1 Introduction
The final phase of lignin formation proceeds as an enzyme-catalysed but chemically driven mechanism influenced by the physicochemical environment of the reaction. The polymerisation involves the coupling of p-hydroxyl cinnamyl alcohol monomers (such as coniferyl alcohol) via phenoxy radicals generated upon phenol dehydrogenation (Fig. 1). These radicals are coupled in a variety of ways to build up the lignin polymer. The validity of this concept has been established by numerous in vitro studies using enzymes or oxidants to produce lignin-like synthetic molecules: the dehydrogenation polymers (DHPs). These synthetic analogues have been found to contain most of the structural units detected in natural lignin, but in very different proportions. As a consequence, the synthesis of a perfect synthetic lignin is still a challenge. This problem illustrates the difficulty to understand and reproduce the real physicochemical conditions (thermodynamic and kinetic) of the polymerisation process within the cell wall. Previous studies have emphasised several factors that may influence the monomer reactivity, such as kinetic effect, competition between dimerisation and cross coupling, specific orientation [1], template effect [2], or changes in the solvation of radicals [3]. All these assumptions are consistent with the fact that the plant cell wall is a complex, heterogeneous and dense medium. Accordingly, the understanding of the lignin biosynthesis requires the use of new polymerisation conditions in order to test the effect of complex physicochemical parameters. For instance, the hydrophilic/hydrophobic character of the cell wall polymers has been seldom taken into account. Cell-wall polysaccharides contain a large amount of hydroxyl groups and can be considered as hydrophilic substances [1], whereas lignins, built of phenyl propane subunits are rather hydrophobic. These polymers are closely associated in plant cell walls and anisotropic structures such as hydrophilic/hydrophobic interfaces can be formed. Another feature poorly taken into account is that cell wall polymers form dense and interpenetrated networks that are difficult to reproduce by model systems in solution. In order to evaluate the effect of these two parameters (local anisotropy and high local concentration) on the monolignol coupling, coniferyl alcohol was polymerised at the air/water interface, which presents the two characteristics described above. In this paper, we report the characterisation of the reaction system (i.e. the air/water interface) during the course of polymerisation. The analysis of the reaction products was also carried out. They were found to be mostly dilignols. Proportions of dilignols formed during interfacial polymerisation were compared to those formed in reference bulk conditions in order to evaluate the effect of the interface.
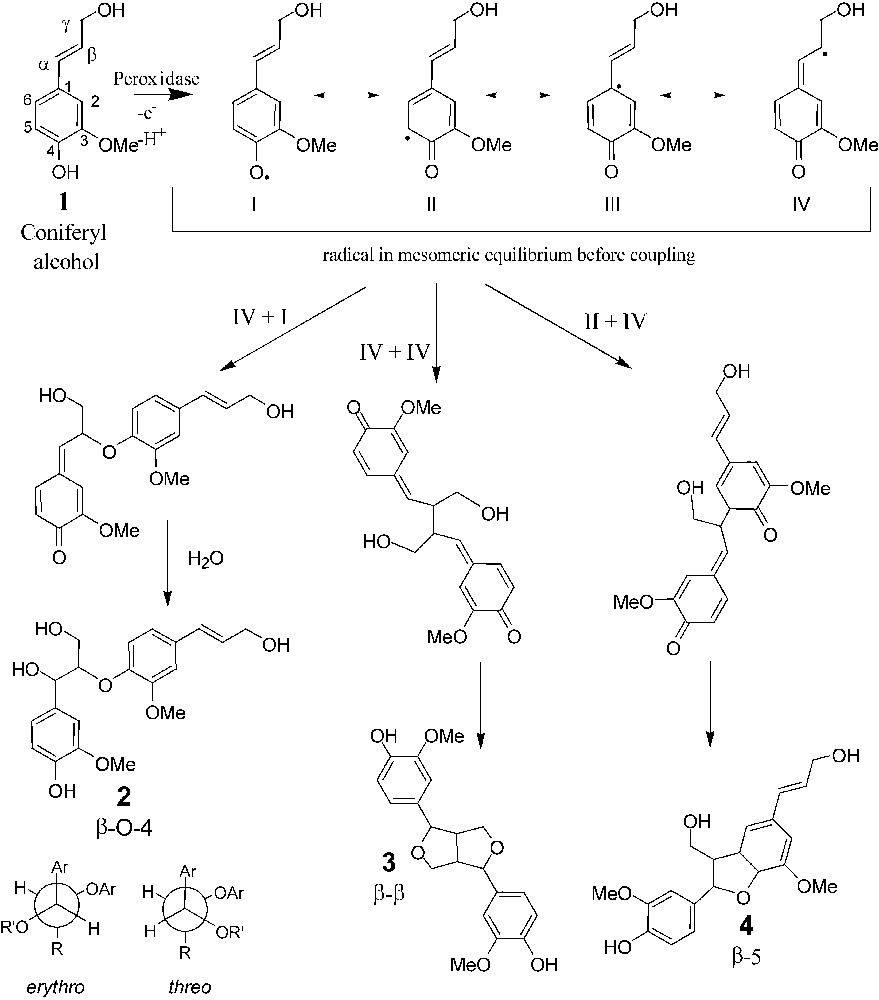
Dehydropolymerisation of coniferyl alcohol. Phenoxy radicals exist as four mesomeric forms that can be coupled to form dimers. β-O-4 exits as erythro and threo forms.
