1 Introduction
The concept of “privileged medicinal scaffolds” has recently emerged as one of the guiding principles of drug discovery [1–4]. These frameworks commonly consist of a rigid heterocyclic ring system that assigns well-defined orientation of appended functionalities for target recognition.
Functionally substituted chromenes often appear as important structural components in both biologically active and natural compounds. It is widely present in alkaloids, flavonoids, tocopherols, and anthocyanins [5–8]. Among different types of chromene systems, 2-amino-4H-chromenes (or 2-amino-4H-benzo[b]pyrans) are of particular utility as they belong to privileged medicinal scaffolds (Fig. 1) serving for the generation of small-molecule ligands with highly pronounced spasmolytic, diuretic, anticoagulant, and antianaphylactic activities [9–11]. The current interest in 2-amino-4H-chromene derivatives bearing a nitrile functionality arises from their potential applications in the treatment of human inflammatory TNF-α-mediated diseases, such as rheumatoid and psoriatic arthritis, and especially in cancer therapy [12–14]. Thus, 2-amino-4H-chromen-3-carbonitriles have strong cytotoxicity against a panel of human cancer lines involving pathways that include microtubule depolarization and tumor vasculature disruption [15]. A chromene analog, Crolibulin™ (EPC2407), currently undergoes clinical trials for the treatment of advanced solid tumors (Fig. 1) [16].
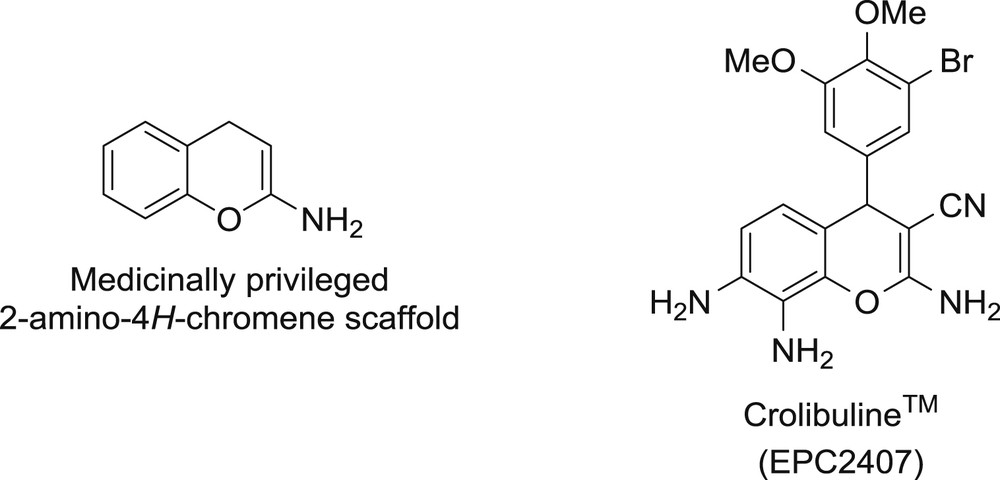
2-Amino-4H-chromene as a privileged medicinal scaffold.
Despite the remarkable progress in the chemistry of chromenes [17], the discovery and development of a new family of compounds with enhanced anticancer properties is of great importance. One of the most powerful approaches to solve this challenge is to employ multicomponent reactions (MCRs) for the synthesis of substituted chromenes. This approach has significant advantages over conventional protocols. By virtue of their inherent convergence and high productivity, as well as their atom-efficient nature, MCRs have naturally become a rapidly evolving field of research [18].
As far as we know, only two multicomponent entries are described for the synthesis of cyclohexylidene-substituted 2-amino-4H-chromene derivatives [19,20]. Both of them employ multicomponent condensation of salicylaldehydes, malononitrile and α,α-dicyanocycloalkenes catalyzed by NEt3. Although it proceeds in a one reaction step, the preliminary synthesis of the Knoevenagel adduct is still required, which implies an additional stage of target compound preparation. Moreover, column chromatography is needed for the purification of desired 2-amino-4H-chromenes in the case of [19]. As for [20], the only one example of the reaction, i.e. the synthesis of 2-[2-(2-amino-3-cyano-4H-chromen-4-yl)-cyclohexylidene]malononitrile was carried out. In addition, the techniques mentioned above were used only for α,α-dicyanocycloalkenes produced from the carbocyclic ketones, which markedly limit the scope of the reaction. Thus, the known procedures have their merits, but a facile and convenient pseudo four-component methodology for the synthesis of 4-[2-(dicyanomethylene)cyclic or heterocyclic]-2-amino-4H-chromenes from salicylaldehydes and cyclic ketones and two molecules of malononitrile is yet to be developed.
