Why did Louis Pasteur (1822–1895) move away from his initial studies of crystallography, measuring the rotation of the plane of polarisation of light after crossing crystals or solutions, to explorations of fermentation, which then led him to study microbiology? In a previous article [1], Pasteur's research on the doubling of tartaric acids and tartrates were analysed based on the entirety of his scientific articles (initial versions, also compared with later editions, notably in collections of his works). It was shown how Pasteur was the direct scientific heir of Jean-Baptiste Biot (1774–1862) and Auguste Laurent (1807-1853), and how he had benefited from the founding ideas of the latter, who was his "tutor" in the laboratory of Antoine-Jérôme Balard (1802–1876).
In the present text, the project is different, and double in scale: all of Pasteur's publications and notes from 1848 to 1880 are used for a better understanding of the transition between his crystallographic research and his studies of fermentations, which mark the end of the period considered in this text; however, not all are cited as such a study was made by Flack [2].
Here we analyse the evolution of scientific thought for a man who has inspired hagiographers as well as detractors, some of them forgetting that the hypotheses, ideas, and theories that scientists use operationally evolve slowly, sometimes contradictorily, as the results of experiments are added. The history of this evolution and that of the progress of atomic theory are elaborated side by side in order to understand how Pasteur "succeeded in tracing a path of certainty through great uncertainties," which, for Debru, "is the very definition of modern experimental science" [3]. Quotes (in italics) are given in cases where they show Pasteur's ideas more faithfully than the commentary of his texts. Many terms used by Pasteur and his contemporaries, when quoted here, are placed in quotation marks so that they are not given a modern meaning, which would be anachronistic or undue. Finally, a synthesis is made between competing hypotheses about the evolution of Pasteur's research, from crystallography to biochemistry and microbiology.
We add that for these analyses, only excerpts from the most prominent publications are given here, because Pasteur repeats himself frequently. With some exceptions, the publications are mentioned in chronological order, in order to clearly separate the facts — established based on publications primarily from the Académie des Sciences (French Academy of Sciences) — and the ideas given in later published texts (by Pasteur or by his historians) and which clearly distort the appreciation of Pasteur's thinking at the time when he conducted his research. With regard to notions of atoms and molecules, anachronisms are avoided, the detailed discussion of the evolution of Pasteur's ideas on this subject having been made in [1]. However, observations related to these concepts will continue to be necessary when they allow a better understanding of Pasteur's thinking at the time he conducted his research, and to better understand the reasons for his change in scientific orientation.
1. Tartaric acids and organic compounds
The study whose results are given here begins with the publication of May 22, 1848 [4], which corresponded to the announcement at the Académie des Sciences of the discovery to which Pasteur would often refer: "racemic acid". Discovered by the Alsatian industrialist Charles Kestner (1803-1870), racemic acid was a mixture of two enantiomers (modern term used to designate compounds whose molecules are mirror images of each other) of "tartaric acid" (or 2,3-dihydroxybutanedioic acid); these compounds are today designated by (2R, 3R), or (+), and by (2S, 3S), or (−) [1].
Pasteur had graduated from the École Normale Supérieure two years earlier, and Balard had welcomed him to his laboratory, where Laurent worked, having taken a sabbatical from his position as professor of chemistry at the University of Bordeaux [5, 6]. Laurent's contributions to Pasteur's initial works — and in particular to the 1848 result — were essential: Laurent taught Pasteur to use crystallography and the measurement of optical activity in conjunction (namely the measurement of the rotation of the plane of polarisation of light when the latter passes through crystals or solutions). Better still, Laurent provided Pasteur with the crystals that he analysed [1]. Yet Laurent was not quoted in the article describing the duplication of tartrates. This led him to send an article to the journal Comptes Rendus de l'Académie des Sciences (in English, **Proceedings of the Academy of Sciences**) a few weeks after the publication of the discovery of the two tartaric acids (+) and (−), in which he indicated his contributions [7] (in the later references to the research which led to the duplication of tartrates, Pasteur rarely quoted Laurent, which is surprising).
The principal scientists who had prepared the discovery announced on 22 May 1848 were René Just Haüy (1743–1822), Louis-Joseph Gay-Lussac (1778–1850), Carl Scheele (1742–1786), Kestner, François Arago (1786–1853), Eilhard Mitscherlich (1794–1863), Biot, and Laurent [1]. Tartaric acid (+) had been studied since 1769, and Kestner's discovery of what was called "paratartaric acid", or "thannic acid" (having been discovered in Thann, Alsace) in 1822 was obviously crucial for the identification of two of the three isomers of tartaric acid (see Figure 1): more precisely, for his study, Pasteur made use of techniques for studying the orientation of the faces of the crystals by using techniques initially developed by William Hyde Wollaston (1766–1828) and by Biot, as well as measurements of the rotation of the plane of polarisation of light, developed by Biot on the basis of discoveries by Étienne-Louis Malus (1775–1812) and Arago.
