1. Introduction
Corrosion of copper is an expensive industrial problem. Various strategies have been developed to address the growing need for the inhibition of copper corrosion [1]. Considerable efforts in corrosion science have been done to find organic molecules that have the potential to be applied as corrosion inhibitors and increase their corrosion inhibition efficiency [2]. Among the methods available for the control of metallic corrosion, the use of synthetic corrosion inhibitors is one of the most practical and cost-effective methods. Diazonium compounds are well known in surface chemistry and are commonly used for the surface modification of metals and alloys to form robust and densely packed organic layers due to the formation of covalent carbon–metal bonds [3]. It was shown that grafting of diazonium salts onto various substrates is a powerful technique for surface coating and provides a significant protection against corrosion [4, 5]. With the many possibilities for R-groups on aryl diazonium salts, a wide range of organic derivatives can be obtained from their covalent binding onto a copper surface. Also, different methods for Cu surface modification with aryl diazonium salts such as electrochemical [4, 6, 7], physical adsorption [4, 8, 9], and photochemical [10] are available.
The great interest of diazonium-based layers lies in their covalently attached organic structures that can be developed for various types of interfacial processes [3]. This aspect can be exploited to generate the layer growth mechanism with long-term efficiency adapted to anticorrosion technology. Generally, the films with covalent bonds act as mixed-type inhibitors by decreasing the anodic and cathodic reactions responsible for the corrosion process [11]. Aryl or alkyl organic films have been shown to be effective at preventing corrosion on iron [12], steel [13] and copper [4] surfaces.
Schematic representation of phenyl aldehyde (multi)layer formation on copper surface by electrodeposition via diazonium (A); pathway corresponds to the surface functionalization to phenylimine-1-butanol or phenylimine-2-propanol (B).
In our previous work, we have showed that the steric effects of the aryl substituent have an important effect on the corrosion inhibition properties of diazonium-based organic layers [14]. In this work, we present the effects of various modifiers using readily available and lower-cost materials covalently attached to the same diazonium-based coating on the long-term layer stability and anticorrosion properties. The first organic layer was electrodeposited after in situ transformation of 4-aminophenyl aldehyde into diazonium and subsequently two amino alcohol derivatives, 2-amino-1-butanol or 1-amino-2-propanol, reacted with the grafted aldehyde moieties to further functionalize the first layer (Figure 1). The purpose of our work is to demonstrate the magnitude of the influence induced by only minor changes in modifier structure on the anticorrosion properties of the layer and thus we have employed compounds with close chemical structure. The study of the copper corrosion inhibition was carried out in acetate buffer solution (pH = 3.5) since acetic acid is one of the abundant species which has been commonly used to represent the effect of all organic acids in corrosion studies, at least as far as it concerns the oil and gas industry [15].
2. Experimental section
2.1. Material and methods
4-Aminobenzaldehyde, amino-2-propanol 93%, 2-amino-1-butanol 97%, sodium nitrite, sodium chloride, potassium ferrocyanide, potassium ferricyanide, potassium phosphate monobasic, sodium phosphate dibasic, potassium chloride, sodium acetate, and acetic acid were obtained from Sigma-Aldrich. Hydrochloric acid (37%) was obtained from Carl Roth, 2-propanol was purchased from Merck Millipore. Chemically pure ethanol and purified water (18 M𝛺⋅cm−1, Millipore) were used to prepare solutions. Acetate buffer (50 mM, pH = 3.5, supplemented with 50 mM KCl) was used for electrochemical evaluation of copper corrosion. All experiments reported in this work were performed in quiescent non-deaerated acetate buffer.
2.2. Apparatus
All electrochemical measurements (chronoamperometry, chronopotentiometry and linear polarization, open-circuit potential and electrochemical impedance spectroscopy) were carried out using a PGSTAT302N potentiostat/galvanostat (Metrohm-Autolab) controlled using Nova 1.10 software and equipped with a conventional three-electrode cell: copper foil as working electrode, platinum foil as auxiliary, and Ag/AgCl/3 M KCl as reference.
