1 Introduction
Bis(indole)-based compounds have recently received much attention as important building blocks for the synthesis of various active biological compounds [1–7]. Bis(indole) derivatives have been prepared via condensation reactions of indole with various aldehydes or ketones in the presence of either protic or Lewis acids [8–12]. Ultrasound irradiation is a powerful technique in synthetic organic chemistry. Enhanced reaction rates, simple experimental procedures, and high yields are the notable features of the ultrasound approach as compared to established methods [13–15]. Organic synthesis in aqueous media is gaining importance in view of the fact that the use of many toxic and volatile organic solvents contributes to pollution. There have been profound research activities in the development of organic reactions in aqueous media, offering key advantages such as rate enhancement and insolubility of final products, which facilitates their isolation by simple filtration [16]. Keggin-type heteropolyacids (HPAs) have been studied extensively for organic synthetic processes as acid or redox catalysts in homogeneous and heterogeneous media [17]. HPAs have catalyzed several organic transformations such as Diels–Alder reaction [18], oxidative dehydrogenation of alcohols and amines [19], olefin hydration [20], synthesis of dihydropyrimidinones [21], esterification reactions [22], preparation of oximes [23] and synthesis of oxazolines, imidazolines, and thiazolines [24]. In conjunction with an ongoing research program involving the synthesis of bis(indole) compounds, we report a simple method for the preparation of bis(indole) derivatives through the Michael reaction of indole with different electron-deficient alkenes. This reaction is catalyzed by 12-tungstophosphoric acid in aqueous media at 90 °C under silent and ultrasound irradiation conditions (Scheme 1).

Synthesis of bis(indole) derivatives catalyzed by 12-tungstophosphoric acid in aqueous media at 90 °C.
2 Results and discussion
In our initial study, the reaction of indole and 2-benzylidenemalononitrile was considered as a model one to optimize the conditions. The reaction was first carried out in H2O in the absence of H3PW12O40 and in reflux conditions. No reaction occurred under silent and ultrasound irradiation conditions (Table 1, entry 1). Similar reactions were then attempted in the presence of 2, 2.5, 3, and 3.5 mol-% of H3PW12O40. The results in Table 1, entries 2–5, show that the use of 3 mol-% of H3PW12O40 at reflux in H2O is sufficient to push the reaction forward. Higher reaction loading of the catalyst had no significant influence on the reaction yield. To find the optimum reaction temperature, the reaction was carried out with 3 mol-% of H3PW12O40 at room temperature, 60 °C, and at reflux temperature, which resulted in the isolation of the product in trace amounts and yields of 65% and 93% (Table 1, entries 7, 6, and 4), respectively. Thus, 3 mol-% of H3PW12O40 and a reaction temperature at reflux were the optimal conditions. In addition, EtOH, MeCN, and MeCO2Et were also tested as solvents. In these cases, 2-((di(H1 indol-3-yl)(phenyl)methyl)malonitrile was formed in lower yields (Table 1, entries 8–10). When H3NSO3 and C10H16O4S were used as catalysts, 2-((di(H1 indol-3-yl)(phenyl)methyl)malonitrile was formed slightly in lower yields (Table 1, entries 13, 14).
Optimization of reaction conditions on the reaction of indole and 2-benzylidenemalononitrilea.
| Entry | Temp (°C) | Solvent | Catalyst (mol %) | Yield (%)b |
| 1 | Reflux | H2O | H3PW12O40 (0) | 0 |
| 2 | Reflux | H2O | H3PW12O40 (2) | 70 |
| 3 | Reflux | H2O | H3PW12O40 (2.5) | 87 |
| 4 | Reflux | H2O | H3PW12O40 (3) | 93 |
| 5 | Reflux | H2O | H3PW12O40 (3.5) | 92 |
| 6 | 60 | H2O | H3PW12O40 (3) | 65 |
| 7 | Room temp | H2O | H3PW12O40 (3) | Trace |
| 8 | Reflux | EtOH | H3PW12O40 (3) | 30 |
| 9 | Reflux | MeCO2Et | H3PW12O40 (3) | 20 |
| 10 | Reflux | MeCN | H3PW12O40 (3) | 28 |
| 11 | Reflux | H2O | H3PMo12O40 (5.4) | 91 |
| 12 | Reflux | H2O | H4SiW12O40 (3.4) | 92 |
| 13 | Reflux | H2O | C10H16O4S (42) | 70 |
| 14 | Reflux | H2O | H3NSO3 (100) | 75 |
a Reaction conditions: indole (1 mmol), 2-benzylidenemalonitrile (0.5 mmol), and solvent (4 mL).
b Isolated yields.
