1. Introduction
URD ABI (Agro-Industrial Biotechnologies Research and Development Unit) [1], a research unit within AgroParisTech [2], is located in the European Center for Biotechnology and Bioeconomy (CEBB) at the heart of the Pomacle–Bazancourt biorefinery [3]. URD ABI drives innovation and its transfer to industrialization, covering the Technology Readiness Level (TRL) scale from 1 to 4. More precisely, URD ABI aims to transform plant and agricultural materials, as well as agro-industrial by-products, into sustainable industrial solutions, aligning with both the bioeconomy concept and several of the United Nations Sustainable Development Goals (UNSDG) [4].
One pillar of URD ABI, biocatalysis has been at the heart of many flagship projects since the unit was founded. Initially limited to the use of commercial enzymes and liquid fermentation, URD ABI’s expertise in the field of biotechnology has rapidly expanded to molecular biology, and enzymology.
In this mini-review, we will use a few examples to illustrate how biocatalysis has evolved within URD ABI and how it is now positioned as a major asset in the development of sustainable access routes to molecules and materials with (very) high added value.
2. Discussion
2.1. Episode 1: First steps in biocatalysis
Initially, biocatalysis activities at URD ABI were limited to the use of commercially available enzymes. To stand out from the stiff competition in this field, we focused on the use of lipases and laccases for the valorization of phenolic molecules, e.g., p-hydroxycinnamic acids and the corresponding benzaldehydes (Figure 1), which until then had remained relatively unexplored.

Naturally occurring p-hydroxycinnamic acids and corresponding benzaldehydes.
At first, the project focused on the production of new bis- and tris-phenolic molecules to replace bisphenol A, a petrochemical criticized for its toxicity (endocrine disruption) and allergenicity. Starting directly from the p-hydroxycinnamic acids that can be readily obtained from local biomasses (ferulic acid in wheat bran and sinapic acid in rapeseed meal or mustard bran) through commercially available esterase-mediated hydrolysis (Scheme 1) [5, 6, 7], we have developed and optimized two chemo-enzymatic synthetic routes leading to a new family of bis/trisphenols and syringaresinol, respectively (Figure 2).

Esterase-mediated production of ferulic acid and sinapic acid from wheat bran and sugar beet pulp, and mustard and rapeseed cakes, respectively.

Ferulic-acid-based bis- and trisphenols and sinapic-acid-derived syringaresinol.
The synthesis of ferulic-acid-based bis- and trisphenols and sinapic-acid-based bisphenol are based on a one-pot two-step Fisher esterification reaction and pallado-catalyzed hydrogenation of ferulic or sinapic acids, followed by transesterification of the resulting saturated ester by diols (1,3-propanediol, 1,4-butanediol, isosorbide) and triol (glycerol) by using Candida antarctica type B lipase immobilized on polystyrene beads (aka Novozyme 435, CAL-B) (Scheme 2) [8].

Chemo-enzymatic synthesis of ferulic-acid-derived bis- and trisphenolic esters.
Coupled with a membrane purification process [9], this solvent-free biocatalysis, carried out under reduced pressure, enables the production of bis- and trisphenols with high yields (90–95%) and purity (98%), and has been validated on a kilogram scale. It is noteworthy to mention that this strategy has also been successfully applied to the production of bisphenol amides [10].
The second chemo-enzymatic synthetic route developed is based on a strategy fairly similar to the previous one. Sinapic acid was esterified and then reduced by DIBAL-H to afford the corresponding allyl alcohol. The latter was then subjected to a laccase from Trametes versicolor, in a buffer solution, to obtain syringaresinol in 93% yield (Scheme 3) [11]. It is important to note here that the high selectivity of the reaction—obtained by using a design of experiment—allows the almost exclusive production of syringaresinol (β–β dimer) and the absence of the β-O-4 dimer, which is generally the major product of this reaction.