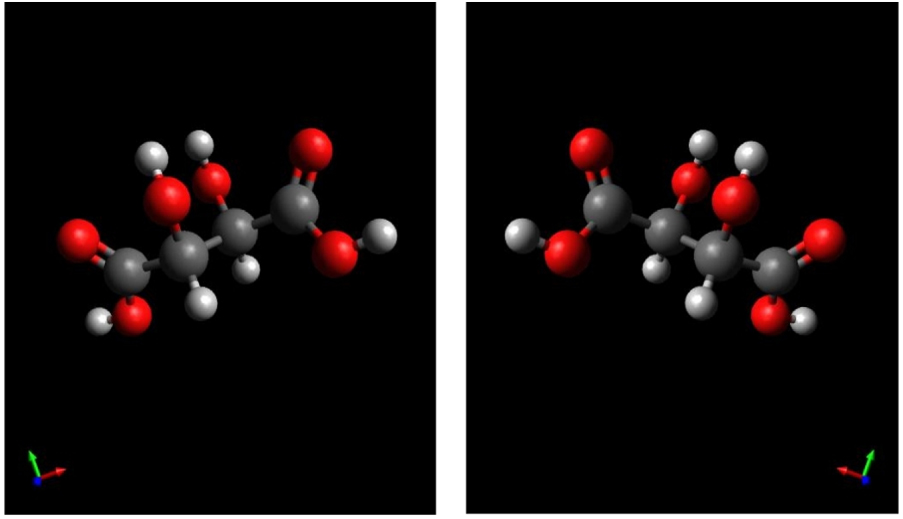
The two optically active isomers (2R-3R) and (2S-3S) of tartaric acid, or 2,3-dihydroxybutanedioic acid. Here, as in the other figures, the representations are modern; in the 1850s and 1860s, when Pasteur studied these compounds, chemists generally had no geometric or stereochemical idea of the form of "molecules", entities which were then often confused with "atoms"; these entities did not correspond to the objects we designate under this name [2]. After the discovery of the "molecular asymmetry" of these two tartaric acids (among three isomers of "tartaric acid"), Pasteur [8] and his contemporaries called these "levoracemic acid" and "dextroracemic acid". Masquer
The two optically active isomers (2R-3R) and (2S-3S) of tartaric acid, or 2,3-dihydroxybutanedioic acid. Here, as in the other figures, the representations are modern; in the 1850s and 1860s, when Pasteur studied these compounds, chemists generally ... Lire la suite
Following Biot, Pasteur reiterated that organic compounds were more frequently optically active than mineral compounds [9]: "Everyone knows, indeed, from the beautiful and numerous researches of M. Biot, that many organic substances have the unique property in the state of dissolution of deviating the plane of polarisation of light rays." He organised his research around this idea.
This period predated the first international congress of chemists by more than ten years, which Friedrich August Kekulé (1829–1896), Charles Adolphe Würtz (1817–1884) and Karl Weltzien (1813–1870) organised in Karlsruhe and where Stanislao Cannizzaro (1826–1910), taking up the ideas of Amadeo Avogadro, circulated a thesis which proposed a clear definition of the concepts of atoms and molecules. On the contrary, in 1848, these notions were very indistinct, to the point of being interchangeable in words, scientific writings and thinking. The term "molecular asymmetry," used by Pasteur and others alongside "optical asymmetry" in the 1850s — and even in the following decades — cannot be interpreted with the meaning of "molecule" that we have today; this is one of the reasons why we propose not to substitute it with the expressions "molecular chirality" or "chirality," unlike the terms "hemihedria" or "rotational activity," which have retained their exact meaning due to being experimental [10, 11]. In [1], it is shown that Pasteur used the word "molecule" with a meaning similar to that given by Haüy [12] when he proposed that the crystals were stacks of "integrant molecules," having generalised the observation in which a small rhombohedral crystal which had broken had made a smaller rhombohedral crystal appear.
This is what is detected in [13], when Pasteur wrote that Haüy "admitted very well that two substances of the same chemical composition [referring to elementary chemical composition, emphasis by us] could have different crystalline forms. What he did not admit, and what seemed inconceivable to him, was that substances having the same chemical composition, and whose molecular arrangement of the elementary atoms was the same [here, it is Pasteur who emphasises], had the same crystalline form." The end of this quotation clearly shows how wrong it would be to believe that Pasteur — like his contemporaries — could have had a clear idea of the concept of an atom or a molecule at the time in history that we are considering here. This can be found in the following: "We can therefore very well, in my opinion, until proof to the contrary, admit that this variety of barytocalcite is only a variety of carbonate barite united by accident atom by atom with lime carbonate" [13, 14]: the word "atom" can only designate an elementary part. Likewise for the following passage: "I therefore think that it is impossible to doubt that a certain molecular group remains constant in all these salts; that the water of crystallisation, that the bases, relegated to the extremities of this group, modify it at these ends only, barely touching, and to the extent of the difference of the angles observed between the facets, to the central molecular arrangement. Certainly I am only confirming here the opinion that all the chemists would state, namely that among all the salts of the same acid, there is something in common. These facts, however, show us, moreover, the close relationship which exists between the crystalline form and the molecular constitution, and the light which can be cast, via crystallographic studies, on the arrangement of atoms" [15, 16]. This quotation raises the question of how Pasteur and his contemporaries imagined these structures on a very small scale [10, 17, 18, 19, 20, 21]. When researching the intellectual evolution of Pasteur, this question is clearly necessary, especially because French chemists, including Pasteur [22], were passionate about the writings of Antoine Laurent de Lavoisier, who, taking up the work of Condillac, had insisted on the importance of nomenclature in chemistry [23].
With this last quotation we can observe that if modern terminology is used, then it is true that crystallography gives information on the arrangement of atoms; on the other hand, it would be anachronistic to see the prefiguration of the works of William Henry Bragg (1862–1942) and William Lawrence Bragg (1890–1971) in 1915 at the end of Pasteur's sentence, since X-rays were not discovered until 1895 by Wilhelm Konrad Röntgen (1845–1923) [24].
Some have considered it "remarkable that, without the clear idea of atoms and molecules, it could have been imagined that molecular chirality [Pasteur speaks of "optical asymmetry" or "molecular asymmetry"] was the result of helical arrangements of objects in crystals." They evoke texts — after the doubling of tartrates — where Pasteur discusses a tetrahedral organisation [25, 26], but it is anachronistic to think that Pasteur correctly understood that certain chemical groups (in the modern sense) formed tetrahedral patterns; this idea was only introduced later, by Joseph Achille Le Bel (1847–1930) and Jacobus Henricus van 't Hoff (1852–1911), and this time with a modern idea of atoms and molecules [10]. Moreover, "molecular asymmetry" is not the result of "helical" arrangements of atoms, neither with the pasteurian meaning, nor with the modern meaning of the word "atom" [1].
