1 Introduction
Aging is accompanied by a progressive and almost universal decline of the physiological functions involved in maintaining the body's composition and homeostasis during action, emotion and, possibly, response to aggression [1]. However, the two adrenal steroid hormones [2,3], cortisol and dehydroepiandrosterone (DHEA), influence the age-related processes of energy metabolism, fat-depot distribution, immune function, and play a significant role in human aging [4–7]. Nevertheless, the functions of DHEA and of its sulfate ester dehydroepiandrosterone (DHEA-S) may be difficult to interpret due to its ubiquitous presence and influence in multiple systems in the human body [4,5]. Although the importance of DHEA-S as a sex hormone precursor in older individuals is established [8], the physiological role of this hormone is still poorly understood and remains to be clearly defined [2,3,9,10].
DHEA-S is also thought to act directly as a neuroactive steroid [11–13], and may have immunoenhancing [9], cardioprotective, antidiabetic, and antiobesity properties [7,14]. Its beneficial effects on lipid metabolism, atherosclerosis [15], osteoporosis, and memory retention have recently attracted widespread attention [16]. Besides, an inhibiting role of the glucose-6-phosphate-dehydrogenase, enzyme involved in carcinogenesis, lipogenesis, and production of toxic oxygen free radicals, has been attributed to DHEA-S [5,17].
Moreover, DHEA and DHEA-S have a pattern of secretion that is age-associated [18]. In normal subjects, serum DHEA-S levels peak by the second decade of life and then decline steadily by an average of about 10% per decade. After the age of 80, DHEA-S levels drop to 10–20% of the peak levels [16,17,19,20]. DHEA-S secretion decline with aging has led to the suggestion that DHEA might prevent this process and even to the unsubstantiated recommendation that elderly people take DHEA as a food supplement in order to remain biologically young [21]. This has generated major interest in its putative role as an ‘anti-aging’ steroid [10,13,16]. Moreover, DHEA and its sulfated metabolite are regarded as biomarkers of aging [22,23].
Cortisol is an integral part of the hypothalamic-pituitary-adrenal (HPA) axis' response to stress. Growing evidence supports the view that chronic cortisol excess may lead to hippocampal atrophy [24] and cognitive impairments [24,25] during aging. Cortisol has also diverse metabolic actions, including regulating lipolysis and visceral fat accumulation [26]. Although the steroidogenic changes in cortisol levels are thought to worsen some age-related diseases, their exact biological significance is not completely understood [8,27,28].
Thus, it is clear that changes in adrenal endocrine function with aging may have far-reaching physiological significance [8]. Moreover, over a lifetime, some adrenocortical hormones change dramatically and others little if at all [6,20,27,28]. Consequently, in recent years, the relation between the adrenal function and aging has been the subject of intense interest [27,28].
The objective of the present research is to study DHEA-S and cortisol levels in relation to the aging process. Therefore, we examined the serum concentrations of those steroid hormones in a sample of 63 elderly subjects aged 65–96 years. We explored the influences of age and gender on those concentrations and on the molar ratios of cortisol/DHEA-S. Then, we studied the relation between changes in DHEA-S and cortisol levels and some parameters like body mass index, physical activity, smoking indications, and lifestyle (urban and rural).
2 Materials and methods
2.1 Subjects
This study invited 63 healthy elderly Tunisian volunteers (30 men and 33 women), aged from 65 to 96 (
All the subjects underwent clinical and laboratory examinations. People suffering from endocrine diseases including diabetes and hypertension as well as Parkinson's disease or other neurological disorders were excluded as far as possible. No subject took any drug or received any medical treatment immediately before or during this study. The study was approved by an ethical committee, and all the subjects gave written informed consent before participating in this work.