Copper foils of 30 × 5 × 0.4 mm (EN-1172-Cu-DHP-R240 from KME Deutschland) were used in this study, all cut from the same sheet. The exposed surface area of 0.25 cm2 of copper electrode was used as working electrode. Prior to each experiment, the copper specimens were washed ultrasonically with 2-propanol for 20 min to get rid of the grease, rinsed with bi-distilled water and dried under argon stream.
The protection of the deposited organic layers against copper corrosion was evaluated by potentiodynamic polarization (Tafel) curves and electrochemical impedance spectroscopy (EIS). The copper working electrode was immersed in a test solution for 300 s at the open-circuit potential (OCP) at the beginning of the experiment. Then, the EIS was conducted on the copper electrode at the OCP. The EIS measurements were conducted in acetate buffer pH = 3.5 at OCP at 50 frequencies logarithmically distributed between 9.5 kHz and 0.1 Hz with an AC amplitude potential of 5 mV. For potentiodynamic polarization experiments, the potential was scanned from −400 to 300 mV vs Ag/AgCl at a scan rate of 5 mV⋅s−1. The results were represented as Nyquist plots and interpolated using Randles equivalent circuit. Static contact angles measurements were performed with water using a CAM 101 goniometer (KSV Instruments).
2.3. Electrodeposition of aryl diazonium salts on copper surfaces
The diazonium moieties were prepared under ice immediately before electrodeposition in 10 mL solution of 5 mM 4-aminobenzaldehyde, 5 mM NaNO2 and 50 mM HCl. The electrochemical deposition of diazonium compound was made by chronopotentiometry at − 0.25 mA for 600 s to generate the strong bonds between copper and phenyl. After phenyl aldehyde layer deposition, the second reaction takes place using a well-known coupling method [16], amino groups from amino-2-propanol or 2-amino-1-butanol react with the aldehyde groups to form imine bonds. To the copper functionalized with the organic layer was added 10 μL of 1 mM amino-2-propanol or 2-amino-1-butanol solution in distilled water and allowing it to react 3 h. Finally, the modified copper surfaces were rinsed with large amounts of water.
3. Results and discussion
3.1. Influence of the electrodeposition process on the copper surface
Electrodeposition of aryl diazonium salts formed in situ from aromatic amines is a versatile approach for layer deposition onto the metal surface. The amine concentration, deposition time and electrochemical parameters are the key parameters for determining the final properties and morphology of the layer. The modified copper electrodes developed for corrosion protection were produced by electrodeposition of a phenyl aldehyde layer using diazonium chemistry followed by the binding of amino alcohols via active sites of carbonyl groups (Figure 1).
The reactions based on radicals’ attack characteristic for diazonium electrodeposition generate mono or multilayer films. However, the formation of the aryl diazonium radicals often predominantly leads to multilayer structures (Figure 1A) [3]. The copper surface was grafted with 2-[(phenylmethylidene)amino]butan-1-ol (Cu-P1B) and (phenylmethylidene)amino-propan-2-ol (Cu-P2P) in two steps: (i) electrografting of phenyl aldehyde on metal surface by diazonium electrodeposition and (ii) amino-2-propanol or 2-amino-1-butanol subsequent reaction with immobilized aldehyde (Figure 1B).
In order to study possible adsorption of 4-aminobenzaldehyde, amino-2-propanol and 2-amino-1-butanol, the copper surface was immersed 24 h in 1 mM of each compound. The adsorption properties of the coatings were determined by recording EIS measurements in acetate buffer (pH = 3.5) at room temperature before and after immersion. No significant changes were observed after copper foils immersion suggesting that no significant non-specific adsorption occurs at the metal surface and no stable layers of organic compounds are formed which hinder charge transfer to the electrode surface.
3.2. Contact angle measurements
The contact angle is the angle that occurs between a drop of water and the solid surface under equilibrium. The hydrophilic properties of the modified surfaces were assessed by contact angle measurements.
