1 Introduction
Rheological investigations on colloidal suspensions or complex fluids help to understand the structural network and to characterize the interactions between the particles. The results’ reproducibility is perfect for model materials such as latex or laponite in fluid (usually water and oils), but for clayey materials, like bentonite, the same technical approach yields more divergent data because of the great variety of compositions of smectites and their high reactivity with the environment.
The specific properties of bentonite (hydration, swelling, water adsorption, viscosity and thixotropy) make it a valuable material in the form of mineral powder for a wide range of uses (agronomy, cosmetics, civil engineering, as a geoliner in environmental sealant in landfills, sewage lagoons or as a compacted barrier for nuclear waste repositories). Bentonite suspensions are useful in many commercial and technological applications, such as drilling fluids, paints and pharmaceuticals [2,8,31]. They exhibit strong colloidal properties, with a three-dimensional organization of the smectite clay crystals within the clay–water system, giving it viscoelastic-fluid behaviour.
The bentonite suspensions display varying rheological properties that are highly dependent on the nature of the clay, suspension preparation, concentration, pH, ionic strength, etc. Of course, bentonite is already a complex clayey material, generated frequently from the alteration of volcanic ash. The nature and the volcanic origin of bentonite deposits give rise to varieties of the mineral that are often extremely heterogeneous, due to the differing depositional environment and subsequent weathering processes according to the region and deposit involved. Bentonite deposits are normally exploited by quarrying. The material is initially crushed and, if necessary, activated with the addition of soda ash (Na2CO3) in the case of natural calcium bentonites. Bentonite is subsequently dried (air and/or forced drying) to reach a moisture content of approximately 15%. According to the final application, bentonite is either sieved (granular form) or milled (into powder and super fine powder form).
Bentonite contains primarily smectite clays (mainly montmorillonite) and other secondary minerals, like feldspar, quartz, calcite and iron oxides. Moreover, smectites constitute a complex microcrystalline mineral whose structure admits many isomorphic substitutions, and where neutrality is respected by the presence of interlayer-exchangeable cations. Since the cation's nature and state of hydration may vary, then the interlayer dimension may fluctuate. The classification criteria are the interlayer charge value (equivalent to the balance of isomorphic substitutions), its origin (tetrahedral or octahedral charge deficit), and the di/tri-octahedral occupation of the octahedral layer. The presence of these minerals can affect a deposit, reducing or increasing its industrial value depending on the application [17,26,29]. Indeed, it is also well known that the mechanism responsible for this rheological behaviour depends on many other factors, like the concentration (usually from 20 to 80 g L−1).
The mineralogical investigations play an important role in the understanding of the rheological properties of bentonite suspensions. The physicochemical properties of smectite layers (size, structural charge, nature of the exchangeable cation and the mean number of layers per particle) as well as the fluid conditions (pH, ionic strength) have been studied to determine their effects on specific properties such as swelling, high adsorption capacity, and strength to shear stress. The existence of a selectivity sequence for the adsorption of cations in the interlayer space of smectite clays has been studied and established [2,8]. The cation exchange becomes increasingly difficult according to the selectivity sequence Ca++ > Mg++ >> NH4+ > Na+ > Li+. Smectite clays display different particulate organizations in suspensions according to their interlayer cation; consequently, their rheological behaviour varies greatly [2,3,5,8,9,13,14,17,26,29,31]. It appears that the rheological technique is sensitive enough to quantify very low microvariations that result from the nature of the interlayer cations in association with the smectite particles [4,8,9,12,16,22,31]. Particularly, the difference between sodium bentonite and calcium bentonite is well known and commonly described in the scientific and technical literature [3,8,14].
Despite this knowledge, the comparison of rheological parameters, even for similar materials like sodium bentonite, as determined by different authors, is difficult because experimental conditions are slightly different and also because the cation exchange is not as simple as it appears. Analytical conditions are so different that it is very difficult to correlate data measured by various authors. This divergence originates from differences in pre-treatments and preparations as well as from different modes of quantification.
Kaufhold et al. [11] have also shown that the quantification of montmorillonite in bentonites is not that easy. Ottner et al. [21] have published a very interesting paper about the results of an inter-laboratory comparison of methods for the quantitative clay analysis of two clay samples. It appears that the inter-laboratory data are equivalent as regards the non-clay mineral analyses, but are strongly divergent as regards the clay mineral analyses. Details about the complete analytical methodology employed are often omitted or not sufficiently described in research papers.