Blood samples for hormones assay were obtained by venipuncture between 07.30–09.30 a.m., after a requested 12-h fasting. The blood was allowed to clot for 30 min at room temperature, and serum was separated by centrifugation at
2.2 Hormonal measurements
Serum total cortisol and DHEA-S concentrations were determined by the electrochemiluminescence immunoassay method ‘ECLIA’, as recommended by the manufacturers (Roche, Germany), and using the Elecsys 2010 and Modular Analytics E170 (Elecsys module) immunoassay analyzers. The measuring range, which was defined by the lower detection limit and the maximum of the master curve of the assay for cortisol and for DHEA-S, were, respectively, 0.5–1750 nmol/l and 0.003–27 μmol/l.
Seeing that DHEA-S may antagonize the actions of cortisol [29,30] cortisol/DHEA-S ratios were calculated after a simple arithmetic division.
2.3 Other measurements
At the health check, height and weight were measured in light clothing without shoes. The body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared (weight/height2). BMI (kg/m2) was used as an estimate of obesity. Consequently, the normal weight was obtained when BMI varied from 19 to 25 kg/m2. Blood pressure was measured twice by using an Accutor after the participant had been seated at rest for 5 min, and the mean of the two readings was used for analysis. Serum total cholesterol, triglycerides, and calcium were also determined at the biochemistry laboratory.
2.4 Statistical analysis
Conventional standard statistical methods (mean and SD, Student's test, ANOVA) were used to analyze the data. All statistical analyses were carried out with programs from SPSS statistical version 11.0 for Windows. So the SPSS was used to determine the relationships between age, sex, cortisol, DHEA-S, and cortisol/DHEA-S ratios. The results are presented as the mean ± SD.
3 Results
Sixty-three elderly individuals were investigated for the association between two adrenal steroid hormones and aging. The average age of the participants was
Table 1 reports all the characteristics of the studied subjects. The men had a lower BMI and higher heights and weights than the women. The BMI declines with increasing age in all subjects. In the studied population, the BMI values were negatively correlated with the cortisol concentrations (Fig. 1), but positively correlated with DHEA-S concentrations (Fig. 2). The cortisol/DHEA-S ratio was positively related to age (
Baseline characteristics of this study's subjects
| Status variable | Men ( |
Women ( |
| Mean ± DS range | Mean ± DS range | |
| Age (year) | 75.73 ± 7.21 (65–92) | 74.66 ± 8.01 (65–96) |
| Height (cm) | 165.43 ± 10.58⁎ (145–190) | 157.27 ± 12.39⁎ (130–185) |
| Weight (kg) | 64.03 ± 10.85 (45–83) | 61.93 ± 14.80(40–100) |
| BMI (kg/m2) | 23.25 ± 2.38⁎ (18.66–27.99) | 25.21 ± 3.83⁎(18.10–35.29) |
| DHEA-S (μmol) | 1.66 ± 1.12⁎ (0.12–4.68) | 1.03 ± 0.62⁎(0.04–2.49) |
| Cortisol (nmol/l) | 282.42 ± 99.46 (94.10–538.60) | 325.54 ± 134.00 (90.69–653.70) |
| Cortisol/DHEA-S ratio | 0.32 ± 0.31 (0.03–1.36) | 0.66 ± 1.03(0.09–5.94) |
| Calcium (mmol/l) | 2.29 ± 0.19 (1.80–2.90) | 2.27 ± 0.22(1.81–2.90) |
| Cholesterol (mmol/l) | 4.41 ± 0.94 (3.0–6.6) | 4.68 ± 0.88(3.1–6.9) |
| Triglycerides (mmol/l) | 1.15 ± 0.80 (0.51–2.5) | 1.60 ± 1.03 (0.53–2.5) |
| Systolic blood pressure (mm Hg) | 138.60 ± 10.80 (125–148) | 136.00 ± 13.20 (123–150) |
| Diastolic blood pressure (mm Hg) | 72.30 ± 8.00 (64–81) | 69.30 ± 7.30 (62–77) |
⁎ P value <0.05.