Among the ideas that Pasteur had to consider for his interpretations was the difference between organic compounds and mineral compounds, identified by Biot [4, 9]. He also was astonished by how quartz rotates the plane of polarisation, on the one hand, while "paratartaric acid" (or racemic acid) and its salts do not. In 1850, Pasteur thus wrote [14]: "The cause that produces hemihedria can have two distinct origins. It can reside in the chemical molecule itself, and be transported to all combinations of that molecule. This is the case for tartaric acid and levoracemic acid. The asymmetry of the shape [of the crystal] may, on the other hand, be only a consequence of the aggregation mode of the molecules in the crystal, which probably is what happens with quartz. In the latter case, once the crystalline structure is destroyed, there is no longer any asymmetry, there is no longer any possible phenomenon of rotational polarisation, and the substance, in the state of dissolution, cannot deviate the plane of polarisation of the light. It will deflect it to the crystallised state. Even in the case where asymmetry exists within the chemical molecule, as a result of a special arrangement of atoms in this molecule, it may occur that the substance does not deviate it in the state of dissolution."
In this extract, the "chemical molecule" seems to be the "integral molecule" of Haüy, a small entity conjectured and misunderstood at the time, as previously reported in [1]. As for the arrangement of the "atoms", it therefore cannot be their current conception, despite the appearance of meaning that the sentence takes a posteriori. Read without anachronism, this sentence contains terms that are too vague to be considered "prescient" [2, 27, 28].
In the end, the main merit of Pasteur, from his thesis years to the publication of the doubling of tartrates, is his particularly continuous scientific activity, even if he repeats himself widely from publication to publication [1]. His ability to synthesise must also be observed, as shown in his article of March 20, 1848 [13], which cites the research of Laurent and others, such as Gabriel Delafosse (1796–1878), who was a pupil of Biot and a professor of Pasteur. In this text, the following crystalline forms are analysed: "all the dimorphic substances" then known to him and Delafosse, namely sulfur, calcium carbonate (aragonite), "baryto-calcite," "potash nitrate," "sodium nitrate," "iron sesquioxide," copper sulfide, silver sulfide, "potash sulfate," mercury iodide, "mesotypes," micas, "lead sulfotricarbonate," nickel sulfate, zinc selenate, garnet, idocrase, naphthalene chloride, "monochlorine naphthalene chloride," pyrite, arsenic acid, and "titanic acid".
In the memoir of May 22, 1848 [4], it is the "tartrates" and the "paratartrates" which are primarily considered, but the young scientist seeks fruitful generalisations: "It will be said, and rightly so: All organic substances that deviate from the plane of polarisation when they are dissolved will therefore enjoy hemihedria. I would very much have liked to present this work to the Academy only after examining organic bases, camphor, and other substances. But here we encounter great difficulties in the search for hemihedria. The beauty of tartrate crystals, their size, served me considerably. However, I have been able to study candy sugar easily, and I can announce from my own research that this substance is hemihedral, and enjoys a high degree of polar pyroelectricity. It was even by studying this latter property that I was assured of the hemihedria, which I then realised via careful observation of the crystalline form. Later, I found that this determination had already been made a long time ago by Dr. Hankel."
At this stage, Pasteur had moved away from the morphological study of the crystals to the study of the possible rotations of the plane of polarisation which they induced, which had led him to better characterise the hemihedria of tartrates. It was a path that appeared fruitful, and which we see him following in his later research.
2. Post-1848
In April 1849, while pursuing his research on tartrates, Pasteur announced that he had discovered that a salt of formic acid was hemihedral [13, 29]: "In closing, I will announce to the Academy a result that seems to offer much interest. I found the double hemihedral character in another organic salt, strontiane formate. This salt crystallises in right prisms with a rhombic base, bearing hemihedral facets on the base edges, and which lead to two identical but inverse tetrahedrons [emphasis by Pasteur]. Then, at the end of that same year, Biot informed Pasteur that "amyl alcohol," produced during the fermentation of potato starch, deviated from the plane of polarisation of light [30]. Pasteur reported difficulties in physically characterising the "compound," beyond the determinations of the elemental composition; this is understandable today, since there are 12 isomeric compounds having the general formula C5H11OH (see Figure 2) [31].
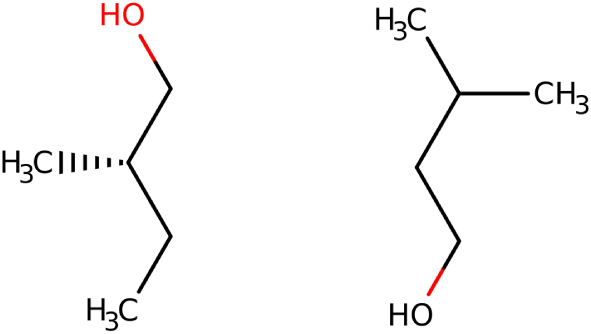
The main amyl alcohols explored by Pasteur: (-)-(S)-2-methylbutan-1-ol, optically active, and 3-methylbutan-1-ol, optically inactive [28, 31].
If fermentation thus appeared in Pasteur's field of scientific study, it is not certain that this immediately changed the course of his experimental research. His publications of 1849 remain devoted to tartaric acids and tartrates; moreover, for a long time, Pasteur remained attached to his 1848 success and pursued direct extensions of it [30, 32].
This observation is historically important, for some saw in the studies of amyl alcohols reasons why, a few years later, Pasteur gradually left the physicochemistry of crystals to research fermentation, then microbiology. On the other hand, others proposed that the transition was rather due to Pasteur's interest in industrial problems when he was appointed to Lille in 1854 [26]. We will take sides on this later.