Coupling rheological and mineralogical techniques gives the opportunity to explore how the structure and texture of smectite suspensions are affected by modifications of the clay layer properties. Mineralogy allows characterising the mineral powder and rheology to determine the macroscopic behaviour of suspensions under shear, which is an indicator of the structure strength. In a previous study [15], the effect of three cations (Ca2+, Li+, and NH4+) on the same bentonite Volclay was investigated with a complete rheological program.
The purpose of the present paper is to analyse the rheological responses of suspensions under shear prepared with three powders (Li-smectites), exchanged and purified in three steps. The influence of the pre-treatment procedures, focusing on the saturation process (with Li cation), and the presence of secondary minerals have a significant effect on the rheological properties as measured by rheometric tests in which all the experimental conditions are well defined and controlled, like the hydration time and the stirring energy applied upon mixing [6].
The <2-μm fraction of a classical Volclay bentonite, used by many authors [1,19,20,24,28] for smectite crystallography investigations constituted the raw material.
We chose to examine the exchange between Ca and Li, shown as one of the most difficult cation exchanges by McBride [17]. The lithium cation was selected in order to examine the behaviour of Li-clay suspensions, because this cation enhances the effect of exchange and the dispersion of the particles (constituted by the simplest structural unit for a smectite, i.e., one layer). Usually the comparison is between Ca and Na cations [3,8]. Li-saturated suspensions are typical of gels that are composed of fully dispersed particles [23]. According to Verbrug and Baveye [31], Li smectite particles are assumed to be ideally constituted of one layer, whereas Ca smectite particles are constituted of many layers. These authors have proposed a Ca–Li exchange isotherm model. Indeed, when the percentage of Li cations added in a Ca-saturated sample reaches 70%, the association of particles in suspension is complex, with areas typical of a gel (i.e. fully dispersed lithium layers) and areas with particles of a few layers maintained by calcium cations in their interlayer spaces. They specified that this complex structural network is the most difficult to reproduce. This choice was also justified by the fact that lithium saturation is the basis of the Hoffman–Klemen test [10], which allows discrimination between montmorillonite and beidellite to prepare another study about the influence of layer charge on the rheological behaviour of a same smectite.
2 Materials and methods
2.1 Materials
We used an industrial powdered bentonite, referred to as Volclay MX-80, packed in bags, and very studied as a compacted barrier for nuclear waste repositories.
The powder from the same bag as the one used for other studies [2,16,24] was used to prepare the samples. In Table 1, the normalized chemical compositions (oxides %) obtained by different authors are gathered. The previous studies have shown that the <2-μm fraction is mainly composed of bi-ionic (Na–Ca)-montmorillonite (83–93%) with minor amounts of quartz, K-feldspar, plagioclases and micas [16]. The proportion of these associated minerals in the coarse fraction of the <2-μm bentonite reaches 15% (measured by dry weighing).
Normalized chemical composition (oxides %) of the infra-2-μm fraction from the initial raw bentonite
Tableau 1 Composition chimique normalisée (en % d’oxydes) de la fraction infra 2 μm extraite de la bentonite brute
| Volclay fraction infra 2 μm (* Ecoclay – Rassineux et al., 2000 [24] – Malfoy et al., 2003 [16]) | |||||||
| 1* | 2* | 3 | 4 | 5 | Average | S.D. | |
| SiO2 | 67.46 | 67.38 | 67.11 | 68.87 | 68.58 | 67.88 | 0.79 |
| Al2O3 | 22.02 | 22.46 | 22.29 | 21.27 | 20.80 | 21.77 | 0.71 |
| MnO | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 |
| MgO | 3.09 | 3.03 | 2.78 | 2.86 | 2.81 | 2.92 | 0.14 |
| CaO | 1.24 | 1.14 | 1.12 | 0.91 | 1.03 | 1.09 | 0.12 |
| Na2O | 1.79 | 1.67 | 2.12 | 1.85 | 1.82 | 1.85 | 0.16 |
| K2O | 0.23 | 0.22 | 0.33 | 0.34 | 0.58 | 0.34 | 0.15 |
| TiO2 | 0.21 | 0.14 | 0.28 | 0.43 | 0.84 | 0.38 | 0.28 |
| Fe2O3 | 3.94 | 3.97 | 3.92 | 3.45 | 3.53 | 3.76 | 0.25 |
2.2 Powder preparation
The <2-μm fraction (equivalent diameter) was extracted from the bulk sample by centrifugation, dried and kept in hermetical flasks. The raw bentonite was mixed with osmotically purified water and the resulting suspensions were left at 20 °C for 24 h. Then, they were centrifuged at 1000 rpm for 2 min, 41 s in a Jouan GR 422 centrifuge. All the samples from the <2-μm fraction were dried at 60 °C and then crushed together manually to constitute the primary stock of studied powder.