Relation between BMI (kg/m2) and serum cortisol concentrations in a Tunisian population sample aged 65–96 years. Points are individual cortisol concentrations (nmol/l). y=266.30−2.85x;

Relation between BMI (kg/m2) and serum DHEA-S concentrations in a Tunisian population sample aged 65–96 years. Points are individual DHEA-S concentrations (μmol/l). y=0.06x−0.12;
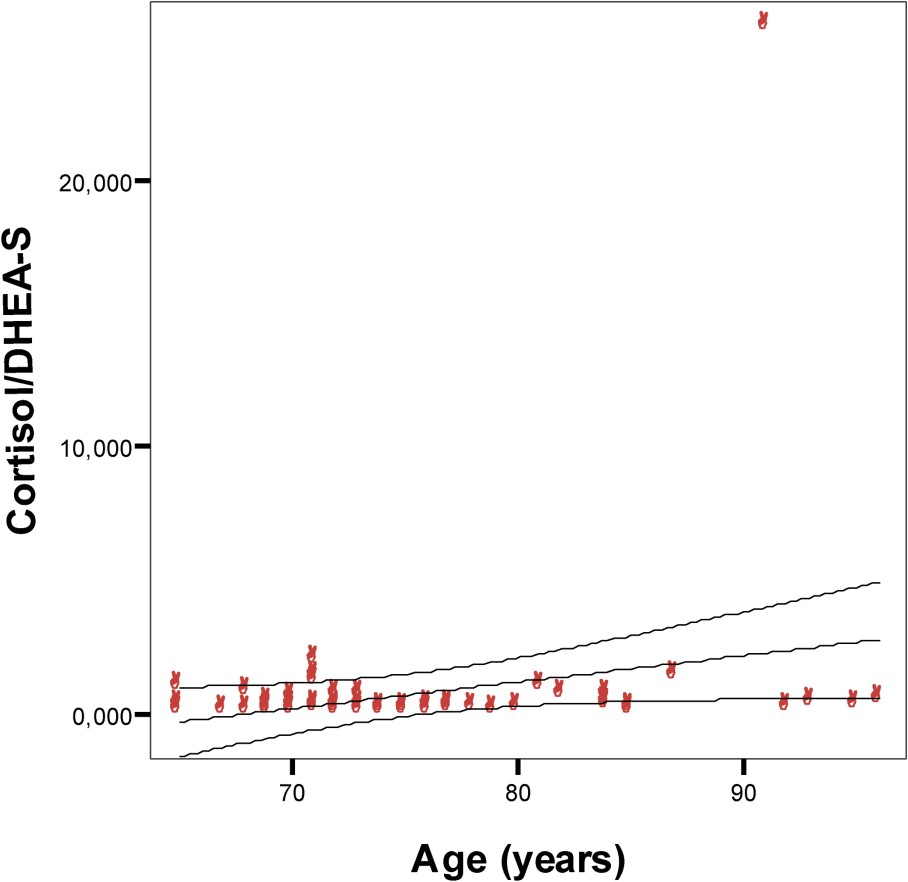
Relation between cortisol/DHEA-S ratio and age in a Tunisian population sample aged 65–96 years. Points are individual cortisol/DHEA-S ratio. y=−6.79+0.10x;
For men, current smoking has no influence on the variation of the morning cortisol rate. In addition, smoking had no relationship with levels of DHEA-S and smokers have lower ratios of cortisol/DHEA-S than non-smokers do. Physical activity and weight were not related to adrenal hormone levels in men or women considering age and BMI.