2 Results and discussion
In the present study we report our results on pseudo four-component transformation of substituted salicylaldehydes 1a–g, cyclic ketones 2a–f and two equivalents of malononitrile into corresponding substituted 2-amino-4H-chromenes 3a–l under facile and mild conditions (Scheme 1).
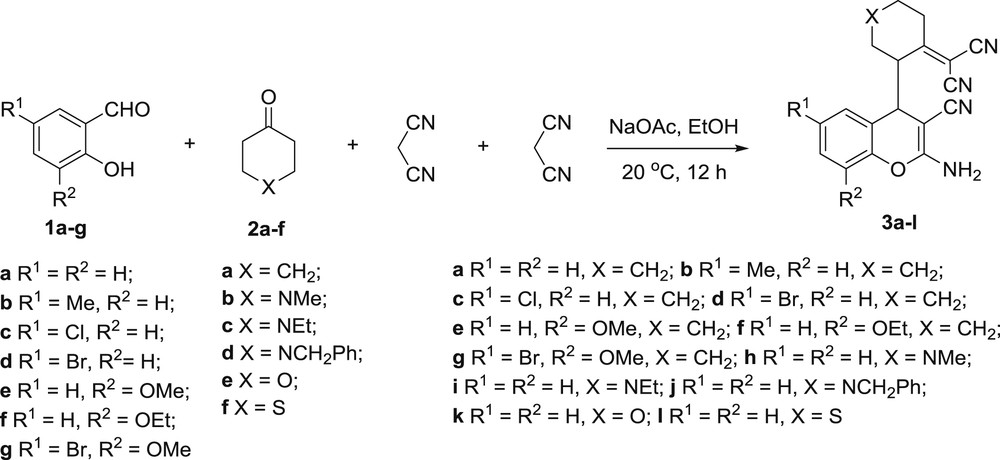
Pseudo four-component transformation of salicylaldehydes 1a–g, cyclic ketones 2a–f and two equivalents of malononitrile into 2-amino-4H-chromenes 3a–l.
At the outset of our studies, some experiments were carried out in order to find the optimal reaction conditions. The condensation of salicylaldehyde 1a, cyclohexanone 2a and two equivalents of malononitrile was chosen as a model reaction (Scheme 2). The results are summarized in Table 1.
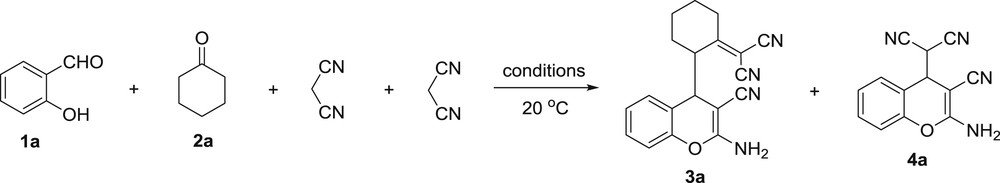
Multicomponent transformation of salicylaldehyde 1a, cyclohexanone 2a and malononitrile into 2-amino-4H-chromenes 3a and 4a.
Multicomponent transformation of salicylaldehyde 1a, cyclohexanone 2a and malononitrile into 2-amino-4H-chromenes 3a and 4aa.
| Entry | Base | Quantity of base (mmol) | Time (h) | Yield of 3ab (%) | Yield of 4ab (%) |
| 1 | – | – | 12 | 0 | 92c |
| 2 | NaOAc | 0.1 | 1 | 74 | 20 |
| 3 | NaOAc | 0.1 | 3 | 83 | 8 |
| 4 | NaOAc | 0.1 | 4 | 85 | 4 |
| 5 | NaOAc | 0.1 | 6 | 88 | 2 |
| 6 | NaOAc | 0.1 | 12 | 90c | 0 |
| 7 | Et3N | 0.1 | 12 | 69c | 0 |
a 1 mmol of salicylaldehyde 1a, 1 mmol of cyclohexanone 2a, 2 mmol of malononitrile, 3 mL of EtOH, 20 °C.
b 1H NMR data.
c Isolated yield.
On the first step, this process was conducted in the absence of a base (Table 1, entry 1) and only (2-amino-3-cyano-4H-chromen-4-yl)malononitrile 4a was isolated with 92% yield (12 h reaction time). Addition of a mild base (NaOAc) dramatically affected the reaction and led to mixtures of 3a and 4a (Table 1, entries 2–5) with the majority of the former. Increasing the reaction time to 12 h led to the formation of individual 2-amino-4H-chromen 3a with 90% yield (Table 1, entry 6). A similar reaction with triethylamine as the catalyst resulted in a lower yield of 69% of 2-amino-4H-chromene 3a (Table 1, entry 7).