When optimizing the model reaction, bis(indole) derivatives were synthesized in high yields under silent and ultrasound irradiation conditions. Ultrasound irradiation accelerated such reactions. The results are summarized in Table 2. It can be observed that the process tolerates both electron-donating and electron-withdrawing substituents in benzaldehydes. In all the cases, the reactions proceeded efficiently at reflux under mild conditions to afford the corresponding products in high yields. All the products were characterized by 1H- and 13C-NMR, IR spectroscopy and elemental analyses.
Synthesis of bis(indole) derivatives in the presence of H3PW12O40 under silent and ultrasonic conditions in aqueous mediaa.
| Entry | X | R | Time Silent/sonicationc | Yield (%)b Silent/sonication | Mp (°C) |
| 1 | CN | C6H5 | 11 h/14 min | 94/93 | 89–90 |
| 2 | CN | 4-NO2C6H4 | 8 h/10 min | 92/91 | 243–245 |
| 3 | CN | 2-FC6H4 | 10 h/12 min | 87/85 | 128–129 |
| 4 | CN | 4-FC6H4 | 10 h/12 min | 86/85 | 132–135 |
| 5 | CN | 4-ClC6H4 | 10 h/12 min | 85/83 | Oil |
| 6 | CN | 4-MeOC6H4 | 12 h/17 min | 85/82 | 126–128 |
| 7 | CN | 3, 4-diMeOC6H3 | 12 h/17 min | 81/80 | 168–169 |
| 8 | CN | 4-HOC6H4 | 12 h/20 min | 80/78 | 87–88 |
| 9 | CN | 4-MeC6H4 | 11 h/15 min | 82/80 | 174–176 |
| 10 | CN | 4-iprC6H4 | 11 h/15 min | 79/77 | 181–183 |
| 11 | CO2Me | C6H5 | 12 h/13 min | 92/91 | Oil |
| 12 | CO2Et | C6H5 | 12 h/13 min | 91/90 | Oil |
a Reaction conditions: indole (1 mmol), electron deficient alkenes (0.5 mmol).
b Isolated yields.
c Constant frequency: 70 W.
As expected, the reaction could be extended to other electron-deficient alkenes. Under optimized conditions, 2-(pyridylmethylene)malononitriles, 3-(pyridyl)acrylates were also chosen as electron-deficient alkenes to react with indole and were found to generate the corresponding indolyl derivatives. The reaction proceeded smoothly as expected in high yields, but while we wanted to obtain the expected bis(indole) derivatives, only indolyl derivatives were obtained (Table 3). A possible reason for this is that the oxidative dehydrogenation of 2-((indolyl)(pyridyl)methylene)malononitriles and of 3-(indolyl)(pyridyl)acrylates in the presence of H3PW12O40 is less reactive than 2-((aryl)(indolyl)methyl)malonitriles and 3-(aryl)(indolyl)acrylates, respectively. 1H- and 13C-NMR spectra of the crude mixture clearly indicate that the formation of the product leads to one enantiomer. Our attempts to detect the second enantiomer in the reaction mixture were not successful. The results are summarized in Table 3. To explore the scope and limitations of this reaction further, we extended our studies to the reaction of pyrrole and 2-benzylidenemalonitrile in the presence of 12-tungstophosphoric acid as a catalyst in aqueous media at 90 °C under the established conditions. The reaction proceeded smoothly and a black sticky mixture was obtained after 3 h. In accordance with the literature, we think that pyrrole as an acid-sensitive compound undergoes polymerization reactions without any occurrence of Michael addition reactions.