Our results revealed that DHEA-S levels were significantly different between three groups: 65–75-, 76–85-, and 86–96-year-olds (
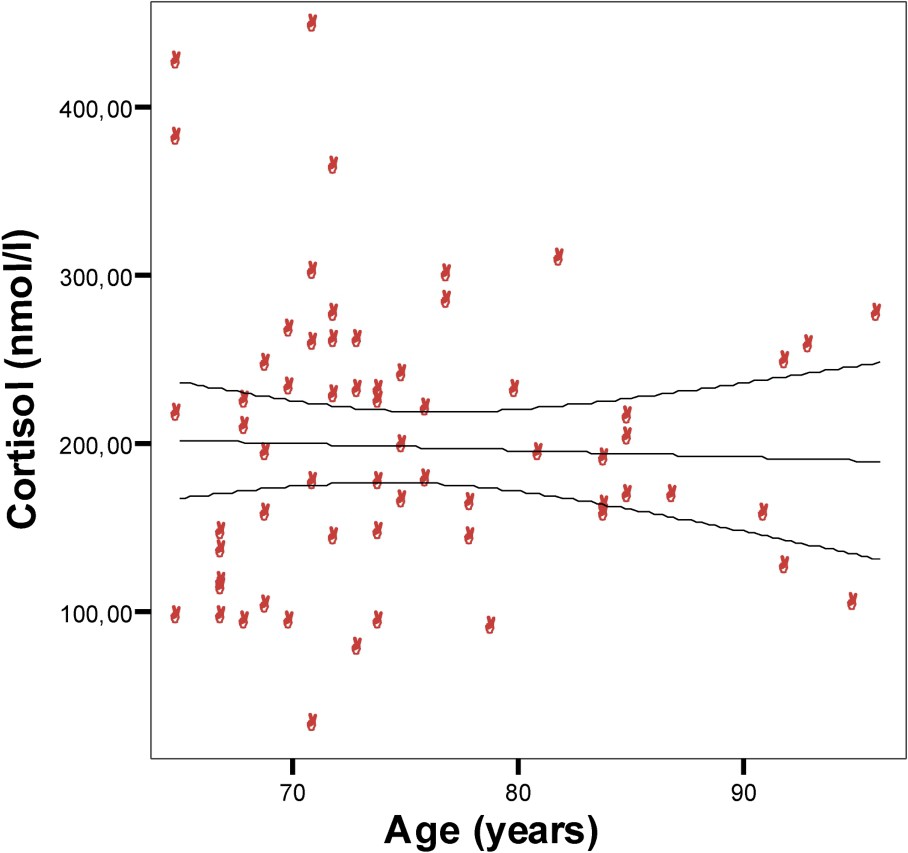
Relation between age and morning serum (08:00) cortisol concentrations in a Tunisian population sample aged 65–96 years. Points are individual cortisol concentrations (nmol/l). y=228.45−0.41x;

Relation between age and morning serum (08:00) DHEA-S concentrations in a Tunisian population sample aged 65–96 years. Points are individual DHEA-S concentrations (μmol/l). y=3.05−0.025x;
There is no influence of lifestyle of rural and urban persons on the morning cortisol and DHEA-S levels in both women and men.
Biologic parameters such as cholesterol (Fig. 6), triglycerides (Fig. 7), and calcium were studied. Those parameters were positively related to DHEA-S, and they show a significant correlation (

Relation between DHEA-S concentrations and cholesterol levels in a Tunisian population sample aged 65–96 years. y=4.41+0.08x;

Relation between DHEA-S concentrations and triglycerides levels in Tunisian population sample aged 65–96 years. y=1.25+0.11x;
4 Discussion
The human adrenal cortex, which undergoes dramatic steroidogenic modifications throughout life [8,22,27,28], secretes not only the essential hormones cortisol and aldosterone, but also DHEA [20]. The production of cortisol and of adrenacorticotrophic hormone (ACTH) is not substantially modified by aging, although a few studies report a slight increase in cortisol with age [6,20,31]. Other reports, however, noticed a cortisol decrease, though smaller than that of other peripheral hormones [32]. Adrenal androgens such as DHEA and DHEA-S have been reported to decline with age [6,20,31]. The DHEA ontogenic secretion pattern is age-associated [1,19], and its fall in elderly subjects has been hypothesized to be involved in the human aging processes [32].
Our results revealed no relation between aging and morning serum cortisol. In fact, the cortisol level has remained unchanged in all subjects. Our findings are compatible with de Bruin et al. [33], and Ravaglia et al. [34], to cite but a few. The latter reported that serum cortisol levels do not change throughout life. It has been suggested that there is an ‘adrenopause’, characterized by low value of blood DHEA-S with maintenance of the cortisol level [13,34–36]. Another study, which found no interaction between age and gender for any cortisol parameter [37], does again agree with our findings.