In 1850, therefore, Pasteur continued to be interested in tartaric acids and "lime" tartrates (the term used by Pasteur is retained here, whereas, for this example, in modern days we would say "calcium," just as we would say potassium, sodium, ammonium, etc.), "potash," "soda," "ammonia," "potash emetic," "ammonia emetic" [33]. However, he extended his research efforts to other organic compounds. This was indeed a promising path, since the living is a source of chirality, in the absence of having the prerogative to rotate the plane of polarisation of light [34]: "The facts that I collected this year relate to asparagine, aspartic acid, the combination of glucose with sea salt, and strontiane formate. […] In examining the crystalline form of asparagine, I have undoubtedly recognised that all crystals of this substance are hemihedral. The hemihedria is, moreover, non-superposable. It was therefore likely that this substance should have the property of rotation, and this is indeed what the experiment has confirmed. […] The connections between asparagine and aspartic acid indicated the probable existence of the rotatory property in aspartic acid. Indeed, aspartic acid deviates from the plane of polarisation of light rays, and its rotational capability has great analogies with that of asparagine. […] Finally, as the recent research of chemists tend to admit that asparagine is the amide of malic acid, I was led to search for rotatory capability in malic acid and malates. The experiment met my expectations again. Malic acid, and the salts derived therefrom, have the property of deviating the plane of polarisation of the light rays; and I have found the non-superposable hemihedria in several malates. But there is a fact that I want to emphasise above all about malic acid. This acid offers, in the peculiarities of its rotational capability, very great analogies with the tartaric acids that are right-handed and left-handed; and these analogies naturally lead to the belief that there are intimate relations of asymmetric molecular arrangements between malic acid and one or the other of the two tartaric acids. It is very likely that there must exist, between malic acid and one of the two tartaric acids, right-handed or left-handed, a common molecular group, with the modification that may be brought, in this group, by the difference in composition of these acids" [author emphasis].
We comment on this passage by observing that asparagine is not the amide of malic acid (the nitrogen atom does not replace the stereogenic carbon atom) (see Figure 3), but, above all, that the hypothesis of "common molecular grouping" between tartaric acids and malic acids is not the cause of the cluster of optical properties; it is rather the tetravalence of carbon, and the tetrahedral organisation of its substituents, which leads to chirality. Of course, noting these errors cannot be a criticism of Pasteur, for ideas on the concept of radicals were still much debated; they had been revised by Laurent around 1844, starting from the theory of the electrochemical dualism of Dumas [35], and it is only in 1860 — that is to say the year of the colloquium of the chemists of Karslruhe — that Auguste Cahours (1813–1891) authorised himself to write [36, 37]: "We give the name of radicals to particular entities whose complex nature can be demonstrated by means of physical forces or by the intervention of reagents, but which, although formed of several elements, have the characteristics of simple entities and fulfill exactly similar functions. They are, in short, compounds which possess the property of forming combinations with simple entities that are entirely similar to those produced by the latter via their mutual union." At that time, Cahours observed that some radicals had been isolated: cyanogen, cacodyle, etc. Others, however, "have at this time only a purely hypothetical existence": methyl, ethyl, acetyl, benzoyl, ammonium, etc.
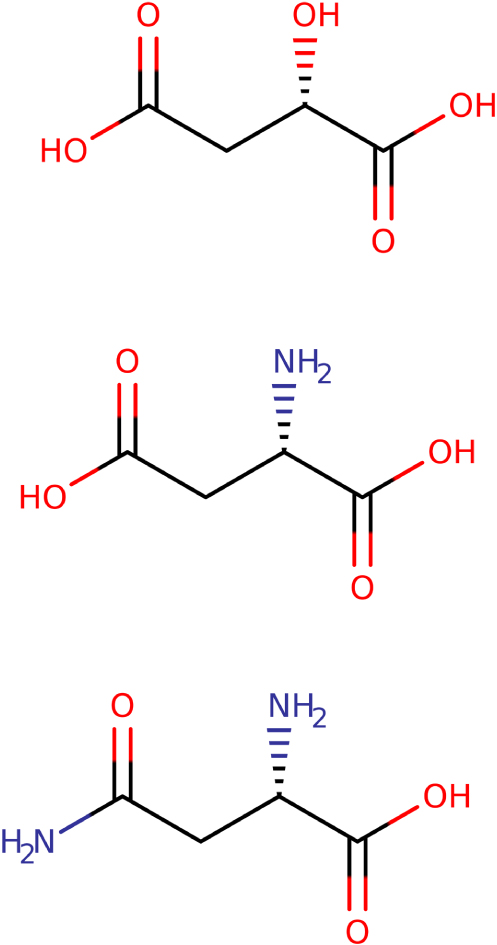
Malic acid, aspartic acid, and asparagine (only the L forms are shown here).
On September 30, 1850, Pasteur published his results of studies on strontiane formate, aspartic acid and aspartates, asparagine, malic acid, malates, fumaric acid and fumarates, maleic acid, and "sea salt glucosate" [38]. As with other previous articles, he publishes almost identical paragraphs in several successive articles, retaining the terms "atoms" or "molecules" with the meanings of his time:
"For a very long time it was completely unknown what could be the cause of this asymmetry of the crystalline form. There is only one work, that of Mr. Delafosse, where, for the first time, there was an attempt to establish that this phenomenon of hemihedria was due to the intimate constitution of the crystal. To account for the phenomenon, Mr. Delafosse considers the internal structure, at the layout of physical molecules, without going as far as the mode of arrangement of atoms in the chemical molecule." Pasteur was too precise not to be aware of the relation, in the end of this excerpt, between the two expressions "physical molecules" and "chemical molecule," but there is, to our knowledge, no discussion on his part of this difference. We are obliged to relate the first term to Haüy's texts (the "integrating molecules"), and associate the second with the explorations of chemists, without being able to explain the relationship between the two terms at this time.
The conclusions of the 1851 text are almost verbatim those of 1850: "The preceding facts lead us to suppose that the hemihedry of strontiane formate is not due to the arrangement of atoms in the chemical molecule, but to the arrangement of physical molecules in the total crystal." This observation reinforces the hypothesis we have just made.
Clearly, the question of the optical activity of the entities is far from being resolved, which is why Pasteur continues his research: [39] "To this day, however, no laboratory methods have led to the production of a substance having an action on the plane of polarisation if it started with compounds that did not themselves possess this faculty. Here we find an echo of his ideas on the optical activity of living compounds.