Thereafter, samples were Ca-saturated using a 2 N CaCl2 solution and kept in contact with saline solutions for 1 h. After three repetitions of this procedure, the chloride excess was repeatedly washed with osmotically purified water followed by centrifugation until the solution was free of chloride, as determined by a negative test with AgNO3. Finally, the purified product was air-dried at 60 °C and mechanically crushed. This Ca-saturated sample was our initial sample.
During the processes, a dark-coloured residue deposit was observed at the bottom of the centrifugation test tubes. X-ray diffraction patterns on the dark residue revealed mica, zeolites, feldspar and quartz. The mass of this residue was measured and was found to account for 10–15%. Li-saturated samples were obtained from the Ca-saturated materials by applying two protocols, which were assumed to ensure interlayer cation exchange.
In the first protocol, the ‘Li-A’ sample was prepared from the initial Ca-saturated material with the standard ‘Hoffman-Klemen’ method used by numerous authors for the same bentonite saturation [10,18,19]. This method consists in keeping the clay material in contact with a 1 N LiCl solution for 1 h. This procedure was repeated three times. Samples were then repeatedly washed with pure water until the supernatant, obtained after centrifugation, was free of chloride. In our study, 3 g of the Ca-clay sample were kept in contact again three times in 30 mL of a 1 N LiCl solution, twice for 1 h, and once for 24 h. Samples were repeatedly washed by dialysis (Spectrapore 6-8000) with osmotically purified water until they were free of chloride.
In the second protocol, the number of contacts with the LiCl solution as well as the saturation time was increased. Ca-saturated samples were stirred five times with the 1 N LiCl solution for 24 h at each contact. Then suspensions were washed as indicated previously. This run product is referred to as ‘Li-B’.
Samples of the Ca-black saturated material were set aside for mineralogical analysis and for determining their influence on the rheological properties of bentonite suspensions. The purified Li-B clay sample, after removal of the dark-coloured residue, corresponding to non-clay associated minerals in the coarse fraction of the <2-μm bentonite (amount 15%), is referred to as ‘Li-C’.
2.3 Preparation of the suspensions
Suspensions of each clay sample at 6% (w/w) were prepared by adding 2.7 g of powdered clay to 45 ml of osmotically purified water in Jouan polyethylene flasks. The mixtures were then briefly hand-shaken to disperse initially the clay in water, after which they were mixed for 5 min at 13,000 rpm using an Ultra Turrax mixer. The resulting suspensions were then rested for 24 h at 20 °C in order to ensure total hydration of the clay. No pH adjustment was made. This procedure was followed rigorously to avoid perturbations in data analysis.
2.4 Methods for powder control
The Ca-saturated starting sample and the three Li-saturated samples were analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), and Flame Adsorption Atomic Spectroscopy (FAAS). For XRD measurements, orientated samples were analyzed in the air-dried (AD) and ethylene-glycol-saturated (EG) conditions. XRD patterns were recorded using a Philips PW 1730 diffractometer (Cu Kα Ni-filtered) equipped with a SOCABIM DACO system. The analytical conditions were: 40 kV, 40 mA, 2–35° 2θ angular range, step size 0.02° 2θ, counting time 3 s per step. The Newmod program [25] was used to simulate XRD patterns and to provide a basis for the interpretation of experimental XRD patterns.
The identification of non-clay accessory minerals in the bentonite was checked using scanning electron microscope (SEM) observations (JEOL JSM 5600 LV) and XRD.
The exchangeable bases (BE) and cationic exchangeable capacity (CEC) were then determined using the method according to the standard AFNOR X 31.130, November 1985. Concentrations of exchangeable cations (Ca2+, Mg2+, K+, Na+, and Li+) were determined using a PerkinElmer 3110 FAAS apparatus. One-hundred-milligram samples (dried at 105 °C) of each material were mixed with 5 ml of a 1 N ammonium acetate solution. The resulting suspension was then centrifuged and the supernatant collected in a 50-mL flask. This process was repeated five times with each sample. The supernatant from each repetition for each sample was collected in a single flask. The volume of the collected supernatant in each flask was then made up to 50 mL using osmotically purified water. Five-millilitre samples of each solution were then mixed with 5 mL of 5% La2O3 and 40 mL of osmotically purified water in a second flask. The CEC was determined from the ammonium-saturated samples by Kjeldahl distillation. The CECs were calculated per weight of a completely dehydrated clay sample. In theory, the CEC and the sum of exchangeable cations (BE) should be equal. The CEC average and standard deviation were obtained with three measurements.