2 Materials and methods
2.1 Experimental system
Coniferyl alcohol, synthesised as previously described [4], was solubilised at 10 mg l−1 in 17-mM phosphate buffer, pH 5.5 to form the sub-phase of the experimental system (Fig. 2). Hydrogen peroxide solution from Sigma (30% w/w in water) was deposited as small drops on the liquid surface until the amount added was equivalent to a concentration of 14.6 mM in the sub-phase. The polymerisation agent was type-VI peroxidase (Sigma, 250–330 units mg−1). It was dissolved in phosphate buffer before spreading by the procedure of Trurnit [5]. The spread quantity of enzyme used in the surface experiments (0.15 mg m−2) was equivalent to 28.8 μg l−1 in the bulk. All glass vessels were cleaned with chromosulfuric acid and rinsed thoroughly with ultra-pure water ( resistivity).

Schematic illustration of the experimental system. S, sub-phase, and , refractive indexes of the air and of the substrate, subscripts ‘i’ and ‘r’ are related to the incident and the reflected beams, and are the electric fields parallel and perpendicular to the plane of incidence, incidence angle, l Wilhelmy plate.
2.2 Surface pressure
The surface pressure was measured by the Wilhelmy method using a mini-balance (KSV, Finland) with a platinum plate.
2.3 Ellipsometry
Ellipsometry analyses optical properties of interfacial thin layers by measuring the change of polarization between the incident and reflected beams. The ellipsometry measurements were done in an air-conditioned room at . They were carried out using a spectroscopic phase-modulated ellipsometer (UVISEL, Jobin Yvon, Arpajon, France) equipped with a xenon arc lamp. Both polarizer and analyser operated at a 50-kHz frequency, and were set to the 0° configuration orientation. The incidence angle was 53.2°. The kinetic measurements were carried out in Brewster conditions at 477 nm. The spectroscopic measurements were monitored between 240 and 820 nm in quasi-equilibrium conditions. The two ellipsometric angles, Ψ and Δ, are linked to the complex ellipticity, ρ, which is the ratio of the Fresnel reflection coefficients, and . is the ratio of the electric field amplitudes parallel to the incident plane, , after and before reflection of the light, whereas refers to the ratio for light with the electric field perpendicular to the plane of incidence, [6] (Fig. 2):
| (1) |
| (2) |
The complex ellipticity measured at this angle is very sensitive to any structure present at the interface. Experimentally, it passes through a positive value close to zero due to the roughness of the interface (capillary waves on a liquid surface). When an adsorption layer occurs with a small thickness, d, such as , the coefficient of ellipticity at the Brewster angle, is defined by:
| (3) |
2.4 Refractive index increment
The specific refractive index increment of DHPs, , was measured using a DSP differential refractometer (Wyatt Technology, USA) and is equal to 0.173 cm3 g−1.