That same year, Pasteur again approached the question of fermentation: starting from crystals produced by Victor Dessaignes (1800–1885), a collector from Vendôme who conducted chemistry in a personal laboratory [40], he did not detect optical activity in "natural" crystals. These crystals had been obtained by fermentation of ammonium fumarate with aspartic acid [41]. Worse, the optical activity of "aspartic acid" depended on pH: dextrarotatory at high pH, levorotatory at low pH.
However, Pasteur continued his research on various organic compounds, convinced of the importance of the discovery he may have made with tartaric acids and tartrates: "Today we see that the combinations active on polarised light can be little altered in their constituent molecular grouping to preserve, without exception, all their chemical properties, losing only, in their constituent molecules, this special asymmetry which produces the right-handed or left-handed character. […] All these four peculiarities, which form so many exceptions to the ordinary laws of the rotational phenomenon, have not yet appeared in any substance. However, active malic acid has offered me these same peculiarities in a more prominent way than tartaric acid."
At the time, he had not found mesotartaric acid, shown by how he conjectures its existence [1]: "There are also strong reasons to believe that there may be a tartaric acid corresponding to inactive malic acid. This acid would be neutral with regards to polarised light like racemic acid, but would differ from the latter in its molecular constitution, and could not be split into two tartaric acids, right-handed and left-handed."
3. 1852: Appointed in Strasbourg
When Pasteur was appointed to the University of Strasbourg in 1852, he continued to publish quickly, always with his fancy style which was classic of the time [1], but in a particularly redundant way from article to article: in a communication from 1852 [42], we find the same sentences as in the previous texts.
It was at this time that Mitscherlich reported to him the presence in Saxony of a factory which may have produced the racemic mixture of tartaric acids industrially [43]. Eager to win the award of the Société de pharmacie de Paris (Pharmacy Society of Paris), Pasteur left for Saxony. Once there, however, he noticed that the manufacturing process no longer produced a racemic mixture since the factory changed the supplier of tartar (wine tartar, not to be confused with calcium carbonate [1]). He visited the quarries and concluded that the problem resulted from refining: the racemic property was eliminated [43].
That same year, he continued his research on quinine, cinchonine and quinidine, observing that quinine was composed of two isomers, like cinchonine. However, he did not understand that quinidine was only the dextrorotatory isomer of quinine. He observed that in the sun, the solutions of these compounds change color. Based on this observation, he started voluntarily heating various compounds in search of an effect on optical activity (see Figure 4). He called the heated quinine "quinicine" and hoped to have found a new drug, but the clinical trials at the Hôpital de la Charité in Paris were a failure [44].
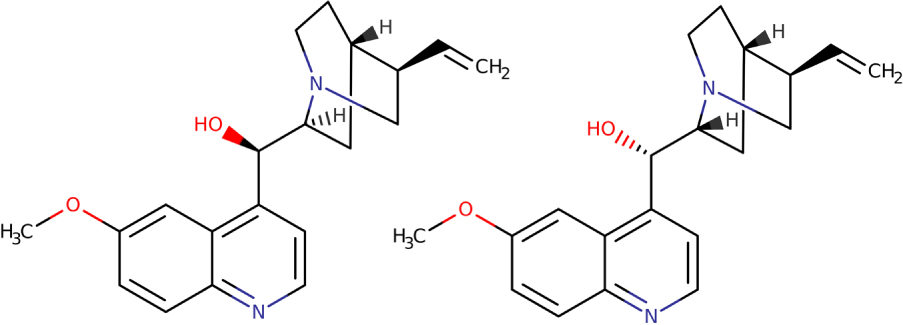
Quinine ((R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol) and quinidine ((S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol).
Above all, in Strasbourg, he continued his research on optical activity, exploring all the samples in the Faculty's collections, and convinced himself that the minerals and chemical substances artificially produced in the laboratory would be dominated by symmetry, and that the only entities that deviated from the plane of polarisation of light would come from plants and animals: the rotational capability would differentiate the animate from the inanimate, life from what is not [42].
At the time, and in particular thanks to Biot, numerous optically active organic compounds had been identified [45], such as amygdalin, various sugars, essential oils, mandelic acid (then called “amygdalic”), camphor, morphine, strychnine, brucine, cinchonine, quinine, albumin, etc. The known active compounds were therefore predominantly organic, with the notorious exception of quartz, but the latter lost its optical activity in the molten state. Pasteur made a "universal" hypothesis that he would keep for a long time [44]: "At the time of their grouping, the elementary atoms [the expression alone suffices to show, once again, how different their notion of an atom was from ours] are subjected to an asymmetric influence and, since all organic molecules which originated in similar circumstances are identical, whatever their origin and place of production, this influence must be universal. It would embrace the entire globe. It is due to the molecular asymmetry of the natural organic products of plants, products that we find in animals almost without alteration, and where it plays a mysterious role of which we have no understanding yet. The universe is an asymmetrical whole, and I am convinced that life, as it manifests itself to us, is a function of the asymmetry of the universe or of the consequences it entails."
In his text, in which he presented in detail his investigations on tartaric acids, Pasteur reported the practices that lead to racemic properties without understanding them, moreover pointing out several times that mother liquors waited several years. Then, while he published notes on quinidine, strychnine, quinine, and quinidine, the mystery of the racemic mixture continued to concern him [46, 47]: "Moreover, one must look at all the hemihedral faces of a crystal as one of the many secondary forms that any substance can always cover. This is because this absolute identity for all that is not hemihedria and sense of the rotational phenomenon [tartrates] exists only as long as the two tartaric acids are united with inactive combinations on polarised light. But if they or their derivatives are placed in the presence of products which have any molecular action on the plane of polarisation, then any identity ceases to take place."
4. The move to Lille
The transition to fermentation research took place in Lille, after Pasteur was appointed dean of the new faculty of science in 1854. Some have said that Pasteur was led to study fermentation because of the industrial activities of the Lille region (alcohol manufacturing or breweries); others have assumed that Pasteur was led to research fermentation by his research on "amyl alcohol" [48, 49, 50]. We believe that there is no need to choose between these two explanations, for they are not mutually exclusive: scientific activity is based on a set of conditions, material and intellectual; and the reading of Pasteur's publications suggests, moreover, that he had already cleared the ground of chirality extensively enough that he could consider it a good scientific strategy to consider new questions, in order to solve or abandon a problem whose solution evaded exploration.