2.5 Methods of analysis for the suspensions
A Rheologica-Stresstech HR control stress rheometer with coaxial cylinder geometry was used to obtain rheological data for the clay suspensions. A film of weakly viscous silicon oil (Rhodia 47 V5) was spread at room temperature on the upper free surface of each suspension to minimize water evaporation.
The experiments were repeated at least four to six times for each sample to ensure reproducibility.
Experimental flow curves were obtained using the ‘quick flow curve’ procedure [2,6,10]. The procedure starts by shearing the suspensions at a shear rate of 500 s−1 for 240 s with the Rheologica-Stresstech HR rheometer, followed by a rest period of 600 s. This is done in order to place the sample suspensions in the same initial structural state. Then, the suspensions are subjected to increasing and decreasing shear stress gradients for 30 min. The existence of a hysteresis between the flow curves obtained with the increasing and decreasing the modes reveals thixotropic properties of the fluid.
The Herschel–Bulkley (HB) model was used for processing the rheological data. This choice is supported by the fit quality and the simplicity and efficiency of this model, which has been used in previous works on bentonite mud.
The Herschel–Bulkley model is expressed as:
- • yield stress values (τ0) are obtained by extrapolation of the linear part of the flow curve, at low shear rate, in a semi-logarithmic representation;
- • k and n are obtained by quadratic minimization after determining the yield stress value given previously.
3 Results and discussion
3.1 Mineralogical characterisation by X-ray diffraction
The Ca-saturated <2-μm fraction (Fig. 1) mainly contains smectite with two well-defined reflections (15.19 Å and 4.48 Å) and three bands (5.02, 3.04 and 2.57 Å). Some accessory minerals are observed, such as quartz (3.34 and 4.26 Å), feldspars (3.23 and 3.18 Å) and cristobalite (4.04 Å). This last accessory mineral is the only one observed.
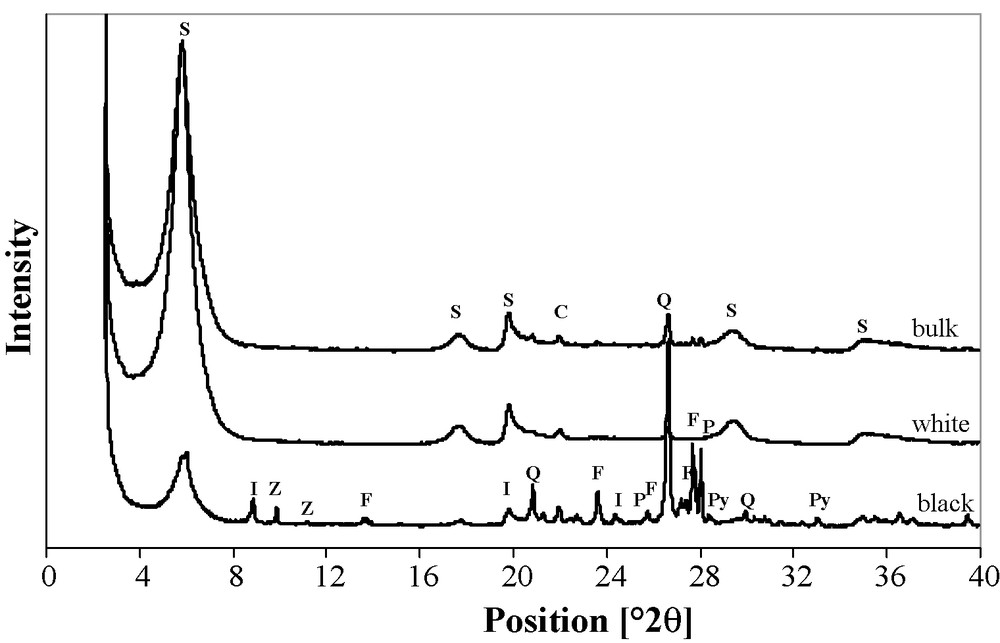
X-ray powder diffraction patterns of <2-μm bulk fraction and of its two white and black Ca-saturated components (S: smectite, I: illite, Z: zeolite, Q: quartz, F: K-feldspar, P: plagioclase, C: cristobalite, Py: pyrite).