2.5 Surface concentration calculation
From the Drude equation [9] and as a first approximation, the absolute value of the Brewster ellipticity is proportional to the surface concentration. The surface concentration was also calculated from the refractive index according to the de Feijter's equation [10]:
| (4) |
| (5) |
2.6 Absorption coefficient calculation
Using the fit parameters, d and , a numerical point-by-point inversion of the Fresnel equations allows us to calculate k, which is the imaginary part of the complex refractive index related to the extinction coefficient, ɛ as previously detailed for absorbing molecules [11,13]. The extinction coefficient of the layer, ɛ, is calculated as:
| (6) |
2.7 Bulk polymerisation
Coniferyl alcohol, dissolved in 17-mM phosphate buffer (5 ml), pH 5.5 was added during 10 min using a peristaltic pump to a stirred solution (15 ml, final volume of the reaction 20 ml) of hydrogen peroxide (Sigma, 30% w/w in water,) and peroxidase type VI in the same concentration as in the surface experiment ( mM, peroxidase: 28.8 μg l−1). After 50 min of additional stirring, an aliquot was taken out of the solution, diluted in methanol and analysed by HPLC.
2.8 HPLC assays
HPLC was performed according to the procedure described by Wallace and Fry [14] (column Spherisorb 5ODS2, 40-min linear gradient 0–100% of water/butanol/acetic acid 98.3:1.2:0.5 to acetonitrile/butanol/acetic acid 98.3:1.2:0.5). Identification and quantification (external calibration) of coniferyl alcohol and associated dehydrodimers was done using reference compounds synthesized according to the procedure described by Okusa et al. [15] and characterized by NMR, as previously published [16].
3 Results and discussion
3.1 Dehydrogenative polymerisation of coniferyl alcohol at the air/water interface
The polymerisation kinetics was simultaneously monitored by surface pressure and Brewster ellipticity measurements on the coniferyl alcohol solution where hydrogen peroxide had been deposited (Fig. 3). Surface pressure and Brewster ellipticity are two physical measurements very sensitive to any structure present at the interface. The last one becomes negative when an adsorption layer is formed at the interface and as a first approximation [9], its absolute value is proportional to the surface concentration. It was observed that the 10-mg l−1 coniferyl alcohol solution used throughout this study has no significant surface properties. Its surface pressure is null and the Brewster ellipticity has a slight positive value due to surface roughness (capillary waves at the air/liquid interface) [8]. When peroxidase was spread on the coniferyl alcohol solution, without hydrogen peroxide, at a surface concentration of 0.15 mg m−2, no surface pressure and no significant modification of the ellipticity were noticed ( has always a significant positive contribution). It was concluded that the peroxidase is in the dilute regime. There are three steps in the course of the polymerisation at the interface (Fig. 3). In the first one, a lag-time of ten minutes is visible by surface pressure measurements, whereas the absolute value of the Brewster ellipticity increases two minutes after the peroxidase was spread. The difference in the delay of the lag time between these two experimental measurements is due to the fact that ellipsometry is a more sensitive technique than the surface balance one. Ellipsometry allows to observe thin layers in the dilute regime where isolated 2D domains of surface active molecules like DHPs are formed at the beginning of the reaction before a homogenous layer appears at the interface [17,18]. The surface pressure is then smaller than 0.1 mN m−1 and not detectable in our conditions. In the second step, the surface pressure and the Brewster ellipticity increase with time, indicating the formation of a continuous interfacial layer. The third step takes place after 40 min, when surface pressure levels off around 5.5 mN m−1 and the absolute value of the ellipticity at (Fig. 3). The polymerisation reaction in a bulk process was also monitored in the same conditions. The surface pressure was not detectable and the absolute value of the Brewster ellipticity levels off at about . This low value of the ellipticity was related to the adsorption of surface-active products from the bulk. However, surface pressure and Brewster ellipticity values are much smaller than when the reaction proceeds at the air/liquid interface. Thus, it can be concluded that the polymerisation reaction mediated by peroxidase spreading occurs near (or at) the air/liquid interface and as a corollary that coniferyl alcohol diffuses from the bulk to the interface. The rate of the surface pressure and of the Brewster ellipticity evolutions depends on the volume concentration of coniferyl alcohol and on the amount of spread enzyme [18], as expected for such a reaction.