Certainly, around Lille, the agricultural and food industries were essential, in particular alcoholic fermentations from beets, but also breweries [50, 51, 52]. At the time of Pasteur's appointment, the Minister of Public Education had insisted on the applications of science, in support of the industries of the region: "I do not need, Sir, to remind you of the importance attached to the success of this new Faculty of Science placed in a city which is the richest center of industrial activity in the north of France" [53]. Consequently, Pasteur made efforts to ensure that the students had not only theoretical training, but also technological and technical knowledge: he organised visits to factories [48, 54] and addressed the phenomena that took place during the fermentation of beet juice in his classes [55]. This supports what I call the "Lille hypothesis" of his scientific evolution. However, this change of workplace, which led to the study of organic compounds, is also consistent with the "universal hypothesis." There is no need to oppose these possibilities; on the contrary, there was for Pasteur a sort of double logic to evolving towards research on fermentation.
What is certain is that shortly after the beginning of the courses of the 1856–1857 academic year, in the autumn, the father of one of the students, M. Bigo, who made alcohol from beet juice, presented to Pasteur his production difficulties: the alcohol obtained from the beets was acidic, of poor quality, and the fermentation vats had a "foul" smell [43]. Pasteur agreed to study the problem, and found himself directly confronted with the question of fermentation [26, 51, 56]. Factual indications of this episode can be found in his laboratory notebooks: the name of Bigo and the name of the street where Bigo's factory was located (namely, rue d'Esquermes, and not d'Esquermay as has been written) [26, 50]. Moreover, it is known that in December 1856, Marie Pasteur, Pasteur's wife, wrote to her father-in-law: "Louis continues to work hard. He is now immersed in beet juice up to his neck. He spends his days in an alcohol factory" [57].
Pasteur identified lactic acid as one of the products that adversely affected the quality of Bigo's mixtures. This is how he was led to research lactic fermentation, which he made part of his course [51]: on February 2, 1857, the first three lines are "Continuation of the study of fermentation. The phenomenon is known to us in its main conditions. Its importance demands more detail "[26]. This is no longer an "amyl" or "universal" hypothesis, but a "lactic" hypothesis, which is only secondarily a "Lille" hypothesis.
What is also certain is that it is from this Lille period (1855–1857) that two articles on amyl alcohol are dated [14]. The first, already mentioned [30], is a short summary of the research presented to the Académie des Sciences in 1855: Pasteur describes the composition and purification of commercial samples of "amyl alcohol", indicating in the summary that commercial amyl alcohol is a mixture of two very similar isomers, perhaps even chemically or physically indistinguishable. At the end of his article, Pasteur wrote that his interest in these compounds arose from crystallographic considerations: the two alcohols are liquid under ordinary conditions, but he had prepared a series of derivatives that crystallised and found that the optically active alcohol derivatives violated his laws [28, 57, 58]. Discovering a refutation of the theories that he himself had proposed, Pasteur had to have been shaken. It is not certain that, with the experimental results available to him at the time, he could imagine how he could reconcile contradictory facts.
What is hardly evoked by historians who were interested in the work in fermentation of Pasteur [26, 50] is that Pasteur was also interested in lactose, in that same year of 1856 [58]: "When Kirchhoff, a member of the St. Petersburg Academy, had published the remarkable discovery of the transformation of starch into sweet matter, Vogel tried Kirchhoff's experiment on milk sugar. He boiled 100 grams of milk sugar with 400 grams of water and 2 grams of sulfuric acid for a few hours, adding a little water occasionally to replace the water that evaporated by boiling. The liquor, saturated by the chalk, evaporated in an oven, giving a thick brown syrup, which became crystalline after a few days." He adds : "This material, analogous to brown sugar, has a much sweeter flavour than is a highly concentrated aqueous solution of milk sugar. This excessively sweet taste led to the suspicion that real sugar had formed, giving rise to alcoholic fermentation. This product had hardly been introduced, under favourable circumstances, with brewer's yeast, so that alcoholic fermentation was established in the strongest way, while milk sugar never ferments. […] Such is the sugar which, with great reason, no doubt was taken for starch sugar; and, at all times, the physiological ideas expressed on milk sugar were based on the purported transformation of this sugar into starch sugar. But, in reality, acid-modified milk sugar is quite different from glucose. I propose to call it lactose. The name milk sugar or lactin would be reserved for the crystallisable sugar of milk."
Here, the confusion of lactose with galactose must not lead to the failure to detect that fermentation issues were again appearing.
This preceded the second thesis, devoted to amyl alcohols, where Pasteur examined the crystallographic question thus posed, and where he writes to have not succeeded in inducing crystalline hemihedria. He concludes that crystalline hemihedria is not a necessary manifestation of "molecular asymmetry" [30, 59]. Let us note in conclusion that this last article evokes "the atomic arrangement of molecules," which gives us an additional indication to analyse Pasteur's structural conceptions at this time.
In 1857 and 1858, Pasteur explained himself on the question of fermentation, a new field with which he partially replaced the first [60, 61, 62]: "I think I must indicate in a few words how I was led to concern myself with fermentation research. […] Perhaps it is surprising to see me approaching a physiological chemistry subject that is apparently far removed from my initial works. However, it relates very directly to it. In one of my last communications to the Academy, I established that amyl alcohol, contrary to what had been thought until then, was a complex matter formed by two distinct isomeric alcohols, one deviating the plane of polarisation to the left, the other devoid of any action. […] I therefore resolved to carry out an in-depth study of the two amyl alcohols, to determine, if possible, the causes of their simultaneous production and their true origin, on which certain preconceptions led me to share the common opinion. […] If [amyl alcohol], when active, originated from sugar, as all chemists admit, its optical action would be borrowed from that of sugar. This is what I am reluctant to believe in the current state of our knowledge. […] The molecular group of amyl alcohol is too distant from that of sugar, if it derives from it, for it to retain an asymmetry in the arrangement of its atoms. Again, these are preconceived ideas. However, they were sufficient for me to decide to study what influence the ferment might have on the production of the two amyl alcohols. These alcohols are always seen to originate in the process of fermentation; this was another invitation to persevere in finding the solution to these questions."