Under optimized conditions in hand, i.e., sodium acetate as the catalyst and a reaction time of 12 h, we then investigated the scope of the reaction. The results are outlined in Table 2. To our delight, salicylaldehydes bearing either electron-donating or electron-withdrawing groups reacted smoothly (Table 2, entries 1–7). As for the carbonyl component, the new types of heterocyclic compounds, such as various piperidin-4-ones, 4H-pyran-4-one and 4H-thiopyran-4-one were involved in this pseudo four-component process as well as cyclohexanone, and gave rise to corresponding substituted 2-amino-4H-chromenes 3a–l in good to excellent yields (Table 2, entries 8–12).
Multicomponent transformation of salicylaldehydes 1a–g, cyclic ketones 2a–f and malononitrile into 2-amino-3-cyano-4H-chromenes 3a–la.
| Entry | Salicylaldehyde | Cyclic ketone | Product, yield (%)b,c |
| 1 | 1a | 2a | 3a, 90 |
| 2 | 1b | 2a | 3b, 71 |
| 3 | 1c | 2a | 3c, 73 |
| 4 | 1d | 2a | 3d, 74 |
| 5 | 1e | 2a | 3e, 78 |
| 6 | 1f | 2a | 3f, 77 |
| 7 | 1g | 2a | 3g, 87 |
| 8 | 1a | 2b | 3h, 75 |
| 9 | 1a | 2c | 3i, 72 |
| 10 | 1a | 2d | 3j, 76 |
| 11 | 1a | 2e | 3k, 90 |
| 12 | 1a | 2f | 3l, 93 |
a 1 mmol of salicylaldehyde 1a–g, 1 mmol of cyclic ketones 2a–f, 2 mmol of malononitrile, 3 mL of EtOH, 20 °C, 12 h.
b Isolated yield.
c Diastereomeric ratio 1:1.
With the above results taken into consideration and the mechanistic data on the multicomponent transformations of salicylaldehydes and C–H acids [21–25], the following mechanism for the pseudo four-component reaction of salicylaldehyde 1, cyclic ketone 2 and two equivalents of malononitrile for the formation of substituted 2-amino-4H-chromene 3 was proposed (Scheme 3).
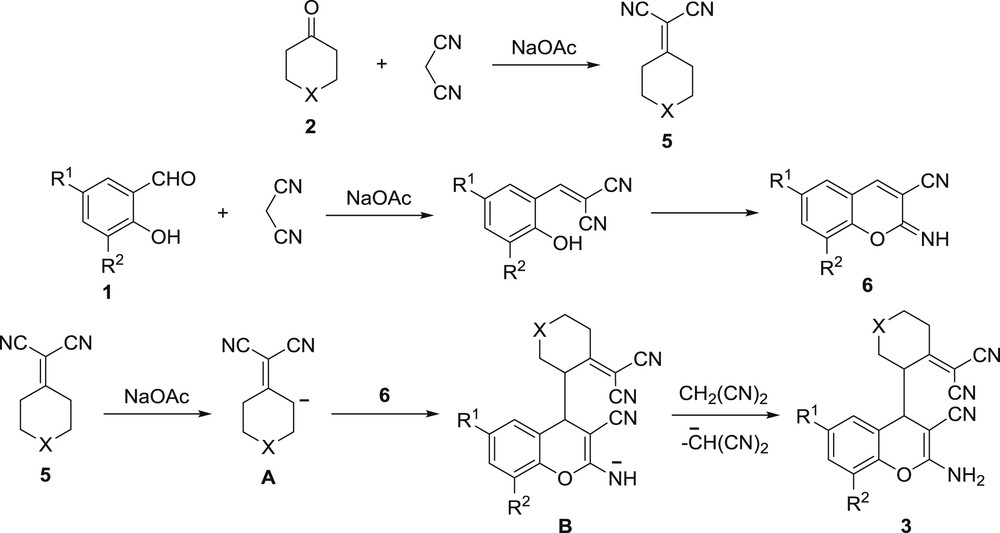
The mechanism of multicomponent transformation.
The first step of this multicomponent process begins with sodium acetate base-induced Knoevenagel condensation of cyclic ketone 2 and malononitrile with the formation of Knoevenagel adduct 5. Another equivalent of malononitrile simultaneously reacts with salicylaldehyde 1 affording cyclic imine 6. Then, by the action of NaOAc, cycloalkylidenemalononitrile 5 forms anion A, which attacks the activated double bond in cyclic imine 6 leading to anion B formation. Further interaction of anion B with malononitrile provides 2-amino-4H-chromene 3 with the regeneration of the malononitrile anion, which starts the next catalytic cycle.