Contact angle goniometry (𝜃) demonstrated an important modification of surface properties before and after 96 h immersion of bare copper foils in a test solution (Figure 2). The contact angle obtained for bare copper foil indicate a weak hydrophobic surface (81.0°) and after 96 h immersion in corrosion buffer, an important change of the contact angle to 22.4° was determined for bare copper foil indicating important modifications due to corrosion. The initial contact angles measured for Cu-P1B and Cu-P2P coatings are characteristic of more hydrophobic surfaces with very close properties due to very close nature of the amino alcohols (102.7° and 97.7°, respectively). The immersion of the functionalized Cu-P1B and Cu-P2P copper surfaces for 96 h in corrosion buffer induced a very small variation of the measured contact angle for Cu-P1B (to 99.1°) and a slightly more important modification for Cu-P2P (to 88.4°). For comparison, the initial contact angle of 86.9° for phenyl aldehyde layer (PhA) decreases to 61.4° after immersion in acetate buffer solution, indicating a more hydrophilic surface and intermediate anticorrosion abilities between unprotected bare copper and Cu-P1B or Cu-P2P copper surfaces.
Contact angle (𝜃) for different copper surfaces functionalized with organic layers before and after 96 h exposure in acetate buffer solution: bare (a,b), P1B (c,d), P2P (e,f) and PhA (g,h).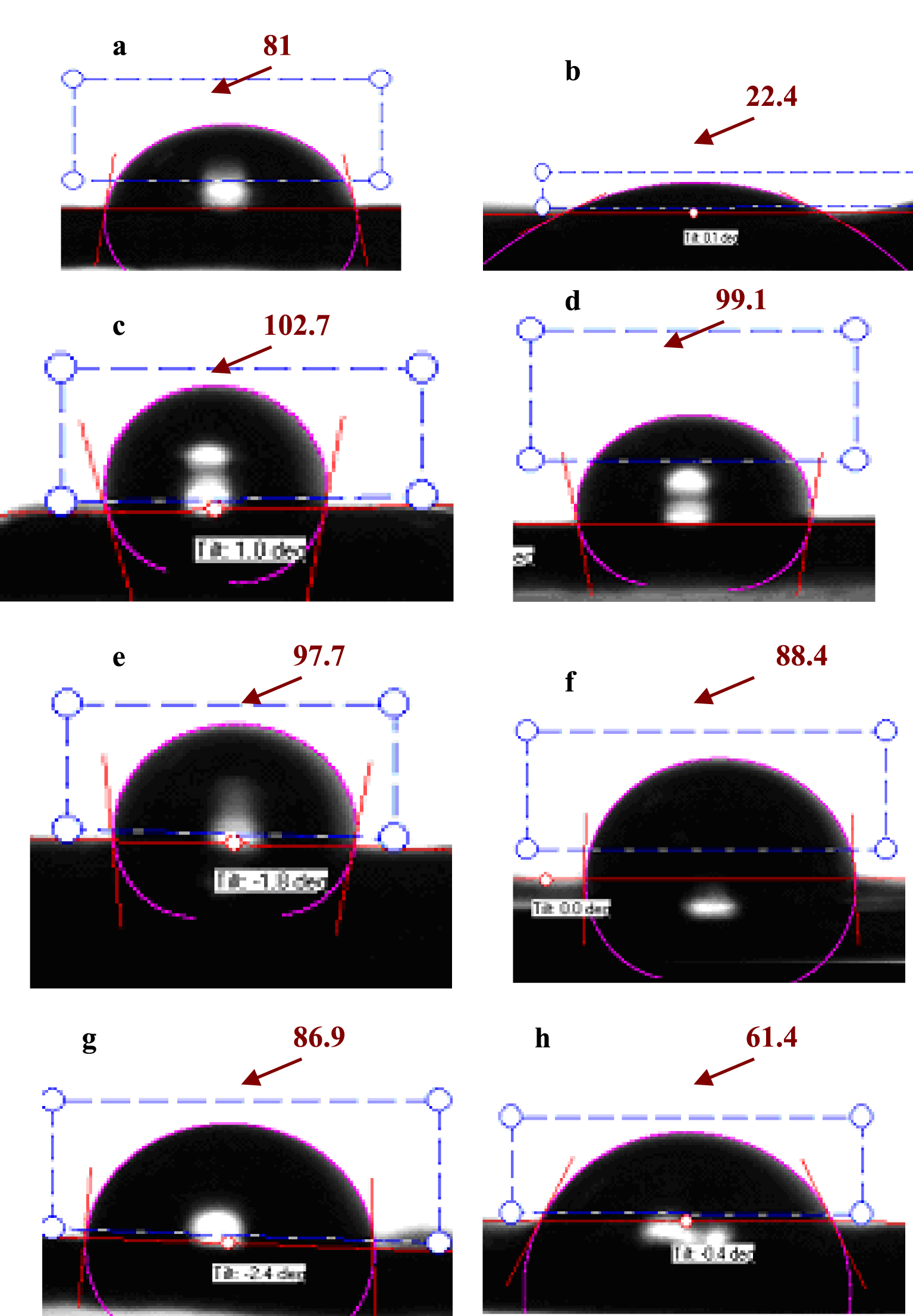
3.3. Electrochemical impedance measurements
The EIS is a convenient tool to confirm and characterize the properties of chemically grafted layers. In case of immersion, experiment type ISO 16773-4:2017 was followed to measure the impedance values of the coatings formed on metals [17]. A modified Nyquist circuit without the Warburg element (Figure 3A) was used to best fit the EIS data [18] since the linear part of the Nyquist plots is absent in numerous cases or only a small part is present. The data were fitted using a subset from measured signals in the few cases where a small linear part was apparent (Figures 3D, E) in order to investigate only the semi arch that is characteristic for the film properties.