Fig. 1. Diffractogrammes de la poudre <2 μm, saturée en Ca et de ses deux fractions blanc et noir (S : smectite, I : illite, Z : zéolite, Q : quartz, F : K-feldspath, P : plagioclase, C : cristobalite, Py : pyrite).
After a better separation, a white major part and a black minor part can be distinguished. Cristobalite is the only accessory mineral still observed within the very smectitic white part, while the black part is relatively enriched in quartz, orthoclase like K-feldspar (3.23, 3.77, 3.28, 3.46 and 6.43 Å), and albite as plagioclase (3.18, 3.76, 3.47 and 6.37 Å) and relatively impoverished in smectite phase, which remains predominant and contains small amounts of mica (9.95, 4.48 and 3.65 Å), zeolite (8.95 and 7.90 Å), probably clinoptilolite, and pyrite (2.71, 3.14, and 2.42 Å).
The XRD patterns of air-dried oriented preparations of the Li-A sample (Fig. 2A) show an intense peak at 12.53 Å and weak peaks around 13.55 Å, indicating a possible interstratification of two types of smectite layers with one or two water layers. The simulation of the 13.55 Å peak is satisfactorily modelled by the association of a one-water-layer interstratified smectite (60%) with a two-water-layer one (40%) [20]. Assuming that two water layers are intercalated between indicidual 2:1 layers for Ca-smectites and one water layer for Li-smectites, these results suggest that the sample is composed of a Li/Ca interstratified smectite. The increased saturation rate (Li-B) improves the (0 0 1) peak intensity and the low peak around 13.55 Å disappears. This trend is more pronounced for the Li-C sample which is cleared of almost all accessory minerals. For the 8 to 32°2θ angular range (Fig. 2B), Li-A and Li-B samples show (0 0 2) and (0 0 4) reflections with dissymmetric profiles whose maxima are centred at 6.20 and 3.09 Å, and small shoulders visible at 6.48 Å and 3.14 Å, respectively. With the Li-C sample, symmetric (0 0 2) and (0 0 4) reflections indicate a better saturation rate and organization of layers.

X-ray diffraction patterns of air-dried oriented preparations from Li- saturated samples (Li-A, Li-B and Li-C). (A) (001) Reflection angular range; (B) (002)- to (005) reflection angular range.
Fig. 2. Diffractogrammes des préparations orientées, séchées à l’air des échantillons saturés Li (Li-A, Li-B and Li-C). (A) Domaine angulaire de réflexion (001) ; (B) domaine angulaire de réflexion (002) à (005).
After ethylene-glycol solvation, the position at 17.04 Å and the symmetrical shape of the (0 0 1) reflections are very similar among Li-A, Li-B and Li-C samples (Fig. 3A). This behaviour suggests the presence of only one type of layered organization. On the other hand, the examination of the 8 to 32°2θ angular range (Fig. 3B) shows that all the (0 0 l) reflections (l = 2 to 6) are detected with a regular shape indicating a very good stacking of the smectite layers.
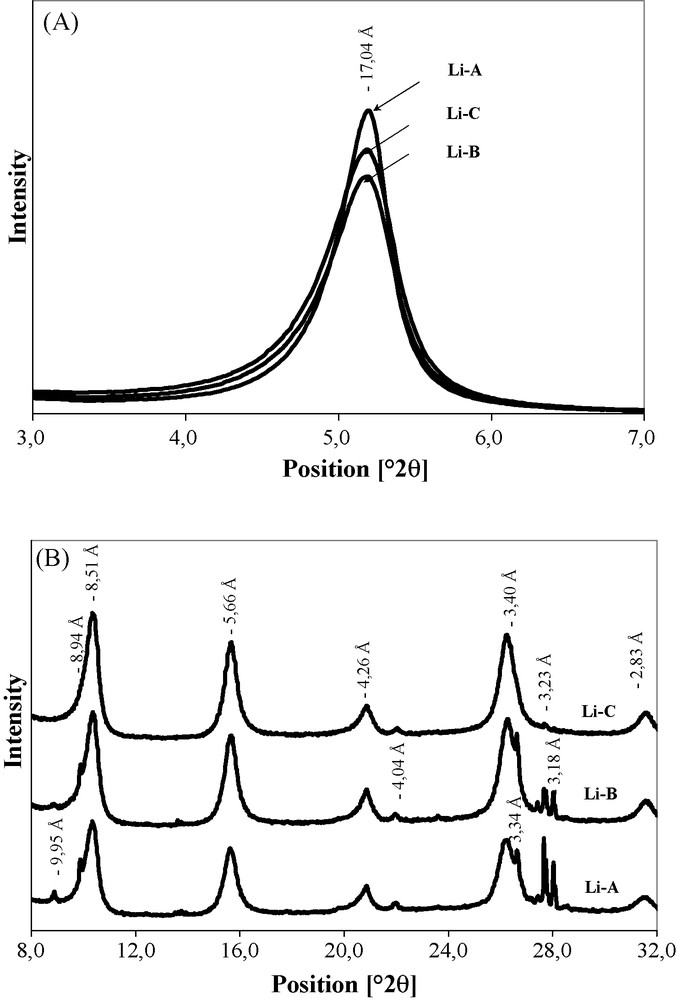
X-ray diffraction patterns of EG-solvated oriented preparations from Li- saturated samples (Li-A, Li-B, and Li-C). (A) (001) reflection angular range; (B) (002) to (005) reflection angular range.