3 Conclusion
The developed facile and efficient catalytic pseudo four-component process can produce an effective transformation of salicylaldehydes, cyclic ketones and two equivalents of malononitrile into medicinally privileged substituted 2-amino-4H-chromenes – the approved basis for the generation of targeting molecules for different biomedical applications. The developed catalytic multicomponent procedure utilizes simple equipment, and requires reasonable starting materials. It is easily carried out, and the reaction products were isolated by an easy work-up procedure and do not need any further purification steps. The application of this convenient multicomponent method is also beneficial from the viewpoint of diversity-oriented large-scale processes.
4 Experimental
4.1 General Remarks
All melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded with a Bruker Avance II-300 spectrometer at ambient temperature. Chemical shift values are relative to Me4Si. IR spectra were registered with a Bruker ALPHA-T FT-IR spectrophotometer in KBr pellets. Mass-spectra (EI = 70 eV) were obtained directly with a Finningan MAT INCOS 50 spectrometer. HRMS mass-spectra (ESI) were measured on a Bruker micrOTOF II instrument; external or internal calibration was done with Electrospray Calibrant Solution (Fluka). All chemicals were purchased from commercial sources and used without further purification.
4.2 General multicomponent procedure
A mixture of salicylaldehyde 1 (1 mmol), cyclic ketone 2 (1 mmol), malononitrile (2 mmol), NaOAc (0.1 mmol) and ethanol (3 mL) was stirred at 20 °C for 12 h. Then the reaction mixture was filtered to isolate the solid product 3, which was then rinsed with ethanol (2 × 2 mL), and dried under reduced pressure.
4.2.1 [2-(2-Amino-3-cyano-4H-chromen-4-yl)cyclohexylidene]malononitrile (3a)
White solid. Yield 0.29 g (90%); diastereomeric ratio 1:1; mp 162–163 °C; HRMS (ESI): calcd for C19H16N4NaO [M+Na]+ 339.1216, found 339.1207; MS (EI): m/z (%) = 171 (100), 146 (25), 81 (32), 55 (36), 41 (25), 39 (29); 1H NMR (300 MHz, DMSO-d6) δ 1.35–1.93 (m, 8H), 1.97–2.18 (m, 3H), 2.26–2.39 (m, 1H), 2.56–2.92 (m, 5H), 3.05 (td, J1 = 12.5 Hz, J2 = 4.8 Hz, 1H), 3.99–4.12 (m, 2H), 6.95 (d, J = 7.3 Hz, 1H, Ar), 7.03–7.40 (m, 11H, Ar, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 18.9, 19.3, 27.8, 28.0, 28.2, 28.5, 31.1, 31.4, 36.1, 36.6, 50.8, 51.5, 51.9, 52.1, 83.1, 84.6, 110.6, 111.6, 111.7, 112.1, 116.3, 116.4, 120.2, 121.0, 123.4, 123.5, 123.8, 124.3, 127.4, 128.4, 128.5, 129.1, 150.1, 150.7, 163.5, 163.6, 185.6, 185.7 ppm; IR (KBr): ν = 3421, 3327, 2940, 2232, 2184, 1581, 1418, 1227 cm−1.
4.2.2 [2-(2-Amino-3-cyano-6-methyl-4H-chromen-4-yl)cyclohexylidene]malononitrile (3b)
White solid. Yield 0.23 g (71%); diastereomeric ratio 1:1; mp 152–153 °C; HRMS (ESI): calcd for C20H18N4NaO [M+Na]+ 353.1373, found 353.1355; MS (EI): m/z (%) = 184 (100), 157 (42), 146 (23), 81 (52), 55 (61), 41 (40), 39 (45); 1H NMR (300 MHz, DMSO-d6) δ 1.35–1.91 (m, 8H), 1.99–2.15 (m, 3H), 2.25 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.22–2.37 (m, 1H), 2.55–2.91 (m, 5H), 3.04 (td, J1 = 12.5, J2 = 4.8 Hz, 1H), 3.90–4.05 (m, 2H), 6.74 (s, 1H, Ar), 6.94 (d, J = 8.1 Hz, 1H, Ar), 7.00 (d, J = 8.1 Hz, 1H, Ar), 7.07 (s, 1H, Ar), 7.09–7.17 (m, 2H, Ar), 7.20 (s, 2H, NH2), 7.22 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 19.0, 19.3, 20.2, 20.3, 27.8, 27.9, 28.0, 28.5, 31.1, 31.5, 36.2, 36.7, 50.8, 51.6, 51.8, 51.9, 83.0, 84.6, 110.7, 111.5, 111.6, 112.1, 115.9, 116.1, 120.3, 121.1, 123.1, 123.2, 127.7, 128.7, 128.9, 129.1, 132.7, 133.4, 148.1, 148.6, 163.6, 163.8, 185.6, 185.8 ppm; IR (KBr): ν = 3446, 3346, 2240, 2182, 1649, 1587, 1407, 1215 cm−1.