(A) Modified Randles equivalent circuit used for fitting EIS data, (B) Nyquist plots measured after immersion in acetate buffer during 24, 48, or 96 h recorded for Cu-P2P, (C) Cu-P1B, (D) Cu-phenyl aldehyde, and (E) Cu-bare surfaces. Lines are the interpolated data.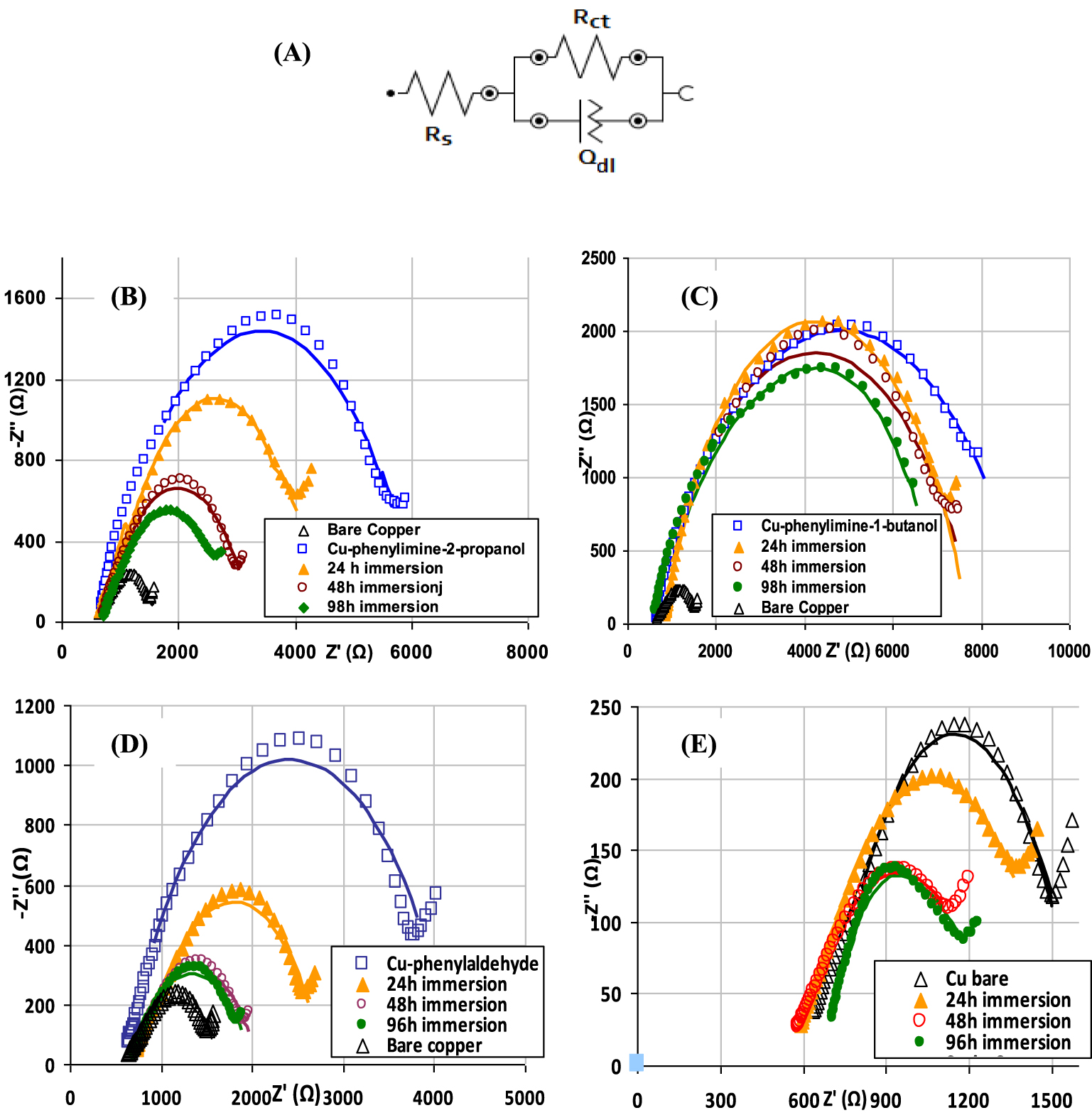
Prior to each electrochemical impedance measurement, the OCP values were measured and set as fixed DC potential for EIS. The Nyquist plots measured at the OCP of copper electrode coated with organic layers after 24, 48, or 96 h immersion in acetate buffer solution are presented in Figure 3. To accurately evaluate the anticorrosion performance of obtained organic films applied on copper surface, it is necessary to analyze and compare the changes of impedance of coating films after immersion in the electrolyte solution over a prolonged time period.
From the impedance measurements, inhibition efficiency (IE) was calculated using the following expression [19].
After each immersion time, we have observed important changes in the measured impedance spectra due to a decrease of the corresponding Rct values and an important increase of Qdl for both the Cu-P1B and Cu-P2P anticorrosion films. These two studied organic coatings exhibit a different behavior for various immersion times in corrosive media (Figure 3).
The radius of the capacitive loop at the interface between the electrolyte and organic film is related to the Rct. As can be seen in Table 1, an Rct = 937 𝛺 was measured for the bare copper–electrolyte interface. The surface coating with PhA leads to an increase of Rct from 937 𝛺 to 4.19 k𝛺 for modified electrode surface by diazonium chemistry. The further enlargement of the capacitive loops for P2P (Figure 3B) or P1B (Figure 3C) coatings on the copper in comparison with the phenyl layer, indicate an important increase in the hindering of charge transfer on the covered copper surface, which reveals the increase in corrosion resistance.