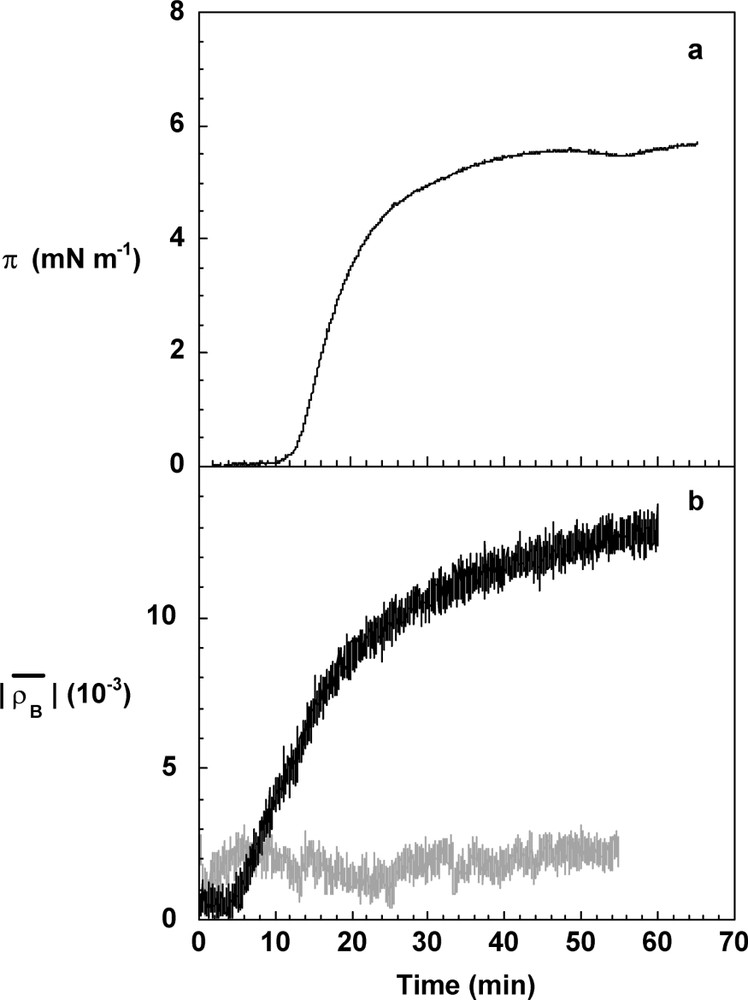
Evolution of the surface pressure, π (a), and of the absolute value of the Brewster ellipticity, (b), during the polymerisation of coniferyl alcohol at the air/liquid interface (black line) and in solution (grey line). The surface pressure during the polymerisation in solution remains undetectable. Coniferyl alcohol was solubilised at 10 mg l−1 in phosphate buffer (50 ml), pH 5.5, in the presence of hydrogen peroxide, 14.6 mM. The used quantity of enzyme was the same in the surface and bulk experiments, and was equivalent to 0.15 mg m−2 or 28.8 μg l−1, respectively.
3.2 Characterisation of DHP layers
The real and imaginary parts of the refractive index and the thickness, d, of the DHP layers were determined after 60 min of reaction from the ellipsometric spectra Ψ and Δ as a function of the wavelength according to the procedure detailed before. The thickness of the DHP layer calculated by fitting a Cauchy law and a one-layer model to the data is and the real part of the refractive index at 589 nm is equal to 1.4723 (Fig. 4a). Thus, the surface concentration of DHP calculated according to the de Feijter equation is 3.3 mg m−2. The imaginary part, k, of the refractive index spectrum of the interfacial layer of DHPs was determined using a numerical point-by-point resolution of the Fresnel equations and the thickness of 41 Å. The absorption spectrum of the layer has a maximum at and a shoulder around . It has the general features of the absorption spectrum of DHPs in a dioxane buffer solution (Fig. 4b). The extinction coefficient spectrum calculated from the ellipsometric parameter k using Eq. (6) and from the surface concentration, Γ, according to Eq. (4) exhibits significantly smaller values than the same spectrum determined for DHPs in solution. The extinction at 280 nm is in the layer and in solution. It can be hypothesized that dehydrodimer structures present in layer experiments have a lower extinction coefficient, since they are chemically different (less conjugated double bonds, see below) than in the bulk experiments. Another hypothesis could be that associations due to the highest local concentrations of DHPs molecules induce a hypochromic effect. Indeed, the volume concentration of DHP in the layer is 804 g l−1, while it is only in the bulk.
