1 Introduction
1,3-Dipolar cycloaddition of nitrones is commonly used in organic chemistry leading to valuable adducts containing multifunctional nitrogen heterocyclic systems with various properties. Cycloaddition with alkynes provides additional access to heterocycles of potential biological interest [1–6]. The Kinugasa reaction [7] affords β-lactams from substituted nitrones and copper(I) acetylides [8] and is usually performed in organic solvents such as DMF, acetonitrile, and sometimes in pure pyridine [7b]. Based on the pioneering work of Kinugasa [7], we developed a Cu(I)-catalyzed asymmetric synthesis of aziridines using terminal acetylenes and a (−)-menthone-based nitrone under microwave irradiation. The expected outcome of this reaction was the β-lactams issued from the known Kinugasa reaction, but the intermediate isoxazoline was not stable under the conditions used and promptly rearranged into the corresponding aziridines. Since the expected β-lactams and aziridines displayed highly similar NMR data and identical mass spectrometry analyses, the structures of the obtained aziridines could be unambiguously identified from X-ray crystallography data.
2 Results and discussion
The initial aim of this work was to prepare a series of β-lactams based on the Kinugasa reaction [7]. Initially, the Cu(I)-catalyzed cycloaddition of nitrone 1 with phenylacetylene 2a (Scheme 1) in acetonitrile was performed at room temperature (Table 1, entry 1). Unfortunately, the reaction failed and each time the starting material remained unchanged. Different conditions were applied with additional microwave irradiation (Table 1, entries 2&6) using nitrone 1 and terminal alkynes 2a,d in DMF and led mainly to aziridines 4a,d in relatively good yields. 1,3-Dipolar cycloaddition of chiral nitrone 1 with phenylacetylene 2a without copper and under microwave irradiation in DMF at 100 °C provided mainly aziridine 4a with a slightly higher yield (63%) than under Cu(I)-catalysis (Table 1, entry 3). Unlike alkynes 2a,d, the reaction of chiral nitrone 1 with terminal acetylenes 2b–c,e under microwave irradiation gave a readily separable epimeric mixture of aziridines 3b–c,e and 4b–c,e (Table 1, entries 4,5, and 7). Also, the reaction failed with propargyl acetate 2f (Table 1, entry 8) probably due to the lower boiling point of this reagent and therefore poor availability in solution under the conditions used.
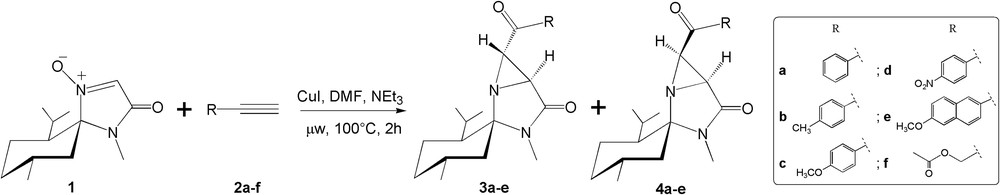
Synthesis of aziridines 3 and 4.
1,3-Dipolar cycloaddition of chiral nitrone 1 with terminal acetylenes 2a–f under different conditions.
| Entry | Conditions | Products (%) | ||
| 1 | 2a | CuI, CH3CN, NEt3, rt, 48 h | NR* | |
| 2 | 2a | CuI, DMF, μW, NEt3, 100 °C, 2 h | 4a (52%) | |
| 3 | 2a | DMF, μW, NEt3, 100 °C, 2 h | 4a (63%) | |
| 4 | 2b | CuI, DMF, μW, NEt3, 100 °C, 2 h | 3b (13%) | 4b (53%) |
| 5 | 2c | CuI, DMF, μW, NEt3, 100 °C, 2 h | 3c (<21%a) | 4c (52%) |
| 6 | 2d | CuI, DMF, μW, NEt3, 100 °C, 2 h | 4d (64%) | |
| 7 | 2e | CuI, DMF, μW, NEt3, 100 °C, 2 h | 3e (12%) | 4e (51%) |
| 8 | 2f | CuI, DMF, μW, NEt3, 100 °C, 2 h | NRb |
a Compound 3c could not be isolated in a pure form, see the experimental section in the Supplementary Material.
b NR: No reaction was observed and starting materials were recovered.
As already described, formation of aziridines 3–4 could be explained by the rearrangement of the 4-isoxazoline intermediate A (Fig. 1), which was formed in situ and not isolated, through a 1,4-migration thermal process [9]. The low thermal stability of the N–O bond in the 4-isoxazoline ring is most probably the reason for this rearrangement under thermal activation [10]. Nevertheless, the ring-opening rearrangement can also be triggered by Cu(I)-species [11] or under radical conditions using Co2(CO)8 [12]. In the present case, the initial 4-isoxazoline intermediate A was therefore generated under thermal conditions with a partial stereocontrol due to the hindered face of the (−)-menthone-based nitrone 1 favoring the approach from the opposite face to the isopropyl group. The presence of CuI in the reaction mixture might have catalyzed the ring-opening of the 4-isoxazoline A [11] but this process can also occur spontaneously under heating being thermal [9] or under microwave irradiation in the present case. Nevertheless, the thermal rearrangements occurred with total selectivity towards the cis-isomers for the aziridine ring system [9], while metal catalyzed aziridines were obtained as diastereoisomeric mixtures with a major cis-isomer [11,12]. These observations would therefore mean that the CuI used in our reaction provided a mixture of diastereoisomers with the ratio observed in agreement with these precedents.