Electrochemical impedance data as a function of immersion time
| Inhibitor/immersion time | Rs (𝛺) | Rct (k𝛺) | Qdl (μMho) | N | IE (%) |
|---|---|---|---|---|---|
| Cu-P1B initial | 583 | 8.6 | 25.8 | 0.583 | 89 |
| Cu-P1B (24 h) | 548 | 7.49 | 12.6 | 0.579 | 87 |
| Cu-P1B (48 h) | 980 | 7.28 | 32.8 | 0.575 | 87 |
| Cu-P1B (96 h) | 682 | 6.45 | 36.7 | 0.569 | 85 |
| Cu-P2P initial | 597 | 5.57 | 13.8 | 0.567 | 83 |
| Cu-P2P (24 h) | 622 | 4.3 | 40.5 | 0.563 | 78 |
| Cu-P2P (48 h) | 698 | 2.6 | 49.1 | 0.59 | 63 |
| Cu-P2P (96 h) | 700 | 2.16 | 53.4 | 0.592 | 56 |
| Cu-PhA | 334 | 4.19 | 36.5 | 0.524 | 77 |
| Cu-PhA (24 h) | 700 | 2.18 | 53.7 | 0.545 | 57 |
| Cu-PhA (48 h) | 690 | 1.38 | 89.2 | 0.552 | 32 |
| Cu-PhA (96 h) | 684 | 1.32 | 105 | 0.545 | 29 |
| Cu-bare (initial) | 207 | 0.982 | 387 | 0.454 | – |
| Cu (24 h) | 219 | 0.937 | 332 | 0.450 | – |
| Cu (48 h) | 226 | 0.916 | 293 | 0.448 | – |
| Cu (96 h) | 270 | 0.892 | 555 | 0.398 | – |
The Nyquist plots for the impedance measurements carried out with bare copper have significantly changed even after 24 h of surface immersion in corrosive medium (Figure 3E). It is obvious that after 96 h exposure in acetate buffer, the substrate material was significantly altered after the corrosion test. The Nyquist plots recorded after deposition of a phenyl aldehyde layer suggest a good initial inhibitor behavior. But after 24 h immersion intervals, the organic layer becomes inefficient (Figure 3D). Thus, the improvement of the anticorrosion coatings using amino derivatives assembled by covalent attachment was necessary.