Synthesis of 2-((indolyl)(pyridyl)methylene)malononitriles and 3-(indolyl)(pyridyl) acrylates in the presence of H3PW12O40 under silent and ultrasonic conditions in aqueous mediaa.
| Entry | X | R | Time Silent/sonicationc | Yield (%)b Silent/sonication | Mp (°C) |
| 1 | CN | 3-pyridyl | 9/10 | 92/91 | 159–161 |
| 2 | CN | 4-pyridyl | 9/10 | 91/90 | 165–166 |
| 3 | CO2Me | 3-pyridyl | 10/11 | 88/86 | 144–145 |
| 4 | CO2Me | 4-pyridyl | 11/12 | 86/84 | Oil |
| 5 | CO2Et | 3-pyridyl | 10/11 | 87/85 | 147–148 |
| 6 | CO2Et | 4-pyridyl | 11/12 | 85/83 | Oil |
a Reaction conditions: indole (1 mmol), 2-(pyridylmethylene)malononitriles or 3-(pyridyl) acrylates (0.5 mmol).
b Isolated yields.
c Constant frequency: 70 W.
A plausible mechanism for the conjugate addition of indole to 2-benzylidenemalonitrile, oxidative dehydrogenation of Michael adducts and second conjugate addition of indole with dehydrogenation adducts is proposed in Scheme 2. H3PW12O40 coordinated with nitrile groups of 2-benzylidenemalonitrile to give 1. The electron-rich β-position of the indole ring then attacked the electron-deficient conjugated carbon–carbon double bond of 1, followed by a hydrogen transfer, and was rearranged to give 2. Then, H3PW12O40 protonated the aromatic ring and got rearranged to give 4. Finally, dehydrogenation of 4 resulted in 2-((indolyl)(phenyl)methylene)malononitriles (5). 2-(di(1H-indol-3-yl)-(phenyl)methyl)malononitrile was obtained by the second conjugate addition of indole with compound 5, and H3PW12O40 was released to catalyze the next cycle (Scheme 2).
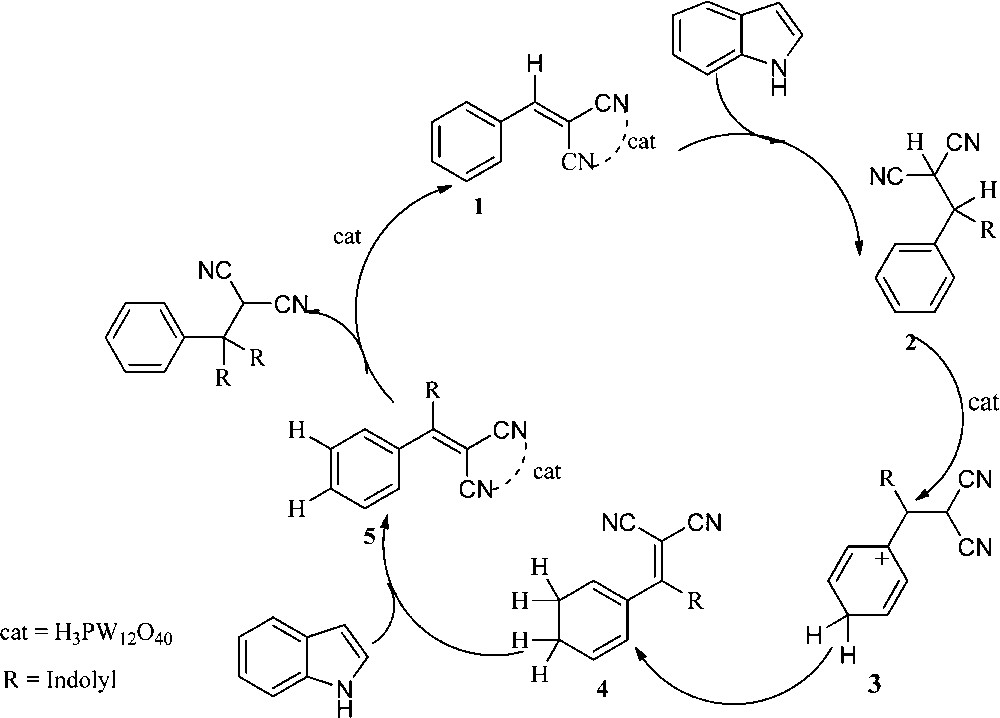
The proposed mechanism for the synthesis of 2-(di(1H-indol-3-yl)-(phenyl)(methyl) malononitrile catalyzed by H3PW12O40.