Fig. 3. Diffractogrammes des préparations orientées, saturées à l’éthylène glycol, des échantillons saturés Li (Li-A, Li-B et Li-C). (A) Domaine angulaire de réflexion (001) ; (B) domaine angulaire de réflexion (002) à (005).
3.2 Cation exchange capacity and exchangeable cations
Table 2 presents the exchangeable cation concentrations (BE) values for each sample with a measurement sensitivity of (±) 5 mequiv/100 g.
Values of each exchangeable cation, their sum BE and the saturation rate of Li for the three smectite powders
Tableau 2 Teneurs en cations échangeables, leurs sommes BE et le taux de saturation Li pour les trois poudres smectitiques
| ±5 mequiv/100 g | [Ca2+] mequiv/100 g | [Mg2+] mequiv/100 g | [K+] mequiv/100 g | [Na+] mequiv/100 g | [Li+] mequiv/100 g | BE mequiv/100 g | % Li |
| Li-A | 23.0 | 5.0 | 0.00 | 0.00 | 50.00 | 78.0 | 64.1 |
| Li-B | 4.0 | 4.0 | 0.00 | 0.30 | 70.00 | 78.3 | 89.4 |
| Li-C | 5.0 | 5.0 | 0.12 | 0.00 | 70.00 | 80.1 | 87.4 |
However, looking in details at the nature and proportion of the individual interlayer cations to refine the knowledge of each powder, many differences are observed according to the saturation method. For Li-A, the standard saturation appears to be incomplete, since the percentage of lithium present in the interlayer is only 64%, with the remaining cations being composed mainly of Ca ions. For Li-B and Li-C, the saturation method seems to be more efficient, since lithium represents 89% of the total exchangeable bases. Indeed, the Li-C sample was obtained by purification of the Li-B sample by extraction of the non-smectitic phase only (dark-coloured residue), which perturbs the CEC value.
Since Hofman Klemen's works [26], it is well known that heating a Li-montmorillonite to 100–200 °C for about 21 days leads to a decrease in CEC. More recently, with shorter heating treatments, but higher temperatures, T = 190 °C [27] and T = 250 °C [32], a great loss in CEC has been observed, 40 to 50% and 25%, respectively. All these studies have shown that with similar temperatures but longer heating treatments or with higher temperatures and heating treatments of similar duration, Li migrates into the octahedral vacant site. In our study, after 12 h of heating at 105 °C, a part of the interlayered Li began to migrate into the octahedral vacant site. Consequently, obtaining a correct value of CEC from Li-saturated montmorillonite applying the conventional process appears impossible. Therefore, the CEC was determined using only Ca-saturated materials heated to 150 °C for a complete dehydration. The CEC of the raw material is 74 ± 4 mequiv/100 g. On the other hand, the latter value is in good agreement with the results by numerous other authors [1,18]. Higher values around 90–95 mequiv/100 g can be found in the literature [20,24,27,32]. Recent works by Dohrmann [7] explain these discrepancies by the great sensitivity of CEC measurements relative to the preparation procedures, such as the drying temperature effect, in particular.
3.3 Effects of pre-treatment procedures on rheological data
For each sample prepared with powders Li-A, Li-B, and Li-C, not less than four rheological tests were conducted. The rheological behaviour measured is typical of a Li-saturated sample (Fig. 4). All suspensions produce flow curves typical of shear-thinning fluids without yield stress and without thixotropy, in agreement with previously obtained results [16] that showed the organization of suspended particles as a function of three cations (Ca2+, NH4+, and Li+) using identical preparation procedures. This means that their mechanical behaviour is dominated by the major cation.