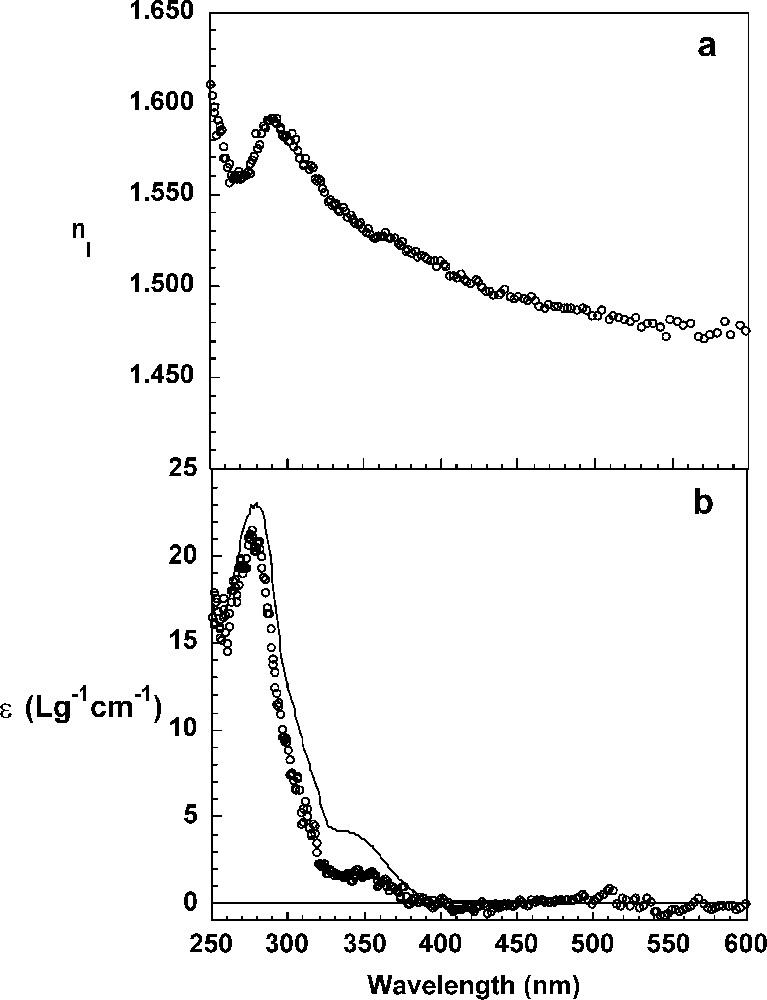
Refractive index, (a), and extinction coefficient, ɛ (b), of the interfacial DHP layer at the air/liquid interface, calculated by numerical point-by-point resolution. The bulk extinction coefficient of DHPs in dioxane/water solution (9:1, v/v) is shown for comparison purposes (continuous line).
3.3 Identification and quantification of the reaction products
After stabilization of the surface pressure (60 min), the interfacial layer was transferred by sweeping in vials containing methanol to stop the reaction and insure a good solubility of the products. A similar procedure was applied for bulk experiments. HPLC analysis of bulk and interfacial samples reveals the presence of unreacted coniferyl alcohol and dehydrodimers (dehydrogenation polymers = DHPs). Quantification of these products indicates that only a minor part of the coniferyl alcohol is incorporated in higher-molecular-weight molecules, since overall recovery yields based on the starting alcohol concentration range roughly between 75 and 85% for both bulk and interfacial polymerisation. As a consequence, it is assumed that the analysis of the dehydrodimers is representative of the reactivity of coniferyl alcohol in these experiments. Since recovery yields present similar values for all the experiments, results are presented as the relative proportion of coniferyl alcohol and of the three main dimers for an easier comparison (Fig. 5). Values are the average of seven independent experiments for interfacial polymerisation and only three different reactions for bulk process, since this latter process was found to be more reproducible.

Relative percentage of coniferyl alcohol and of the three main dimers formed in surface and bulk experiments, as determined by HPLC.