3 Conclusions
In conclusion, we have developed a facile approach to prepare bis(indole) derivatives by reaction of indole with electron-deficient alkenes. This reaction is catalyzed by 12-tungstophosphoric acid as a highly stable, effective and readily available catalyst under silent and ultrasound irradiation conditions. The procedure reported here has the advantages of mild reaction conditions, short reaction time, high yields of products, operational simplicity, and avoidance of toxic catalysts and solvents.
4 Experimental
Melting points were determined by Büchi melting point B-540 B.V.CHI apparatus in open capillaries. They were uncorrected. IR spectra were recorded as KBr pellets on a Bruker Eqinox 55 spectrometer. 1H- and13C-NMR spectra were obtained in CDCl3 with Me4Si as the internal standard with a Bruker Avance 500 MHz spectrometer. Elemental analyses were carried out with a Costech ECS 4010 CHN analyzer. Column chromatography was performed on a silica gel (230–400) mesh. Analytical TLC was performed on pre-coated plastic sheets of silica gel G/UV-254 with a thickness of 0.2 mm. Electron-deficient alkenes were prepared by treatment of benzaldehyde derivatives and active methylene compounds in aqueous media, at room temperature.
4.1 General procedure for the synthesis of bis(indole)
A mixture of indole (0.12 g, 1 mmol), electron-deficient alkenes (0.5 mmol) and catalyst (3 mol%) was refluxed at 90 °C in water (4 mL) under silent and ultrasound irradiation conditions for the appropriate time. The completion of the reaction was monitored by TLC. After cooling, the resulting precipitate was filtered, and the crude product was purified by column chromatography to obtain a pure one. The results are summarized in Tables 2 and 3. All the products are unknown compounds, which were characterized by mp, IR, elemental analyses, 1H-, and 13C-NMR spectra.
4.1.1 2-(di(1H-indol-3-yl)(phenyl)methyl) malononitrile
Red crystals; mp 89–90 °C. IR (KBr): ν = 3409, 3050, 2923, 2160, 1606, 1475 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 5.94 (s, 1H, CH), 6.67 (s, 2H, CH), 7.07 (t, J = 6.4 Hz, 2H, ArH), 7.23 (t, J = 6.4, 2H, ArH), 7.27–7.35 (m, 7H, ArH), 7.45 (d, J = 6.9 Hz, 2H, ArH), 7.88 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 40.61, 77.70, 111.41, 112.69, 119.58, 120.11, 120.30, 122.27, 124.00, 126.50, 127.51, 128.60, 129.11, 137.10, 144.40 ppm; anal. calc. for C26H18N4: C 80.81, H 4.69, N 14.50. Found: C 80.51, H 4.49, N 14.20 (Table 2, entry 1).
4.1.2 2-(di(1H-indol-3-yl)(4-nitrophenyl)methelyl)malononitrile
Yellow crystals; mp 243–245 °C. IR (KBr): ν = 3422, 3052, 2923, 2187, 1610, 1507, 1456, 1341 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 5.93 (s, 1H, CH), 6.64 (s, 2H, CH), 6.92 (t, J = 7.4 Hz, 2H, ArH), 7.09 (t, J = 7.4 Hz, 2H, ArH), 7.26 (d, J = 7.9 Hz, 2H, ArH), 7.34 (d, J = 8.1 Hz, 2H, ArH), 7.46 (d, J = 8.3 Hz, 2H, ArH), 8.06 (d, J = 8.7 Hz, 2H, ArH), 9.30 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 36.01, 48.90, 111.01, 113.00, 113.28, 119.78, 120.10, 122.01, 123.78, 124.00, 128.01, 130.05, 138.50, 148.01, 149.11 ppm; anal. calc. for C26H17N5O2: C 72.38, H 3.97, N 16.23. Found: C 72.15, H 3.65, N 16.43 (Table 2, entry 2).