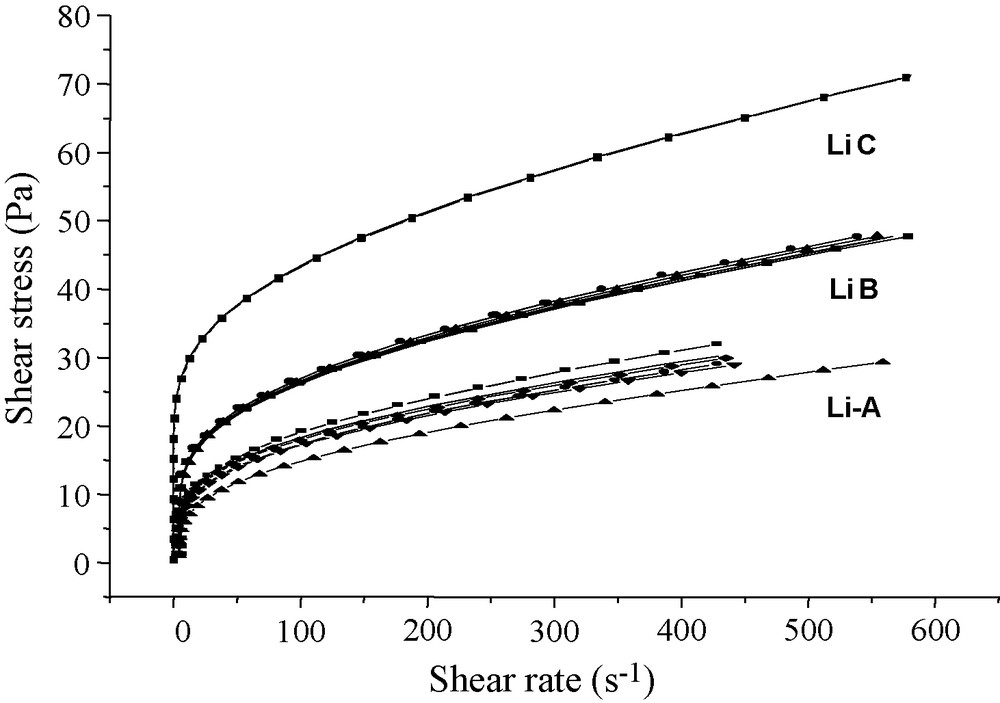
Flow curves of the three Li smectite suspensions at 6 weight% (Li-A; Li-B; Li-C).
Fig. 4. Courbes d’écoulement des trois suspensions de smectites Li à 6 % en poids (Li-A ; Li-B ; Li-C).
Coupling fine mineralogical analyses and rheological measurements allows us to observe that the presence of micrometric heterogeneities in the studied samples has a great influence on the determination of the rheological parameters of the suspensions, despite the control of all other conditions (concentration, hydration time, mixture energy, pH, conductivity, initial infra-2-μm powder...), which are the same for each sample. We consider also that the chemical composition of the powders stayed constant and similar for all samples prepared in the same conditions. The observed fluctuations of the mechanical behaviour of the Li-bentonite suspensions may then be due to the uncompleted saturation of the interlayer cation Li and to the presence of non-clay minerals in the infra-2-μm (equivalent diameter) fraction of the studied material. Furthermore, the dispersion of measurements between suspensions prepared from the same sample material has decreased with the purification quality.
Table 3 shows the evolution of the mean values of k (consistency) and n (shear-thinning index) for samples Li-B and Li-C and C.
Mean values of rheological parameters (n and k, without units) with standard deviation σ and dispersion δ = (max − min)/min
Tableau 3 Valeurs moyennes des paramètres rhéologiques (n et k, sans unités) avec écart-type et déviation
| n consistency | k flow index | |||||
| Mean | σ | δ (%) | Mean | σ | δ (%) | |
| Li-A (six samples) | 0.33 | 0.012 | 5.5 | 4 | 0.363 | 13.7 |
| Li-B (four samples) | 0.28 | 0.005 | 2.5 | 8 | 0.308 | 5.6 |
| Li-C (four samples) | 0.18 | 0.001 | 0.8 | 20 | 0.071 | 0.5 |
The mean values of k increase significantly in correlation with the Li saturation level or the extraction of non-clay minerals. The consistency is doubled between Li-A and Li-B, and multiplied by 2.5 between Li-B and Li-C. In the same way, n is reduced by a factor of 1.1 between Li-A and Li-B and by 1.5 between Li-B and Li-C. The dispersion of the consistency values reaches a maximum for Li-A (13.7%) and a minimum for Li-C (0.5%). The dispersion of the shear-thinning index values follows the same progression as for the consistency values, with the maximum values for Li-A (5.5%) and the lowest values for Li-C (0.8%).