Chemo-enzymatic synthesis of syringaresinol from sinapic acid.
As the original aim was to propose biosourced, non-toxic alternatives to bisphenol A, the study of the toxicity of the aforementioned compounds was realized in collaboration with INSERM Montpellier and showed that they did not exhibit any activity, regardless of their concentration (Figure 3) [12].

Endocrine disruption activity of bisphenol A and bio-based bis- and trisphenols.
Moreover, possessing free phenol moieties, all compounds exhibit valuable antioxidant activities, both in solution or in plastics [13, 14]. Interestingly, the antioxidant activity was further enhanced through a highly selective laccase-mediated oligomerization of ferulic-acid-based bisphenols (Scheme 4) [15].
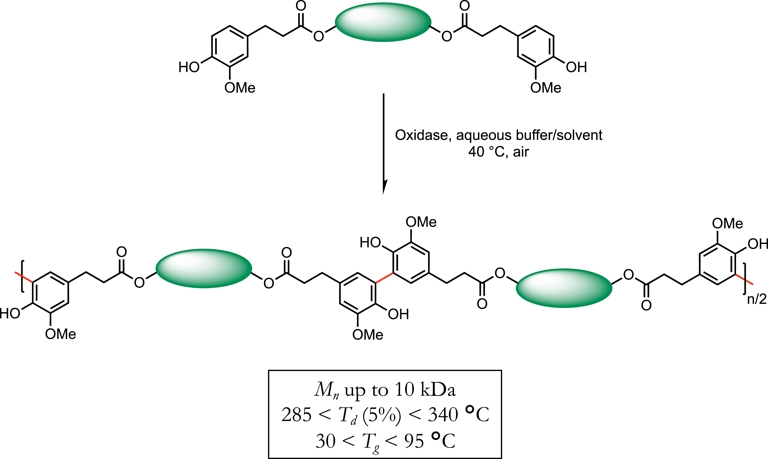
Laccase-mediated regioselective oligomerization of bisphenols.
Subsequently, in collaboration with INRAE, Rensselaer Polytechnic Institute, and ICGM, we demonstrated that, of all the phenolic compounds developed, syringaresinol led to epoxy-amine resins with thermo-mechanical properties similar to those of bisphenol A-based ones [16], whereas the bisphenolic esters provided degradable epoxy-amine resins [12, 17]. It is worth mentioning that these bisphenols were also used as monomers for the production of bio-based non-isocyanate polyurethanes (NIPUs) [18, 19]. High added-value applications have been developed from these molecules. For example, butane-1,4-diyl bis(3-(4-hydroxy-3-methoxyphenyl)propanoate) (BDF), the bisphenol derived from ferulic acid and 1,4-butanediol, has been used to develop (i) new polylactic acid (PLA)/BDF blends with shape-memory elastomer properties [20, 21], (ii) self-healing materials [22], and (iii) reversible adhesives [23].
More recently, still in the field of phenolic compounds, biocatalysis has been put to good use in a project dedicated to the production of naturally occurring sinapoyl malate (Scheme 5), which is proving a multifunctional phenolic compound with UV-filtering, antioxidant and anti-tyrosinase properties for the cosmetics and biocontrol sectors [24].

Sustainable three-step synthesis of sinapoyl malate.
Although highly active, sinapoyl malate was difficult to formulate due to the p-hydroxycinnamic acid ester core and the two acid moieties. To overcome this problem, one solution was to add hydrophobic moieties to the two malic esters. However, such a selective (trans)esterification of these malic acids/esters, without affecting the α,β-unsaturated ester, was not conceivable by purely chemical means. Fortunately, by exploiting the inability of CAL-B to transesterify p-hydroxycinnamic acids, we were able to graft the fatty chains in a completely regioselective manner to produce new and more easily formulated analogs (Scheme 6) [25].