He also adds: "I was led to attend to fermentation following my research on the properties of amyl alcohols and on the very remarkable crystallographic peculiarities of their derivatives. […] I propose to establish in the first part of this work that, just as there is alcoholic fermenting, brewer's yeast, which is found everywhere where there is sugar that splits [an important word for the history of atomism, since it is the same as that which was used for the separation of tartaric acids from racemic, but which has a different meaning in modern language] into alcohol and carbonic acid, so there is a particular ferment, a lactic yeast always present when sugar becomes lactic acid, and that, if any nitrogenous plastic can transform the sugar into this acid, it is because it is for the development of this ferment a suitable food. […] Let us now see the characteristics of this substance, the production of which is correlative with the phenomena included under the name of lactic fermentation. […] Under the microscope, it is comprised of small globules or very short segments, isolated or in clusters, constituting irregular flakes."
We will not discuss here the controversy about the nature of fermentation and the role of microorganisms or inanimate ferments, but we will emphasise that, in this scientific debate, Pasteur based his views on his "universal" hypothesis of life, as indicated in the rest of the text: "These were for me the opportunity and the reason for new experiments on fermentation. But, as often happens in such circumstances, my work has gradually expanded and deviated from its first direction; so that the results that I publish today seem foreign to my previous studies. The connection will be more evident in those that follow. I hope to be able to relate the phenomena of fermentation to the molecular asymmetry inherent in organic substances."
In other words, there is a continuity between his experiments on crystals and his research on yeasts; the common thread is asymmetry [60]. Moreover, instead of adopting a historical interpretation that might be wrong because it is concluded with excessive certainty, how can we not cautiously recall [63] that the texts in which Pasteur evokes "molecular asymmetry" had gradually moved away from experimental reports to become structured research works? This led Geison to distinct views, when he detected differences between Pasteur's notes, notebooks, and publications [50].
In any case, Pasteur began by obtaining the left-handed tartaric acid from the racemic, proof that his concerns of chirality were— clearly — not far removed. Then, in 1858 [64], he showed that succinic acid is "a normal, necessary product of alcoholic fermentation" [65]: "After a few days of fermentation, the initially inactive [racemic] liquid has a substantial rotatory power to the left, and this capability gradually increases as the fermentation continues, reaching a maximum. The fermentation is then suspended. There is no trace of right-handed acid in the liquor, which, evaporated and mixed with its volume of alcohol, immediately gives an abundant crystallisation of left-handed ammonia tartrate. This is undoubtedly an excellent way to prepare left-handed tartaric acid."
Reading the memoirs of this time reveals that it was Pasteur's abilities as a chemist that allowed for his innovative research on fermentation: Pasteur moved away, certainly, from researching fermentation of lactic acid (sour milk), because research on this theme is rare, unlike that of alcoholic fermentation, and because no lactic yeast had been discovered. It should be emphasised, however, that lactic acid is a by-product of optically active amyl alcohol. And his hypothesis on the chirality of life continues to move him: "If I were to be told that, in these conclusions, I go beyond the facts, I would answer that this is true, in the sense that I place myself frankly in a way which […] cannot be definitively demonstrated. […] It is my opinion, according to my current knowledge of the subject, that anyone who will judge with impartiality the results of this work and those which I will publish shortly will recognise along with me that fermentation shows itself to be correlative of life, of the organisation of globules, not of the death or putrefaction of these globules, nor does it appear as a phenomenon of contact, where the transformation of sugar would be carried out in the presence of the ferment without giving it anything, without taking anything from it." Here, it will be observed that there were considerations for living entities in the research on optical activities, and considerations for the living with fermentation: this is the link that clearly needs to be noted in order to understand the transition from crystallography to biochemistry.
Pasteur was then launched into researching fermentation, now called alcoholic fermentation, which he would have initially avoided as it had been studied previously: [66] "I beg you to be kind enough to announce to the Academy a curious and very unexpected result. It is the constant presence of glycerin among the products of alcoholic fermentation." He first discovered [67] that there was no lactic acid in alcoholic fermentation. Then, he attacked Justus von Liebig by name, noting that, contrary to what the latter had published, there was no ammonia in alcoholic fermentation [68].
5. After 1860
In 1860, Pasteur was launched into experiments on the causes of fermentation [69, 70] when he lectured at the Société Chimique de Paris (Chemical Society of Paris), now the Société Chimique de France (Chemical Society of France) [71]: there he insisted on the issues of chirality related to fermentation, such as in the fermentation of tartaric acids. He mentions "amyl alcohol" only to say that he discovered such an inactive alcohol, which is the beginning of a series of compounds. In this emphasis on the fermentation of tartaric acids, and not on amyl alcohol, we propose to see less a desire to reconstruct history, but rather a manifestation of a new scientific interest. Moreover, in a lecture he gave to the Central Society of Veterinary Medicine in 1880 [70], while tracing the evolution of his career, Pasteur said nothing about "amyl alcohol" and he clearly indicated that it was the observation of the enantioselective metabolism of tartaric acids by microorganisms that had directed him towards fermentation. This observation is interesting because it highlights Pascal's personal choice out of multiple causes.
Alongside the exploration of the transition to the study of fermentation, we have so far followed the evolution of Pasteur's meaning of the words "atoms" and "molecules." However, what did he think of these in 1860? Consider three extracts [71]:
- "Remember the definition of the chemical species that I mentioned earlier: it is the collection of all individual entities that are identical in nature, the proportion and the arrangement of elements. […] In isomeric entities, their nature and proportion are the same. The arrangement alone differs. The great interest of isomerism has been to introduce into science this principle that the entities can be and are essentially different by this alone that the arrangement of atoms is not the same in their chemical molecules."