4.1.3 2-((2-fluorophenyl)di(1H-indol-3-yl) methyl)malononitrile
Light pink crystals; mp 128–129 °C. IR (KBr): ν = 3392, 3050, 2930, 2165, 1580, 1438, 1219 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.28 (s, 1H, CH), 6.73 (s, 2H,CH), 7.09 (m, 3H, ArH), 7.13 (t, J = 9.1 Hz, 1H, ArH), 7.28 (m, 4H, ArH), 7.39 (d, J = 8.1 Hz, 2H, ArH), 7.46 (d, J = 7.9 Hz, 2H, ArH), 7.89 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 36.00, 42.11, 111.40, 112.20, 113.01, 116.01, 118.68, 119.71, 120.20, 122.39, 124.01, 127.31, 128.01, 129.01, 130.68, 137.10, 159.01 ppm; anal. calc. for C26H17N4F: C 77.21, H 4.24, N 13.85. Found: C 77.50, H 4.51, N 13.55 (Table 2, entry 3).
4.1.4 2-((4-fluorophenyl)di(1H-indol-3-yl) methyl)malononitrile
Red crystals; mp 132–135 °C. IR (KBr): ν = 3403, 3052, 2928, 2185, 1601, 1416, 1217 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 5.90 (s, H, CH), 6.33 (s, 2H, CH), 7.00 (m, 2H, ArH), 7.19–7.27 (m, 4H, ArH), 7.30 (m, 2H, ArH), 7.80 (d, J = 8.1 Hz, 2H, ArH), 8.00 (d, J = 7.9 Hz, 2H, ArH), 9.30 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 39.90, 48.00, 112.01, 112.78, 113.01, 116.00, 119.01, 120.69, 121.20, 123.79, 128.41, 130.00, 136.78, 138.00, 159.11 ppm; anal. calc. for C26H17N4F: C77.21, H 4.24, N 13.58. Found: C 77.00, H 4.60, N 13.44 (Table 2, entry 4).
4.1.5 2-((4-chlorophenyl)di(1H-indol-3-yl)methyl)malononitrile
Viscous oil; IR (KBr): ν = 3411, 3052, 2923, 2165, 1616, 1415, 744 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 5.89 (s, 1H, CH), 6.66 (s, 2H, CH), 6.93–7.04 (m, J = 8.1 Hz, 4H, ArH), 7.21 (d, J = 7.9 Hz, 2H, ArH), 7.44 (d, J = 7.3 Hz, 2H, ArH), 7.80 (d, J = 8.1 Hz, 2H, ArH), 8.00 (d, 2H, ArH), 8.60 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 37.60, 50.01, 112.00, 113.01, 113.79, 118.49, 120.01, 121.49, 121.68, 125.50, 128.29, 130.01, 131.87, 138.00, 140.01 ppm; anal. calc. for C26H17N4Cl: C 74.19, H 4.07, N 13.31. Found: C 74.45, H 4.34, N 13.52 (Table 2, entry 5).
4.1.6 2((3,4-di methoxyphenyl)di(1H-indol-3-yl)methyl)malononitrile
Light yellow crystals; mp 168–169 °C. IR (KBr): ν = 3399, 3055, 2926, 2250, 1593, 1456 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 3.80 (s, 3H, CH3), 3.89 (s, 3H, CH3), 5.88 (s, 1H, CH), 6.67 (d, J = 1.8 Hz, 1H, ArH), 6.71 (s, 2H, CH), 6.81 (d, J = 8.2 Hz, 1H, ArH), 6.88 (dd, J1 = 8.4 Hz, J2 = 1.8 Hz, 1H, ArH), 7.05 (t, J = 7.5 Hz, 2H, ArH), 7.21 (t, J = 7.5 Hz, 2H, ArH), 7.4 (d, J = 8.1 Hz, 2H, ArH), 7.45 (d, J = 7.9 Hz, 2H, ArH), 7.96 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 38.01, 50.00, 57.30, 57.38, 110.01, 112.00, 112.78, 115.00, 117.68, 119.70, 120.01, 122.00, 123.01, 123.67, 128.71, 133.00, 136.48, 148.00, 150.00 ppm; anal. calc. for C28H22N4O2: C 75.32, H 4.97, N 12.55. Found: C75.52, H 4.63, N 12.22 (Table 2, entry 7).