Recent evidence challenges the view that glucocorticoid function is not altered by aging [14,37]. Nevertheless, others studies reported gender-specific age-related alterations of the HPA axis [8,38]. Zhao et al. [39] found that cortisol rates increase significantly with age, in healthy elderly subjects of both sexes. Laughlin et al. found higher levels of cortisol in women than in men throughout the 50–89 years age range [8]. Moreover, some studies have demonstrated a 20–50% increase in 24-h mean cortisol levels between 20 and 80 years of age [13,14,37]. Many 24-h studies have now demonstrated higher cortisol levels in older than in younger adults.
To our knowledge, HPA axis overactivity, related to stress and possibly to aging, leads to increased cortisol and ACTH levels [40,41]. The progressive elevation of morning cortisol levels in healthy elderly individuals of both sexes could be independent of adiposity. Moreover, in certain cases, the random morning sampling may have underestimated the association of age with cortisol [8]. Other biological factors, such as the corticosteroid-binding globulin (CBG) may be behind the increase of total cortisol [8].
This divergence may be also due to differences in geographic and ethnic origins and/or diet and life style that may affect blood hormone patterns [39,42]. These factors should be further explored as they could have significant implications in all age-related processes that differ with sex [39]. However, other biological measurements can explain this discrepancy [43]. Thus, the well-known age-related change in adrenal androgens persisted into advanced age for both men and women, and exhibited a gender-specific pattern [8].
The secretion and circulating levels of the adrenal steroid DHEA and its sulfate (DHEA-S) decrease with aging [19]. Serum levels of DHEA-S are the highest of all steroids in humans [44,45]. Its long half-life (≈8–10 h), limited diurnal variations, and lack of noticeable changes of metabolism in aging make the serum level a convenient marker of its adrenal production [44]. In addition, the decline of DHEA-S with age has led to considering the intriguing possibility that its serum levels are related to the development of age-associated ‘normal’ changes [46]. Moreover, the decline of this glucocorticosteroid with aging has led to the suggestion that DHEA-S could be implicated in longevity [9].
In an elderly Tunisian population sample, the levels of DHEA-S appeared to be related to aging and were significantly low. Our results confirm the findings of the several studies that have investigated the association between the DHEA-S and aging and of those that found clear differences in DHEA and DHEA-S between young and elderly humans [8,33]. In addition, the uneven decrease of serum DHEA-S in our different age groups may be explained by the significant influence of genetic components on DHEA-S levels, as suggested by Rotter et al. [47]. Several other biological factors, namely sex, have been found to influence DHEA-S concentrations [19,48].
Our study indicates that DHEA-S concentrations are higher in men compared with women. DHEA-S concentration declines with age [13] and remains 10–20% higher in men than in women [49]. This difference according to sex is in agreement with today's understanding of the course of physiological DHEA production in humans [50]. Moreover, the physiological effects of DHEA-S may differ in men and women and may depend on the predominance of other sex hormones such as oestrogens [51]. The effect of DHEA-S level seems to be less important in women than in men, possibly because of different hormonal metabolisms of DHEA and DHEA-S [31,36]. Thus, gender differences may exist biologically and DHEA-S may play a distinct role in the sexes for hormonal reasons [35].
Our results are in agreement with previous studies [7,8,31,35], which found a significant inverse correlation of DHEA-S concentration with age, a higher DHEA-S level in men, and significantly lower values of DHEA-S in women. Other case studies of subjects aged 85 and over found that DHEA-S values in women tended to be higher than those in men are, but the difference did not achieve statistical significance [17,34].
DHEA-S may act as a glucocorticoid antagonist to cortisol in a number of systems [25,29,30]. In agreement with some studies [45], our data show that in 63 subjects, there is a relation between the cortisol/DHEA-S ratios and age. However, the significance of the higher molar ratio of cortisol/DHEA-S in women and its increase with age in both sexes is not clear [8].