- "Are the atoms of the right-handed [tartaric] acid grouped according to the turns of a dextrorotatory helix, or placed at the vertices of an irregular tetrahedron, or arranged according to a given asymmetric assembly? We cannot answer these questions."
- "Allow me to represent roughly, albeit fundamentally with accuracy, the structure of quartz and that of natural organic products. Imagine a rotating staircase with steps made of cubes, or any other object with a superposable mirror image. Destroy the stairs, and the asymmetry will be gone. The asymmetry of the staircase was only the result of the method of assembling its elementary steps. Such is the case with quartz. […] Imagine, on the contrary, that the same rotating staircase is formed of steps made from irregular tetrahedra. Destroy the stairs, and the asymmetry will still exist. "
The two lessons date from 20 January and 3 February, while the Karlsruhe Congress took place from 3 to 5 September: Pasteur, having not explored the specific question of atomic theories, had no reason to change his point of view. Moreover, its terms remain poorly fixed in 1863 [43]: "Now the atomic groups that make up the molecules of all chemical species are objects and assemblies that we find around us. A priori, therefore, we can believe that they too must be divided into our two categories: groups of atoms that have a plane of symmetry and a mirror image that is superposable on itself, and groups of atoms that do not have a plane of symmetry and a mirror image that is not superposable on itself. In other words, there must be groups of symmetrical atoms and other asymmetrical ones, that is to say right-handed and left-handed groups, groups that are the inverse of each other. These are, for example, the right-handed tartaric group and the left-handed tartaric group. There are a host of groups of asymmetric atoms that are still awaiting the artificial or natural production of their inverses. We have the right-handed sugar; we do not know the existence of the left-handed sugar. We have the left-handed albumin; we are not aware of the right-handed albumin."
Here Pasteur speaks of "groupings of atoms" or "groups of atoms", and no longer of "molecules"; it is not clear what relationship he makes between entities. In 1874, in a letter addressed to Michel Eugène Chevreul (1786–1889), Pasteur evoked a "definition of the (chemical) species" [72]. However, the analyses presented in [1] indicate that, even in the 1910s, ideas remained unclear on this subject.
In any case, his initial ideas on "molecular asymmetry" did not leave him after his change of field of research, and they imply that in 1871, that is to say 15 years after having stopped his research on chirality, he still believed in a universal basis of chirality, and resumed experiments to establish it [73]. He supported this idea in 1875: "To transform an inactive entity into another inactive entity, which has the ability to simultaneously resolve itself into a right-handed entity and its symmetrical partner, is in no way comparable to the possibility of transforming an inactive entity into a simple active entity. We have never done this; on the contrary, living nature does it constantly before our eyes" [74].
In 1883, Pasteur continued to promote his "universal" idea of the physical origin of chirality: [75] "What must be done to imitate nature? We must break with your methods, which are, from this point of view, outdated and powerless. It is necessary to seek to make asymmetrical forces act, to resort to actions that are solenoid, magnetic, to luminous asymmetrical movement, to actions of substances that are themselves asymmetrical. When, pulled along, chained I should say, by the almost inflexible logic of my studies, I passed from the research of crystallography and molecular chemistry to the study of ferments, I was entirely thinking of introducing asymmetry into chemical phenomena."
Or again: "These experiments accentuate the deep dividing line between the mineral kingdom and the organic kingdom, since in order to imitate what nature does, that is to say to prepare a right-handed or a left-handed body, we are forced to involve very particular actions, actions of asymmetry. The dividing line we are talking about is not a question of pure chemistry and the production of such and such products; it is a question of forces: life is dominated by asymmetrical actions whose enveloping and cosmic existence we sense. I even feel that all living species are primarily, in their structure, in their external forms, functions of cosmic asymmetry. Life is the seed and the seed is life. Now, who could say what the fate of these "seeds" would be, if we could replace in these seeds the immediate constituents, albumin, cellulose, etc., etc., with their inverse asymmetric constituents? The solution would consist, on the one hand, in the discovery of spontaneous generation […] yes, there is a deep separation between the organic kingdom and the mineral kingdom. This dividing has two manifestations: on the one hand, we have never made a synthetic product, mineral or organic […] on the other hand, asymmetry presides over chemical actions that give rise to the immediate essential constituents of plant life."
Pasteur's attempts to produce molecular chirality via physical forces did not succeed [51, 73]. Nevertheless, he did not abandon his belief in the role of such universal forces in the genesis of molecular chirality, and even later he conducted experiments to produce optically active compounds using magnetic fields, but no biological factors [73]. In the meantime, fermentation research had led him to study microorganisms, and to all the achievements for which he is rightly famous: acetic fermentation, silkworm disease, anthrax, rabies, etc.
In the end, examination of the texts gathered here shows how false hypotheses based on insufficient theories could allow a remarkably active scientist to progress, to accumulate experimental data, which, long after he had reoriented himself, were enlightened by the works of other scientists: the tetrahedrons of Joseph Achille Le Bel (1947-1930) and Jacobus Henricus Van 't Hoff (1852–1911) had nothing to do with those of Pasteur, but they allowed for the opportunity to understand "molecular asymmetry", and to advance the ideas of "chirality" [1]. In a way, Pasteur justified himself in anticipation in this regard when, in 1878, he brought together earlier works on "molecular asymmetry" to publish them under the title: "Etudes de chimie moléculaire ou recherches sur la dissymétrie dans les produits organiques naturelles" ["Studies of molecular chemistry or research on asymmetry in natural organic products"] (the book was not completed) [75]. He wrote: "But, as Lavoisier says somewhere, it is the fate of all those who work to see a new step that they should take as soon as they have taken the first one, and they would never give anything to the public if they waited until they had reached the end of the career which appears to them sequentially and which seems to be expanding as they progress." This is indeed what he did.
Conflicts of Interest
The author has no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0