As previously reported [16], the three major reaction products are representative of the β-5, β–β and β-O-4 intermonomeric linkages, whereas 4-O-5 or 5–5 coupling modes were not detected, if they occur. This finding emphasizes the higher reactivity of the β position, as reported in the literature. The relative proportion of the three compounds agrees with previously published data [16]. β-5 Dehydrodimer is the major compound, whereas the two other molecules are formed in approximately equal amount. Results of the bulk and interfacial experiments reveal some differences. The amount of β-5 dehydrodimers is roughly 15% lower in interfacial experiments than in the bulk ones. This results in the increase of the β-O-4, β–β, and CA amounts (4, 8 and 3%, respectively). These amounts are low, but significant, since they are larger than standard errors (Fig. 5). Another difference between the two processes was also evidenced by the change observed of the threo/erythro ratio of the β-O-4 dehydrodimer. In the case of the bulk process, this ratio is equal to 0.63 in good agreement with previous studies [15] and it increases to 0.75 in interfacial polymerisation. Again, the difference between these two values is low, but statistically significant. These results can be interpreted according to two different hypotheses. In interfacial experiments, CA diffuses from the sub-phase to the surface, where it polymerises, whereas in the bulk experiments, CA is pumped into the stirred solution via a Teflon tubing. Since the addition rate of the monomers is a well-known and critical factor in lignin polymerisation, as illustrated by the difference evidenced between ‘Zulauf’ (ZL) and ‘Zutropf’ (ZT) dehydrogenopolymers [19,20], it can be hypothesized that interfacial polymerisation is a ‘Zutropf’-like process, whereas in the bulk it is a more ‘Zulauf’-type, locally at the end of the pumping tube, even though the reaction time for the interfacial reaction is somehow very short (20 min). One of the main structural differences between ZL and ZT DHP is the increase of the β-O-4 linkage content in the ZT process similarly to the increase found at the interface [19,20]. The second hypothesis is based on the fact that the reaction occurs at the air/water interface (or very close), as demonstrated in the first section. In this local environment, the physicochemical properties such as solvation are different from those observed in the bulk. It has been demonstrated that changes in polarity of the polymerisation medium induced by adding organic solvents modify the reactivity of the coniferyl alcohol [21,22]. For, instance the formation of a higher proportion of β-O-4 linkages was reported in the case of a methanol/water mixture [21]. Two arguments support the second hypothesis. The first one is related to the fact that the Zutropfverfahren process results in higher molar mass DHPs, since the monomer concentration is lower and consequently the coupling between monomers and oligomers is favoured at the expenses of dimerisation. If interfacial polymerisation was controlled by the diffusion process, the yield of the dehydrodimers should be lowered, as previously reported in the case of dialysis experiments [23]. As mentioned previously, the bulk and interfacial yields were very similar. The second feature supporting the hypothesis of the modification of the local environment is the change of the threo/erythro ratio of the β-O-4 dehydrodimer between the two synthesis methods (0.63 for bulk polymerisation and 0.75 in the interfacial process). Variation of the threo/erythro ratio has been studied and was found to be related to some physicochemical conditions of the reaction, such as pH [24,25], via the modification of the mechanism of water addition on the quinone methide (see Fig. 1). Based on this previous work, it seems that the addition rate is not a factor influencing the threo/erythro ratio and, to our knowledge, such an assumption was never reported in the literature. Accordingly, the increase in this ratio can also be understood as a local modification of the coupling environment, for instance a modification of the solvation of radicals by water, which may affect the reactivity of the quinone methide.
4 Conclusion
Polymerisation of coniferyl alcohol at the air/water interface confirms the sensitivity of coupling reaction of lignin monomers to the physicochemical environment. This emphasizes the need for understanding and characterizing the physicochemical conditions occurring in the plant cell walls during lignification. Among all the possible scientific investigations, model systems such air/water interfaces may afford some opportunities for us to understand the lignification process by changing in a simple and defined manner the polymerisation conditions. In the case of the air/water interface, this will be done by studying the polymerisation in the presence of various adsorbed molecules (surfactant, polymers...).
Acknowledgments
Thanks to N. Grozev for his contribution to a preliminary part of this work and the French Ministry of Foreign Affairs for financial support.