Lipase-mediated regioselective transesterification toward fatty esters of sinapoyl malate.
CAL-B has also been used for the valorization of levoglucosenone (LGO), a chiral molecule with high added value obtained through the pyrolysis of cellulose [26]. Because of its chirality, α,β-unsaturated ketone and ketal, LGO is an ideal platform molecule for organic chemists. Among the many chiral molecules that can be synthesized from LGO, (S)-γ-hydroxymethyl-α,β-butenolide (HBO) and its reduced derivative ((S)-γ-hydroxymethyl-γ-butyrolactone, 2H-HBO) are probably the ones that open up the widest range of possibilities (Scheme 7).

LGO, a valuable chiral chemical platform.
These chiral butyrolactone/butenolide can be used to produce drugs [27], aromas [28], polymers [29, 30] etc. Until the URD ABI studied its production from LGO, HBO was obtained by Baeyer-Villiger oxidation of LGO, either in the presence of peracids [31] or in the presence of zeolites [32] (Scheme 8). In both cases, although effective in terms of yield, these two chemical processes are nevertheless dangerous and toxic for humans as well as for the environment due to the use of toxic and/or explosive reagents and toxic solvents.

Hazardous and toxic chemical synthetic routes toward LGO.
To overcome these limitations, we designed and optimized a sustainable alternative chemical pathway based on the use of CAL-B and hydrogen peroxide in ethyl acetate (AcOEt) to produce in situ peracetic acid, which oxidizes LGO to HBO in high yields without the need for harsh oxidizing agents in large excess or hazardous petroleum-based organic solvents (Scheme 9) [33]. It is noteworthy to mention that, to recycle CAL-B, HEPES buffer can be used.

Lipase-mediated Baeyer-Villiger oxidation of unsaturated and saturated LGO into HBO and 2H-HBO, respectively.
As the above examples show, the implementation of biocatalytic reactions using commercially available enzymes has been a key element in the strategy of URD ABI for the production of high added-value molecules and materials using sustainable processes. However, due to the relatively limited number of enzymes on the market, the high potential of biocatalysis could not be fully exploited within URD ABI. Aware of these limitations, it was decided to acquire the capacity to produce enzymes on site and thus go even further in terms of innovation.
2.2. Episode 2: Fully harnessing the potential of molecular biology and biocatalysis
With these new capacities, and in collaboration with the University of Florida, we first explored another biocatalytic strategy for the conversion of LGO into HBO via cyclohexanone monooxygenase (CHMO). This strategy has the advantage of (i) using oxygen from air as oxidative species and therefore significantly improving the system in terms of safety compared to H2O2 and, (ii) not implementing organic solvent such as AcOEt. However, the use of the CHMO collection from Stewart Lab did not lead to positive results and no activity was observed. However, using Cyrene™ as substrate allowed us to observe the formation of 2H-HBO. We then explored the biodiversity using the basic local alignment search tool (BLAST) and the DNA sequence coding for the CHMO from Acinetobacter sp. NCIB 9871 as the parent sequence in an attempt to find new CHMOs. A hit from Pseudomonas aeruginosa strain Pa1242 was identified with a query cover and identity percentage of 97 and 67% respectively. As CHMOs are NADPH-dependent, a quite expensive cofactor, we further implemented the system in whole cells to take advantage of the cells’ capacity to regenerate NADPH by themselves (Scheme 10). Doing so allowed us to reach a concentration of 20 g/L (160 mM) over 48 h or a consumption rate of 401 ± 36 mg/L/h [34, 35].