4.2.3 [2-(2-Amino-6-chloro-3-cyano-4H-chromen-4-yl)cyclohexylidene]malononitrile (3c)
White solid. Yield 0.26 g (73%); diastereomeric ratio 1:1; mp 174–176 °C; HRMS (ESI): calcd for C19H15ClN4NaO [M+Na]+ 373.0827, found 373.0816; MS (EI): m/z (%) = 206 (33), 204 (100), 179 (32), 177 (92), 146 (29), 81 (72), 55 (86), 41 (65), 39 (67); 1H NMR (300 MHz, DMSO-d6) δ 1.40–1.89 (m, 8H), 1.98–2.17 (m, 3H), 2.25–2.38 (m, 1H), 2.59–2.93 (m, 5H), 3.08 (td, J1 = 13.2, J2 = 5.1 Hz, 1H), 4.00–4.16 (m, 2H), 7.04 (d, J = 2.2 Hz, 1H, Ar), 7.10 (d, J = 8.4 Hz, 1H, Ar), 7.17 (d, J = 8.4 Hz, 1H, Ar), 7.31–7.45 (m, 7H, Ar, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 18.8, 19.3, 27.7 (2C), 28.0, 28.5, 31.0, 31.4, 35.9, 36.3, 50.4, 51.2, 51.6, 51.7, 83.1, 84.9, 110.6, 111.4, 111.5, 112.0, 118.1, 118.4, 119.9, 120.7, 125.5, 125.6, 127.0, 127.6, 128.0, 128.2, 128.3, 128.4, 149.0, 149.5, 163.3, 163.5, 185.1, 185.3 ppm; IR (KBr): ν = 3446, 3347, 2239, 2186, 1651, 1584, 1406, 1266, 1183 cm−1.
4.2.4 [2-(2-Amino-6-bromo-3-cyano-4H-chromen-4-yl)cyclohexylidene]malononitrile (3d)
White solid. Yield 0.29 g (74%); diastereomeric ratio 1:1; mp 164–166 °C; HRMS (ESI): calcd for C19H15BrN4NaO [M+Na]+ 417.0321, found 417.0302; MS (EI): m/z (%) = 250 (100), 248 (94), 223 (49), 221 (47), 146 (25), 81 (55), 55 (53), 41 (45), 39 (49); 1H NMR (300 MHz, DMSO-d6) δ 1.39–1.87 (m, 8H), 1.98–2.18 (m, 3H), 2.24–2.37 (m, 1H), 2.58–2.93 (m, 5H), 3.08 (td, J1 = 13.5, J2 = 5.1 Hz, 1H), 3.99–4.16 (m, 2H), 7.04 (d, J 8.4 Hz, 1H, Ar), 7.11 (d, J = 8.4 Hz, 1H, Ar), 7.16 (d, J = 2.2 Hz, 1H, Ar), 7.35 (s, 2H, NH2), 7.40 (s, 2H, NH2), 7.46 (d, J = 2.2 Hz, 1H, Ar), 7.48–7.56 (m, 2H, Ar) ppm; 13C NMR (75 MHz, DMSO-d6) δ 18.8, 19.2, 27.7 (2C), 27.9, 28.5, 31.0, 31.4, 35.9, 36.2, 50.4, 51.2, 51.6 (2C), 83.2, 84.9, 110.6, 111.5 (2C), 112.0, 115.4, 115.9, 118.5, 118.7, 119.9, 120.7, 126.0 (2C), 130.0, 131.1, 131.2, 131.3, 149.5, 149.9, 163.2, 163.4, 185.1, 185.2 ppm; IR (KBr): ν = 3478, 3311, 2233, 2192, 1651, 1586, 1416, 1230, 1180 cm−1.