The Nyquist plots in Figure 3B show decreasing protective properties of the P2P coating in time due to slight changes of film properties that lead to decrease of the measured Rct. Thus, the arcs of the capacitive loops decrease in size and the corrosion resistance reached a lower value after 96 h immersion in buffer acetate. On the other hand, the evaluation of corrosion protection of the P1B film in the corrosive electrolyte, depicted in Figure 3C, indicates that this organic layer maintains its barrier properties with time probably due to an increase organic film density, thus enhancing the corrosion resistance of coating system–metal interface. Significantly smaller changes in comparison with P2P in the impedance spectra, even for 98 h immersion in acetate buffer solution, were obtained for the Rct values decrease, which indicates that the P1B coated layer can serve as an effective corrosion inhibitor for copper. The impedance parameters calculated from the fitting models are summarized in Table 1.
3.4. Potentiodynamic polarization measurements
Potentiodynamic polarization curves of the copper foils have been measured to study the kinetics of the anodic and cathodic reactions. Potentiodynamic polarization curves of the bare and modified electrodes are presented in Figure 4.
IE was calculated using the following expression [20].
The evaluation of corrosion behavior for bare and coated copper electrodes was obtained by immersing the electrodes in a test solution for different times. The studied coatings have relatively close molecular structures, but the overall properties of the electrochemical behaviors are significantly different. It can be observed from Table 2 that copper electrodes coated with P1B and P2B films have higher IE% values than the IE for PhA-coated electrodes (Figure 4C). Compared to the modified electrodes, bare Cu has the highest corrosion current density. Regarding the long-term effect of acetate buffer on the bare copper surface, bare copper underwent severe corrosion after 48 h of immersion, as a result of which the icorr value increased drastically from 32 to 104 μA/cm2 (Figure 4D). This drastic change in current density values suggested that acetate ions severely corrode the bare metal.
The anodic and cathodic Tafel slopes recorded for P2P film on copper were changed after immersion in the test solution. We have observed that the organic coatings still block the reaction sites of the metal surface after 24 h, but the coated copper is affected after 48 or 96 h of immersion, both on the anodic and cathodic reaction mechanisms (Figure 4B). For the longest tested immersion times, the reduction of polarization slopes and shifting of Ecorr toward more cathodic domains occurs. On the other hand, the P1B film gives protection by blocking effect offering high resistance to corrosion media even after 96 h of immersion (Figure 4A).