4.1.7 2-((4-hydroxyphenyl)di(1H-indol-3-yl)methelyl)malononitrile
Red crystals; mp 87–88 °C. IR (KBr): ν = 3407, 3055, 2922, 1611, 1455 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 5.40 (s, 1H, CH), 5.30 (s, 1H, OH), 6.67 (s, 2H, NH), 6.8 (d, J = 8.2 Hz, 2H, ArH), 7.00 (m, 2H, ArH), 7.14 (d, J = 7.9 Hz, 2H, ArH), 7.6 (m, 2H, ArH), 7.90 (d, J = 8.2 Hz, 2H, ArH), 7.97 (d, J = 8.2 Hz, 2H, ArH), 8.30 (s, 2H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 39.00, 49.01, 111.01, 113.00, 116.01, 119.58, 121.11, 123.01, 128.20, 129.01, 129.28, 132.69, 136.50, 138.01, 155.50 ppm; anal. calc. for C26H18N4O: C 77.59, H 4.51, N 13.92. Found: C 77.65 H 4.71, N 13.62 (Table 2, entry 8).
4.1.8 2-((1H-indol-3-yl)(pyridine-3-yl) methyl)malononitrile
White crystals; mp 159–161 °C. IR (KBr): ν = 3407, 3075, 2902, 2258 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 4.80 (d, J = 6.9 Hz, 1H, CH), 4.99 (d, J = 6.9 Hz, 1H, CH), 7.00–7.90 (m, 7H, ArH), 8.42 (s, 1H, CH), 8.61 (s, 1H, CH), 10.37 (s, 1H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 29.50, 42.01, 110.90, 112.21, 112.89, 116.10, 118.61, 120.01, 122.68, 123.29, 124.00, 134.12, 136.10, 136.88, 150.01, 150.10; anal. calc. for C17H12N4: C 74.98, H 4.44, N 20.58. Found: C 74.55, H 4.61, N 20.55 (Table 3, entry 1).
4.1.9 Ethyl 2-cyano-3-(1H-indol-3-yl)-3-(pyridine-3-yl)propanoate
Light pink crystals; mp 147–148 °C; IR (KBr): ν = 3411, 3040, 2980, 2252, 1732, 1580, 1440, 1260 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.06 (t, J = 7.1 Hz, 3H, CH3), 4.08 (q, J = 7.1 Hz, 2H, CH2), 4.34 (d, J = 6.2 Hz, 1H, CH), 5.01 (d, J = 6.2 Hz, 1H, CH), 6.80–7.90 (m, 7H, ArH), 8.45 (d, J = 3.9 Hz, 1H, CH), 8.63 (s, 1H, CH), 10.04 (s, 1H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 14.10, 38.01, 40.88, 63.39, 111.11, 112.79, 116.10, 118.59, 119.90, 122.11, 122.59, 123.00, 124.01, 133.20, 135.29, 136.90, 148.21, 149.89, 165.19 ppm; anal. calc. for C19H17N3O2: C 71.46, H 5.37, N 13.16. Found: C 71.16, H 5.67, N 13.36 (Table 3, entry 5).
4.1.10 Ethyl 2-cyano-3-(1H-indol-3-yl)-3-(pyridine-4-yl)propanoate
Viscous oil. IR (KBr): ν = 3402, 2981, 2250, 1741, 1599, 1458 cm−1; 1H-NMR (500 MHz, CDCl3): δ = 1.06 (t, J = 7.1 Hz, 3H, CH3), 4.08 (q, J = 7.1 Hz, 2H, CH2), 4.34 (d, J = 6.2 Hz, 1H, CH), 5.01 (d, J = 6.2 Hz, 1H, CH), 7.18 (m, 4H, ArH), 8.6 (d, J = 7.9, 2H, ArH), 7.28 (d, J = 8.2 Hz, 2H, ArH), 8.63 (s, 1H, CH), 10.04 (s, 1H, NH) ppm; 13C-NMR (125 MHz, CDCl3): δ = 14.10, 38.11, 40.10, 63.39, 112.01, 117.40, 120.69, 121.71, 122.59, 123.20, 124.51, 124.69, 137.11, 141.10, 150.01, 153.41, 164.69 ppm; anal. calc. for C19H17N3O2: C 71.46, H 5.37, N 13.16. Found: C 71.23, H 5.14, N 13.54 (Table 3, entry 6).
Acknowledgments
The authors thank the Research Council of Yazd University for financial support.