4.2.5 [2-(2-Amino-3-cyano-8-methoxy-4H-chromen-4-yl)cyclohexylidene]malononitrile (3e)
White solid. Yield 0.27 g (78%); diastereomeric ratio 1:1; mp 151–152 °C; HRMS (ESI): calcd for C20H18N4NaO2 [M+Na]+ 369.1322, found 369.1312; MS (EI): m/z (%) = 201 (99), 200 (100), 146 (25), 81 (72), 55 (66), 41 (47), 39 (44); 1H NMR (300 MHz, DMSO-d6) δ 1.35–1.90 (m, 8H), 1.97–2.15 (m, 3H), 2.25–2.38 (m, 1H), 2.57–2.91 (m, 5H), 3.02 (td, J1 = 13.6, J2 = 5.5 Hz, 1H), 3.81 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.98–4.08 (m, 2H), 6.52 (dd, J1 = 7.4, J2 = 1.6 Hz, 1H, Ar), 6.84 (dd, J1 = 7.5, J2 = 1.6 Hz, 1H, Ar), 7.01–7.19 (m, 4H, Ar), 7.25 (s, 2H, NH2), 7.31 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 18.9, 19.3, 27.8, 27.9, 28.2, 28.6, 31.0, 31.4, 36.2, 36.7, 50.8, 51.4, 51.9, 52.1, 55.7, 55.9, 83.0, 84.6, 110.8, 111.5, 111.6, 111.7, 111.8, 112.1, 118.8, 120.2, 120.3, 121.0, 123.8, 124.2, 124.4, 124.6, 139.3, 140.0, 147.4, 147.5, 163.5, 163.6, 185.7, 185.8 ppm; IR (KBr): ν = 3459, 3341, 2932, 2231, 2181, 1637, 1580, 1412, 1207 cm−1.
4.2.6 [2-(2-Amino-3-cyano-8-ethoxy-4H-chromen-4-yl)cyclohexylidene]malononitrile (3f)
White solid. Yield 0.28 g (77%); diastereomeric ratio 1:1; mp 152–154 °C; HRMS (ESI): calcd for C21H20N4NaO2 [M+Na]+ 383.1478, found 383.1464; MS (EI): m/z (%) = 215 (98), 214 (100), 146 (33), 81 (70), 55 (63), 41 (40), 39 (47); 1H NMR (300 MHz, DMSO-d6) δ 1.31 (t, J = 7.3 Hz, 3H, CH3), 1.36 (t, J = 7.3 Hz, 3H, 2CH3), 1.40–1.90 (m, 8H), 1.97–2.15 (m, 3H), 2.25–2.38 (m, 1H), 2.57–2.91 (m, 5H), 3.02 (td, J1 = 13.6, J2 = 5.5 Hz, 1H), 3.94–4.20 (m, 6H), 6.51 (d, J = 7.5 Hz, 1H, Ar), 6.83 (d, J = 7.5 Hz, 1H, Ar), 6.97–7.18 (m, 4H, Ar), 7.22 (s, 2H, NH2), 7.27 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 14.5, 14.6, 18.9, 19.3, 27.8, 27.9, 28.2, 28.6, 31.0, 31.4, 36.3, 36.7, 50.8, 51.4, 51.9, 52.1, 64.2, 64.5, 83.0, 84.5, 110.7, 111.6, 111.8, 112.1, 112.7, 113.4, 118.9, 120.1, 120.3, 121.0, 123.7, 124.1, 124.5, 124.7, 139.6, 140.5, 146.4, 146.7, 163.5, 163.6, 185.7, 185.8 ppm; IR (KBr): ν = 3411, 3333, 2942, 2238, 2186, 1649, 1583, 1412, 1202 cm−1.
4.2.7 [2-(2-Amino-6-bromo-3-cyano-8-methoxy-4H-chromen-4-yl)cyclohexylidene]malononitrile (3g)
White solid. Yield 0.37 g (87%); diastereomeric ratio 1:1; mp 181–182 °C; HRMS (ESI): calcd for C20H17BrN4NaO2 [M+Na]+ 447.0427, found 447.0409; MS (EI): m/z (%) = 280 (100), 278 (95), 253 (44), 251 (42), 146 (27), 81 (57), 55 (51), 41 (49), 39 (47); 1H NMR (300 MHz, DMSO-d6) δ 1.37–1.87 (m, 8H), 1.95–2.15 (m, 3H), 2.22–2.35 (m, 1H), 2.57–2.92 (m, 5H), 3.05 (td, J1 = 13.2, J2 = 5.1 Hz, 1H), 3.83 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.95–4.12 (m, 2H), 6.74 (d, J = 2.0 Hz, 1H, Ar), 7.03 (d, J = 2.0 Hz, 1H, Ar), 7.24 (d, J = 2.0 Hz, 1H, Ar), 7.26 (d, J = 2.0 Hz, 1H, Ar), 7.35 (s, 2H, NH2). 7.40 (s, 2H, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 18.8, 19.2, 27.7, 27.8, 27.9, 28.6, 31.0, 31.4, 35.9, 36.3, 50.5, 51.1, 51.7, 51.8, 56.2, 56.4, 83.1, 84.8, 110.7, 111.4, 111.5, 112.0, 114.6, 114.7, 115.3, 115.7, 119.8, 120.7, 121.3, 122.3, 126.3 (2C), 138.8, 139.5, 148.2, 148.4, 163.2, 163.4, 185.2, 185.3 ppm; IR (KBr): ν = 3468, 3387, 3324, 2954, 2230, 2182, 1651, 1574, 1421, 1214 cm−1.
