil-in-water emulsions, as shown in Table 3. Moreover, it turns out that both values of G′ and G′′ increase with the copolymer concentration and that G′′ values are slightly higher than the values of G′.
4 Conclusions
In this preliminary study, the concept of block copolymer stabilized non-aqueous emulsions was confirmed for the system PEG 400/Miglyol 812 with a volume composition of 70/30. Submicron droplets of Miglyol 812 in a PEG 400 continuous phase were obtained in the presence of a PBut-b-P2VP block copolymer acting as an efficient emulsifier. This copolymer was selected on the basis of the solubility parameter concept.
The non-aqueous emulsion characteristics, such as droplet size and rheological behaviour were determined as a function of the copolymer concentration. With increasing copolymer concentration, smaller droplets and higher viscosities were obtained. Moreover, a linear evolution was observed between G′ and G′′ and it could be concluded that the viscous properties of these emulsions dominate over their elastic properties.
An original aspect of this study is the water dispersibility of these non-aqueous emulsions without additional surfactant. In fact, the PEG 400 continuous phase is water miscible and the PBut-b-P2VP copolymer provides the electro-steric stabilization of the aqueous emulsion as the P2VP sequences are protonated at pH 1. From a practical point of view and for biomedical and cosmetic applications, this type of oil-in-water submicron emulsion may be of interest for the encapsulation of water-sensitive components.
Further work is in progress concerning the possibility to extend the present concept of water dispersible non-aqueous emulsions to oil-in-water emulsions stabilized by block copolymers comprising a sequence that is soluble in PEG 400 as well as in water at pH 7.
Acknowledgments
The authors would like to thank to Dr. J.-P. Lerch for providing the copolymer sample.