(A) Tafel polarization curves of CU-P1B, (B) Cu-P2P, and (C) Cu-phenyl aldehyde modified electrodes before and after 24, 48, and 96 h exposure in a 0.05 M acetate buffer solution. (D) The curves for bare copper electrode are presented for comparison.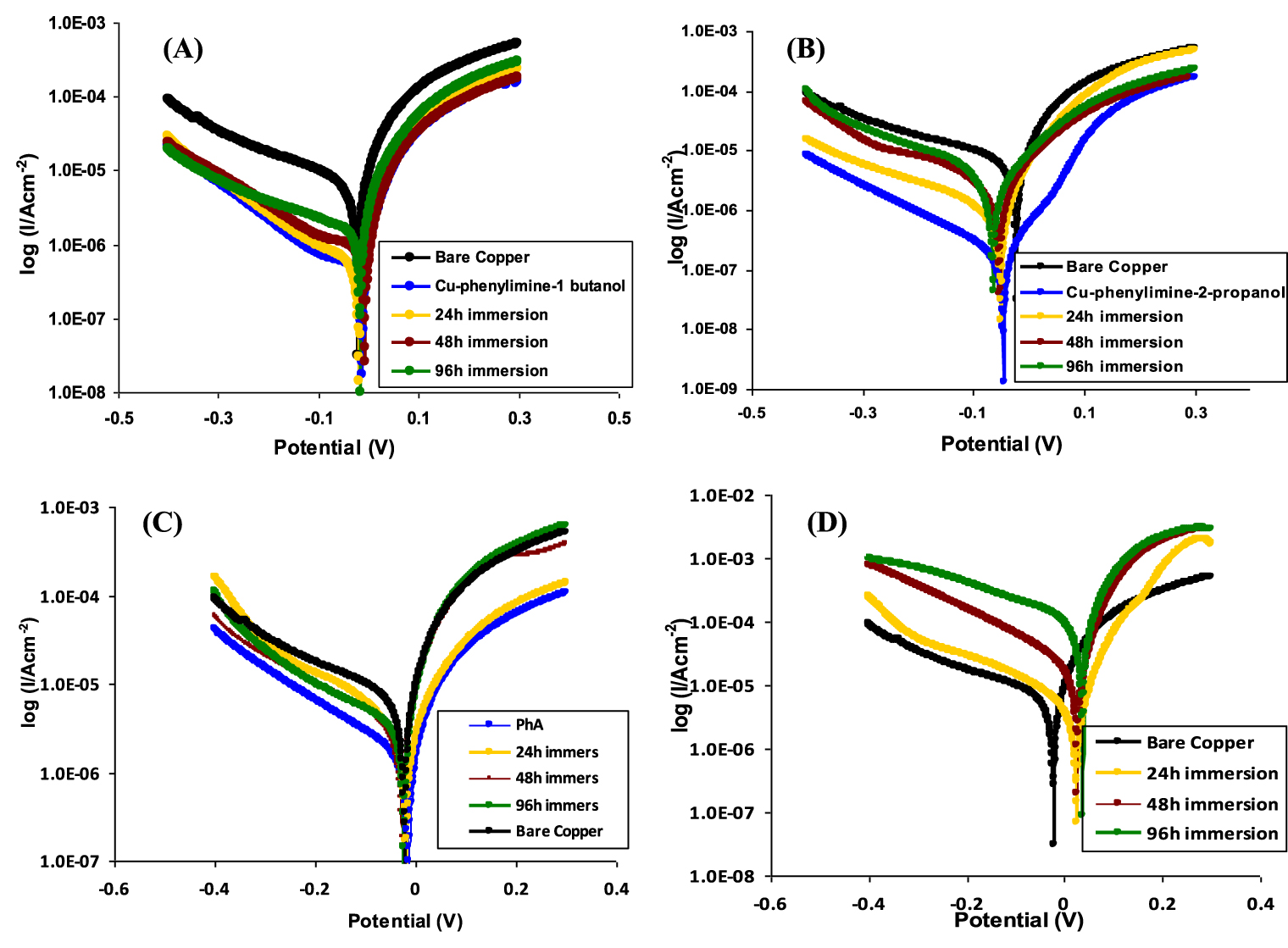
Both P2P and P1B decrease the icorr values in comparison with the bare copper surface, as shown in Table 2, leading to increased IEs. Close examination shows that increased immersion times slightly increase the icorr values for Cu-P2P, while there are no significant changes for Cu-P1B. The corrosion inhibition mechanism can be classified as an anodic or cathodic type only if the displacement in corrosion potential is more than 85 mV with respect to corrosion potential of the blank [21]. The maximum observed variation of corrosion potential was 62 mV for P2P after 92 h immersion, which indicated that both the studied organic inhibitors belong to the mixed-type inhibitors.