4.2.8 [4-(2-Amino-3-cyano-4H-chromen-4-yl)-1-methylpiperidin-3-ylidene]malononitrile (3h)
White solid. Yield 0.25 g (75%); diastereomeric ratio 1:1; mp 155–157 °C; HRMS (ESI): calcd for C19H17N5NaO [M+Na]+ 354.1325, found 354.1317; MS (EI): m/z (%) = 171 (100), 161 (23), 81 (38), 55 (45), 41 (43), 39 (45); 1H NMR (300 MHz, DMSO-d6) δ 1.94 (dd, J1 = 12.5, J2 = 2.7 Hz, 1H), 2.03–2.19 (m, 3H), 2.24 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.53–2.64 (m, 2H), 2.65–2.78 (m, 2H), 2.81–3.02 (m, 2H), 3.05–3.20 (m, 2H), 3.20–3.33 (m, 2H), 4.03–4.13 (m, 2H), 6.99–7.18 (m, 4H, Ar), 7.20–7.43 (m, 8H, Ar, 2NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 31.4, 31.5, 37.1, 37.5, 44.1, 44.6, 51.5, 51.6, 52.2, 52.3, 55.2 (2C), 55.4, 55.8, 84.1, 85.7, 110.4, 111.4, 111.5, 111.8, 116.2, 116.5, 120.3, 121.0, 123.1, 123.4, 123.7, 124.5, 127.7, 128.5, 128.7, 129.1, 150.1, 150.8, 163.6, 163.7, 181.3, 181.6 ppm; IR (KBr): ν = 3442, 3321, 3200, 2799, 2231, 2188, 1644, 1595, 1418, 1269 cm−1.
4.2.9 [4-(2-Amino-3-cyano-4H-chromen-4-yl)-1-ethylpiperidin-3-ylidene]malononitrile (3i)
White solid. Yield 0.25 g (72%); diastereomeric ratio 1:1; mp 137–138 °C; HRMS (ESI): calcd for C20H19N5NaO [M+Na]+ 368.1482, found 368.1478; MS (EI): m/z (%) = 175 (22), 171 (100), 81 (42), 55 (46), 41 (35), 39 (33); 1H NMR (300 MHz, DMSO-d6) δ 1.08 (t, J = 7.3 Hz, 3H, CH3), 1.90–2.23 (m, 2H), 2.32–2.83 (m, 4H), 2.84–2.96 (m, 1H), 3.14–3.27 (m, 1H), 3.27–3.42 (m, 1H), 4.00–4.10 (m, 1H), 7.01–7.18 (m, 2H, Ar), 7.20–7.43 (m, 4H, Ar, NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 11.6, 12.0, 31.5, 31.6, 37.1, 37.5, 50.5, 50.8, 51.5, 51.6, 52.1, 52.4, 52.5, 52.8, 53.4, 53.5, 83.9, 85.5, 110.4, 111.3, 111.5, 111.8, 116.3, 116.5, 120.2, 120.9, 123.2, 123.6, 123.7, 124.4, 127.6, 128.5, 128.7, 129.0, 150.1, 150.9, 163.7, 163.8, 181.9, 182.3 ppm; IR (KBr): ν = 3436, 3321, 3205, 2808, 2231, 2197, 1655, 1591, 1415, 1226 cm−1.