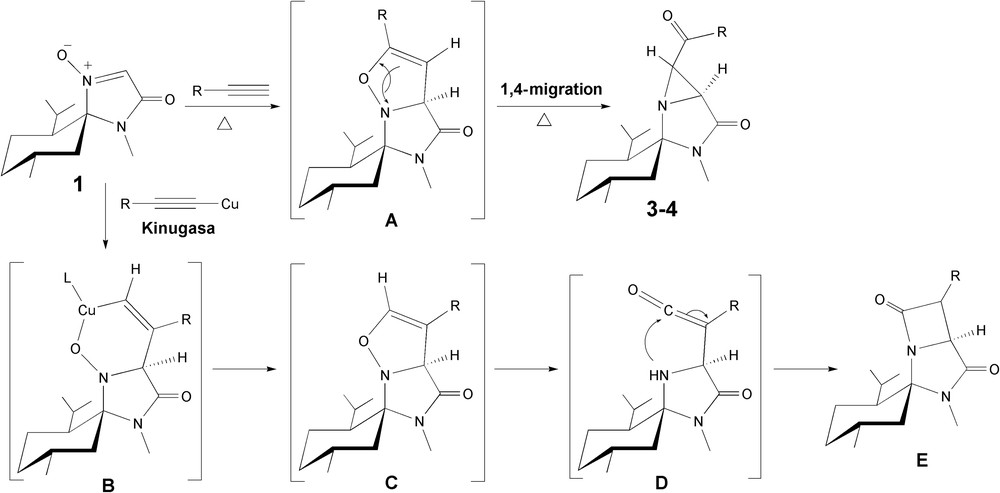
Different paths to the aziridine or β-lactam residues depending on the initial regiochemistry of the 1,3-dipolar cycloaddition.
The activation of the alkyne with copper was not observed since this approach would have led to the opposite regioisomer C, through the 6-membered ring metallocycle intermediate B [13]. This regioisomeric 4-isoxazoline C would then evolve to the ketene intermediate D which would lead to the β-lactam E.
This difference in regioselectivity observed in the presence or not of Cu(I) species is probably the reason why the expected Kinugasa-type β-lactams could not be observed. The reaction proceeded as if the Cu(I)-acetylide (R–C≡C–Cu) could not be formed or was not reactive enough with the (−)-menthone-based nitrone 1, therefore allowing the terminal alkyne to react with the nitrone.
The structures of diastereoisomers 3 and 4 have been unambiguously established by X-ray diffraction analysis and by NMR spectroscopy. The cis-configuration at the aziridine ring was clearly identified in compound 3b (Fig. 2). The diastereoisomers of the same alkyne (i.e. 3b/4b or 3c/4c or 3e/4e) could not provide both crystals suitable for X-ray diffraction and therefore the comparison of the stereochemistry induced at the aziridine ring was possible with the only two X-ray data collected for these compounds 3b and 4a which crystallized and were of opposite configuration at the aziridine ring.

ORTEP representation of the crystal structures of 3b [14] (left) and 4a [15] (right).
These X-ray crystallography results provided a basis for the interpretation of NMR data. As exemplified for isomers 3b/4b (Fig. 3), the aromatic protons of the 4-methylphenyl moieties resonating between 7.25 and 8.00 ppm provided different signals. More importantly, signals resonating between 3.10 and 3.60 ppm can be considered as very different from the trans-3b aziridine isomer to the cis-4b isomer, both in terms of chemical shifts but also 3J coupling constants. NMR data therefore clearly indicated the different configurations observed by X-ray diffraction for each diastereoisomer. This NMR pattern could be observed for all other compounds in the series.

Partial 1H NMR spectra (400 MHz, CDCl3) of compounds 3b and 4b.
3 Conclusion
1,3-Dipolar cycloaddition of nitrones with alkynes provides access to either β-lactams under Cu(I)-catalysis or to aziridines by simple heating. We have demonstrated here that when a chiral nitrone is too hindered, the Cu(I)-catalysis cannot act and therefore the thermal pathway dominates the reaction and provides aziridines as diastereoisomeric mixtures. The aziridines obtained could be readily characterized by standard multi-dimensional NMR spectroscopies and mass spectrometry. But the chirality and structure of the aziridine backbone could be ascertained by X-ray diffraction analysis of two diastereoisomers of opposite configurations (cis and trans). The unmasking of the menthone chiral auxiliary is now under investigation to provide a novel entry towards chiral aziridine-based amino-acids.