Aging itself is known to cause a decrease in muscle mass and an increase in abdominal adipose tissue [52], but it is still unknown if DHEA-S may affect body composition. In agreement with several studies [34] and in opposition to others [7,50], a relationship between DHEA-S concentrations and BMI was observed in healthy elderly Tunisian subjects. Still, the highest BMI rates were correlated to the highest DHEA-S concentrations in women, but not in men. This result could be explained by the status of the body's energy reserves, as well as by a modification of the eating habits of the old person. In fact, research [34,53] found a direct relationship between DHEA-S and BMI in men, and explained this relation by a better nutritional status. Moreover, to our knowledge, in aging humans, the failure of adrenal androgen secretion is accompanied with other endocrine-metabolic features that include changes in body composition including the increase of fat mass and the loss of muscular mass [52]. Thus, the DHEA-S may be regarded as a marker of general health status and could therefore be related to aging [25].
Our population exhibited no relation between DHEA-S and cortisol levels and the lifestyle of rural and urban persons, on the one hand, and physical activity on the other. These findings concerning the physical activity contrast with the data reported by Ravaglia et al. [34] and by Berkman et al. [54], suggesting a direct relationship between DHEA-S levels and physical performance.
Our study found no relationship between DHEA-S levels and cigarette smoking. These findings contrast with the data that showed that smoking was positively associated with DHEA-S [51]. Mazat et al., however, found an interaction between smoking habits and DHEA-S levels in men, but not in women [31]. Thus, tobacco consumption may have a direct effect on DHEA-S level, despite the controversial results obtained: several authors found a higher level of DHEA-S in smokers [15,55], while others did not [56], or even found the opposite [19,57]. The relation between cortisol levels and smoking has not been fully investigated yet.
Our results also revealed a positive relationship between circulating DHEA-S and lipid profile, on the one hand, and DHEA-S and calcium, on the other hand. Concerning the lipid profile including total cholesterol and triglycerides, we found a positive correlation between these biological parameters and the DHEA-S concentrations. These findings were in agreement with the epidemiological data reported by Barrett-Conner et al. [58] suggesting a higher cardiovascular risk in old subjects, especially in postmenopausal women with higher DHEA-S levels. Nevertheless, our findings were in disagreement with the data reported by Ravaglia et al. [34] suggesting a slight negative relationship between DHEA-S and triglycerides in women over 90, and no relationship between the variation of DHEA-S and HDL-cholesterol. Ravaglia et al. observed no relationship of DHEA-S with lipid profile in men. Rudman et al. [48] and Hauner et al. [59] failed to find a correlation between DHEA-S levels and cardiovascular risk in elderly men. Barrett-Conner et al. [15], however, showed an inverse relationship between DHEA-S and lipid profile in men over 50. The findings about the lipid profile and its relation with the DHEA-S concentrations are still contradictory.
As for calcium, it was shown that the osseous mineral loss that occurs at the menopause is, at least, partly due to the rapid decline of the DHEA [60]. In the same way, DHEA-S levels are positively associated with bone mineral density at three sites in women, but not in men. The gender specificity extends to bone metabolism as well [61].
In elderly Tunisian population, the aging process appears to affect cortisol and DHEA-S secretions in different ways: we found that morning cortisol level did not change and that the DHEA-S level decreases with age. We also found a sex-related difference in morning DHEA-S and cortisol levels. Further exploration is needed mainly for cortisol because of its important implications in all age-related processes that differ with gender. Moreover, DHEA-S plays different roles according to gender. In fact, the metabolism of DHEA-S somewhat differs from men to women [31,36]. Up to now, we are unable to draw any deduction as to the role of these observed differences, mainly because the DHEA role in aging is still controversial [36]. However, endocrinological abnormalities have been pointed out as causes and/or consequences of aging itself.
DHEA-S has a pattern of secretion that is age-related [18], and the decline of this steroid has been pursued as a major factor in the development of age-associated disorders [62]. The use of DHEA-S, as a therapeutic agent, can be recommended for old people to correct some disorders caused by aging. Nevertheless, the knowledge of the mechanisms involved in DHEA-S action and the interaction of DHEA-S and cortisol or DHEA-S and other neurohormones is indispensable.