The electrochemical kinetic parameters from potentiodynamic polarization of copper in 0.05 M acetate buffer solution: corrosion current density (icorr), corrosion potential (Ecorr), anodic Tafel slope (𝛽a), cathodic Tafel slope (𝛽c), polarization resistance (Rp), corrosion rate (CR), and polarization resistance (PR) obtained from Tafel plots
| Inhibitor/ immersion time | Ecorr (mV) | icorr (μA/cm2) | 𝛽c (mV/dec) | 𝛽a (mV/dec) | CR (mm/year) | PR (k𝛺) | IE (%) |
|---|---|---|---|---|---|---|---|
| Cu-P1B initial | − 10 | 3.30 | − 169 | 48 | 0.07 | 26.0 | 89.7 |
| Cu-P1B (24 h) | − 7 | 3.87 | − 124 | 57 | 0.09 | 23.0 | 87.9 |
| Cu-P1B (48 h) | − 6.2 | 4.65 | − 154 | 93 | 0.11 | 17.4 | 85.4 |
| Cu-P1B (96 h) | − 10 | 5.40 | − 414 | 39 | 0.12 | 10.8 | 83.1 |
| Cu-P2P initial | − 44 | 4.70 | − 646 | 206 | 0.108 | 21 | 85.3 |
| Cu-P2P (24 h) | − 48 | 7.03 | − 599 | 82 | 0.161 | 17.9 | 78 |
| Cu-P2P (48 h) | − 51 | 12.11 | − 316 | 116 | 0.280 | 12.1 | 63.9 |
| Cu-P2P (96 h) | − 62 | 14.56 | − 201 | 141 | 0.334 | 9.99 | 56.6 |
| Cu-PhA (initial) | − 13 | 7.4 | − 336 | 81 | 0.17 | 15.3 | 76.9 |
| Cu-PhA (24 h) | − 18 | 14 | − 248 | 94 | 0.32 | 8.98 | 56.3 |
| Cu-PhA (48 h) | − 22 | 21.3 | − 592 | 75 | 0.49 | 5.41 | 33.6 |
| Cu-PhA (96 h) | − 28 | 22.5 | − 988 | 66 | 0.5 | 4.8 | 29.9 |
| Cu-bare (initial) | − 10 | 32.10 | − 591 | 72 | 0.74 | 3.40 | – |
| Cu-bare (24 h) | 25 | 38.96 | − 454 | 89 | 0.87 | 3.36 | – |
| Cu-bare (48 h) | 26 | 104.62 | − 297 | 64 | 2.40 | 0.885 | – |
| Cu-bare (96 h) | 37 | 5090 | − 554 | 89 | 117 | 0.264 | – |
As indicated in Table 2, the changes in 𝛽c values were higher than that of 𝛽a, which indicated that the inhibitors act as mixed type with predominant cathodic effectiveness. Our results indicated that copper foils modified with P1B films presented improved anticorrosion properties because this coating gives protection by barrier effect on the anodic and cathodic reaction of the metal substrate interface. Our interpretation of such a behavior is that the inhibitor grafted onto the metal surface offers moderate electrolyte permeability in a buffered solution with acetate ions and acetic acid molecules. Increasing immersion time does not significantly change the current values for both CU-P1B and (B) Cu-P2P and therefore the IE measured do not vary significantly. This layer by layer depositions on the copper surface results in stable organic films with good IE.
4. Conclusions
Molecular phenyl aldehyde (multi)layers were electrochemically formed based on diazonium chemistry on Cu and were covalently modified with either amino-2-propanol or 2-amino-1-butanol. Contact angle measurement revealed the hydrophobic nature of surfaces modified by the inhibitor molecules applied in the corrosive solution. The P1B film–metal interface system was determined to be more hydrophobic than the P2P coating on the copper surface by water contact measurements. The corrosion inhibition behavior of P1B and P2P films was investigated in acetate buffer after different immersion times. The layer functionalization had an important effect on the anticorrosion properties in comparison with the phenyl aldehyde (multi)layers. Interestingly, there is a marked difference between the effects of the two studied modifiers despite their relatively similar structure. Position of the hydroxyl group attached on the alkyl molecular fragment influences the formation and efficiency of the protection coatings on the copper surface. The protection efficiency calculated from EIS measurements after 24, 48, or 96 h of immersion in corrosion electrolyte was much higher for P1B in comparison with P2P. Based on the Tafel polarization results, the investigated organic coatings can be classified as mixed type inhibitors.



 CC-BY 4.0
CC-BY 4.0