4.2.10 [4-(2-Amino-3-cyano-4H-chromen-4-yl)-1-benzylpiperidin-3-ylidene]malononitrile (3j)
White solid. Yield 0.31 g (76%); diastereomeric ratio 1:1; mp 161–162 °C; HRMS (ESI): calcd for C25H21N5NaO [M+Na]+ 430.1638, found 430.1633; MS (EI): m/z (%) = 237 (24), 171 (100), 91 (53), 81 (42), 77 (65), 55 (37), 41 (31), 39 (42); 1H NMR (300 MHz, DMSO-d6) δ 1.92–2.07 (m, 2H), 2.14–2.29 (m, 1H), 2.31–2.44 (m, 1H), 2.58–2.83 (m, 3H), 2.85–3.01 (m, 3H), 3.08–3.39 (m, 5H), 3.46–3.67 (m, 2H), 3.86–4.04 (m, 2H), 4.11–4.21 (m, 1H), 6.41 (d, J = 7.3 Hz, 1H, Ar), 6.77 (t, J = 7.3 Hz, 1H, Ar), 7.01–7.47 (m, 20H, Ar, 2NH2) ppm; 13C NMR (75 MHz, DMSO-d6) δ 31.2, 31.8, 36.9, 37.6, 51.3, 51.4, 52.1, 52.2, 52.8 (2C), 53.8, 54.7, 60.4, 61.1, 84.0, 85.8, 110.4, 111.2, 111.5, 111.8, 116.2, 116.3, 120.3, 121.1, 123.1, 123.4, 123.7, 124.1, 127.1, 127.4, 127.8, 128.1 (2C), 128.4 (3C), 128.5, 128.6, 129.3 (2C), 129.7 (2C), 137.6, 137.7, 149.9, 150.8, 163.6, 163.7, 181.4, 181.8 ppm; IR (KBr): ν = 3474, 3346, 2816, 2236, 2177, 1636, 1574, 1412, 1225 cm−1.
4.2.11 2-[3-(2-Amino-3-cyano-4H-chromen-4-yl)tetrahydro-4H-pyran-4-ylidene]malononitrile (3k)
White solid. Yield 0.29 g (90%); diastereomeric ratio 1:1; mp 175–176 °C; HRMS (ESI): calcd for C18H14N4NaO2 [M+Na]+ 341.1009, found 341.1004; MS (EI): m/z (%) = 171 (100), 148 (18), 143 (58), 118 (30), 115 (22), 91 (22), 78 (27), 39 (27); 1H NMR (300 MHz, DMSO-d6) δ 2.53–2.85 (m, 3H), 2.97–3.08 (m, 1H), 3.22–3.37 (m, 2H), 3.46–3.56 (m, 3H), 3.75–3.82 (m, 1H), 3.96–4.00 (m, 1H), 4.19–4.39 (m, 3H), 7.06–7.40 (m, 12H, Ar+NH2); 13C NMR (75 MHz, DMSO-d6) δ 31.8, 32.0, 36.3, 36.8, 39.5 (2C), 51.6, 52.1, 67.3, 67.6, 67.7 (2C), 84.2, 85.7, 110.3, 111.3, 111.5, 111.8, 116.3, 116.5, 120.1, 120.9, 122.5, 123.2, 123.8, 124.5, 127.6, 128.6, 128.8, 129.2, 150.2, 150.8, 163.6, 163.9, 179.9, 180.1; IR (KBr): ν = 3470, 3344, 2863, 2235, 2179, 1635, 1576, 1417, 1264, 1101 cm−1.
4.2.12 2-[3-(2-Amino-3-cyano-4H-chromen-4-yl)tetrahydro-4H-thiopyran-4-ylidene]malononitrile (3l)
White solid. Yield 0.56 g (91%); diastereomeric ratio 1:1; mp 181–182 °C; HRMS (ESI): calcd for C18H14N4NaOS [M+Na]+ 357.0781, found 357.0774; MS (EI): m/z (%) = 171 (100), 164 (29), 143 (61), 137 (14), 115 (22), 99 (11), 47 (31), 39 (20); 1H NMR (300 MHz, DMSO-d6) δ 2.84–3.21 (m, 14H, alyph), 4.28–4.33 (m, 2H, CH), 7.07–7.58 (m, 12H, Ar+NH2); 13C NMR (75 MHz, DMSO-d6) δ 29.7, 30.2, 31.3, 31.4, 32.4, 32.8, 36.2, 36.5, 50.1, 51.0, 51.1, 51.7, 85.2, 86.9, 110.4, 111.2, 111.4, 111.7, 116.3, 116.6, 120.0, 120.6, 122.8, 122.9, 123.8, 124.6, 127.6, 128.6, 128.7, 128.9, 150.0, 150.9, 163.8 (2C), 181.2, 181.6; IR (KBr): ν = 3443, 3334, 2231, 2180, 1634, 1577, 1485, 1413, 1255, 1188 cm−1.
Acknowledgements
The authors gratefully acknowledge the financial support from the Presidential Scholarship Program for the State Support of young Russian scientists – PhD (project No. MK-899.2014.3).