We recall the results of Verbrug and Baveye [30], who have specified the complex association of particles in suspension when the percentage of Li cations added in a Ca-saturated sample reaches 70%. This complexity can explain the rheological data dispersion.
Between Li-A and Li-B, the heterogeneity is linked to the Li level saturation. The Li-A and Li-B bentonite samples are considered equivalent. They have the same mineralogical component (XRD and SEM observations) and the same CEC value, but their exchangeable cation distribution differs. The <2-μm fraction of the Li-A and Li-B saturated bentonite contains non-clayey minerals that are difficult to identify directly by XRD measurements. The mineralogical identification of these minerals (quartz, mica, feldspar and zeolites) was performed by SEM. The interlayer cations present in the Li-A sample consist mainly of Li (64%) and Ca (30%). Analysis of the rheological behaviour of the Li-A system shows an incomplete monovalent-for-divalent cation exchange during the saturation process of the smectite phase.
The Li-B sample is fully saturated with lithium cations and its observed rheological behaviour indicates a viscosity of the suspensions up to 1.7 times greater than that of the corresponding Li-A suspensions. The k and n arithmetic values vary significantly, indicating a most consistent suspension that flows with more difficulty. Data scattering is reduced to 5.5% for k and 2.5% for n. Although the improvement in the saturation degree provided by the new saturation method is perceptible, the dispersion of rheological data is still significant. Considering that saturation is almost perfect, the reproducibility of the tests is also better (5.6% of error for k and 2.5% for n).
The difference between Li-B and Li-C is the presence or not of other minerals (dark coloured). They have the same BE, but different CECs, with equal saturation states. Besides, Li-C exhibits similar CEC and BE values, so no other associated minerals can increase the difference between the two parameters. The CEC value is typical of a low-charge smectite, so it can be accepted that the Li-C saturated sample is composed of a nearly pure smectite phase. For the Li-C sample, cleared from the non-clay fraction, the reproducibility of the rheological tests is nearly perfect (0.5% of error for k and 0.7% for n). Moreover, the viscosity is 1.4 times and 2.4 times higher than the one measured in the Li-B and Li-A samples, respectively. Here, the increase in k associated with the decrease in n confirms that the structural network is very strong. The evolution of the rheological parameters due to the increase in the smectite content is remarkable and is evident from the perfect superposition of the curves from the four tests of this sample (Fig. 4). This means that the structural network is equivalent for each one of the tested samples, which is not the case for the Li-A and Li-B samples.
Consequently, the classical saturation procedures were slightly modified by introducing a systematic control (BE measurements) and by increasing the contact time with the chloride solution to optimize the saturation processes.
Although Li saturation tends to expand the range of the rheological parameter values, the rheometric technique using a high-resolution rheometer allows detecting microheterogeneities within the bentonite. Fine mineralogical investigations allow refining the nature of the heterogeneity.
4 Conclusions
This study has shown that the rheological behaviour of Li-smectite suspensions (60 g/l), prepared from a same bentonite, purified mineralogically and chemically with different methods, is always rheofluidifiant, without yield stress and thixotropy, whereas the quantitative determination of the rheological parameters is completely different. A systematic control of mineralogical and physical parameters (XRD and BE) has allowed us to observe an incomplete saturation and the presence of accessory minerals that disrupt the organization of Li particles (assumed to be ideally constituted of one layer) in the suspensions and modify significantly their rheological parameters, also hindering reproducibility.
It appears that the rheological measurements are very sensitive and useful to detect the effect of heterogeneities within the powders. When willing to compare rheological data of bentonite, attention is drawn to the necessity of describing accurately the pre-treatment processes, especially when procedures for exchanging interlayer cations are used, as they significantly influence the structural properties of the clay, and hence its technical properties. Thus, ‘standard’ procedures must be verified and constantly adjusted to the studied sample, as some are less easily saturated than others are. A good interpretation of the rheological measurements needs the detailed knowledge of the mineralogical composition, highly responsible for clay suspensions organization.