Bioconversion of Cyrene™ into 2H-HBO.
As it was previously discussed in this review, it is possible to access aromas from LGO, such as the flavoring agent dairy lactone (butter, peach, or fruity aroma depending on the concentration used) [28, 36]. The transfer from a classical organic step toward a biocatalytic one is of particular interest in such a pathway. Indeed, naturality is progressively becoming a requested standard for almost all industries nowadays and it is particularly the case for the food industry. The implementation of a classical organic synthesis step in a pathway causes the natural character of the targeted molecule to be lost. With this first proof of feasibility, we decided to go further in trying to substitute each step by a biocatalytic system in order to access natural dairy lactone. Therefore, we explored the possibility of transforming LGO into Cyrene™ by old yellow enzymes (OYEs) as it could add a second biocatalytic step toward dairy lactone and provide Cyrene™ through a method that does not use transition metals such as palladium. Indeed, some industries that could use Cyrene™ as substitute for DMSO, DMF or NMP are required to have very small traces of transition metal, parts per billion, and a biocatalytic pathway would be able to provide such a grade. The OYE 2.6 from Pichia stipitis was chosen as it displayed the best activity from the set of OYEs tested [37, 38]. Contrary to the transformation of Cyrene™ into 2H-HBO, the whole-cell strategy for scaling up the process could not be applied because of the high toxicity of LGO toward cells. Therefore, a regeneration system of NADPH has been implemented using glucose dehydrogenase and glucose as the sacrificial agent. The process was scaled up to five liters with a final concentration of 250 mM (Scheme 11).

Biocatalytic transformation of LGO into Cyrene™.
Although the system was proven to be efficient, its cost remains relatively high, mostly due to the use of NADP+, which represents more than 70% of raw material cost. Therefore, we looked into a strategy that could allow us to reduce the production cost. We identified that the purification steps of NADP+ production represents the majority of the cost as it is usually performed through ion exchange chromatography followed by a desalting step using activated charcoal. In order to overcome such drawbacks, we developed and patented a membrane filtration strategy that allows purifying high molecular weight adenosine-based cofactors in a 3-to-1 process that enables (i) purifying, (ii) desalting, and (iii) recycling the by-products. The proof of feasibility was made on coenzyme A, FAD, NAD, and NADP+ [39, 40]. In the case of NADP+, we added a final step involving an apyrase from potatoes in order to simplify the profile of by-products (Scheme 12), allowing us to reach higher purity and productivity [41].

Simplification of the reaction medium using apyrase.
Another approach investigated to mitigate coenzyme-associated expenses was the immobilization of the coenzymes on cellulose nanocrystals (CNCs). We used ATP as a case study and were able to graft it by a click chemistry approach onto CNCs. We then used the resulting material in a biocatalysis involving a pantothenate kinase from E. coli for the production of pantethine diphosphate and showed that the biocatalysis was occurring as observed when using free ATP (Scheme 13). It must be noted that both mono and dephosphorylated products were observed. Such results open up real perspectives for cofactor immobilization [42].

Biocatalytic production of pantethine diphosphate using CNC-ATP.
Unlocking the access to coenzymes is crucial for biocatalysis as it can give access to a very large variety of biocatalytic activities.
3. Conclusion
By integrating chemistry and biocatalysis, a powerful approach has been implemented for developing sustainable processes aimed at producing bio-based chemicals and materials. This interdisciplinary synergy leverages the precision of chemical methods alongside the selectivity and eco-friendliness of biological catalysts, such as enzymes and microorganisms. Biocatalysts enable highly specific transformations under mild reaction conditions, often reducing energy requirements and minimizing the need for toxic solvents or harsh chemicals. When combined with advanced chemical methods, biocatalysis can enhance reaction efficiency, yield, and selectivity, opening new pathways for the valorization of renewable biomass into high-value chemicals, polymers, and advanced materials. Key advancements in enzyme engineering, metabolic pathway optimization, and hybrid chemo-enzymatic processes have expanded the repertoire of bio-based products that can be produced sustainably, offering promising alternatives to petrochemical-derived products. This combined approach supports circular economy principles by using renewable feedstocks and designing biodegradable end-products, ultimately reducing environmental impact and reliance on limited resources.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgements
Authors would like to thank the Région Grand Est, the Conseil Départemental de la Marne and the Grand Reims for their financial support.






 CC-BY 4.0
CC-BY 4.0